Research Article Open Access
Study on the Rare Earth Containing Phosphate Rock in Guizhou and the Way to Concentrate Phosphate and Rare Earth Metal Thereof
Qin Zhang1, Yueqing Qiu1, Jianxin Cao1, Yibo Wang2, Jinping HU3, Wei Cheng1 and Tiancun Xiao1,3*1Guizhou University-CSCSTUK Clean Energy Centre, School of Mines, Guizhou University, Caijiaguan Campus, Guiyang City, 550003, Guizhou, China
2Department of Chemistry, University of Alberta, Canada
3Inorganic Chemistry Laboratory, Oxford University, South Parks Road, OX1 3QR, UK
- *Corresponding Author:
- Tiancun Xiao
Inorganic Chemistry Laboratory
Oxford University, South Parks Road
OX1 3QR
Tel: +44-(0)1865-272660
E-mail: xiao.tiancun@chem.ox.ac.uk
Received Date: November 18, 2014; Accepted Date: December 09, 2014; Published Date: December 15, 2014
Citation: Zhang Q, Qiu Y, Cao J, Wang Y, HU J, et al. (2014) Study on the Rare Earth Containing Phosphate Rock in Guizhou and the Way to Concentrate Phosphate and Rare Earth Metal Thereof. J Powder Metall Min 3:126. doi: 10.4172/2168-9806.1000126
Copyright: © 2014 Zhang Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
Guizhou province is one of the main phosphate resources base in China, characterized with the co-existence of rare earth in the phosphate rock. In this work, the phosphate rock samples in Zhijin County, Guizhou province have been collected and analyzed using ICP, X-Ray diffraction, Scanning microscope, Infrared spectra and X-ray photoelectron spectroscope. It is found that the total content of rare earth elements in the phosphate rock is about 0.1 wt%, amongst which Y, La, Ce, Pr, Nd and Sm are the main rare-earth components and co-sit the lattice of the apatite. The phosphate content in the as-collected rock is more than 20 wt%, and impurities such as quartz, calcite and clay are also present. The raw phosphate rock physically was treated with flotation to remove the impurities such as quartz and calcite; a refined phosphate with rare earth elements of more than 0.1 wt%. The refined phosphate rock is then treated with excessive H3PO4 to dissolve the apatite; the residue from the dissolution gives an earth enriched precipitation, which contains up to 2 wt% rare earth elements. Compared to the conventional way to use H2SO4 to extract phosphate, the treatment of the refined phosphate with phosphoric acid leads to significantly improved rare earth oxide extraction.
Keywords
Phosphate; Rare earth; Calcite; Quartz; Apatite
Introduction
A vast phosphate rock sedimentary reserve has been discovered in Guizhou province, south west of China, which is named as Kaiyang Phosphate Rock Reserve [1,2]. The characteristics of the phosphate reserve are that its phosphate content is relatively low, but it has some heavy rare earth elements such as yttrium and light rare earth such as lanthanum and cerium. Therefore this reserve is regarded as a vast phosphate reserve as well as a resource for rare earth oxides. However, the phosphorus content is in the range from 17 wt% to 24 wt%, which is poor to medium grade phosphate rock reserve [3-5].
It is estimated that there is about 1.248 billion tons of phosphorus deposit in the region which also contains about 1.446 million tons of rare earth oxide. The phosphorus deposit is distributed in 3 layers concurrence siliceous apatite deposit layer, siliceous phosphorus rock deposit, layering apatite deposit.
In terms of composition, the phosphorus rock reserve can be classified into agglomerate siliceous phosphate, phosphate rock containing dolomite and bioclast. The phosphate rock is mainly composed of calcite floroapatite, which is present in the form of noncrystalline, cryptocrystalline and colloid rock. Their main gangue is calcite, dolomite, and ankerite [5-7].
The impurities in the reserves include dolomite, calcite, quartz, clay, sphalerite and pyrite. The key feature of Kaiyang Phosphorite reserve is that it contains some amount of rare earth elements especially yttrium and light rare earth such as La, Ce, however, the content is lower [8-10].
Up to now, there has been lots of research into upgrading and extracting phosphate from the rock, which normally adopts the milling, flotation followed by acid leaching approach. This is a general processing adopted for all the phosphate rock [2], which mostly enriches the phosphate, but the rare earth element may be lost with the gangue. There has been little detailed structure and characteristic analysis on the Kaiyang phosphorus rock and the way to benefit both phosphate and rare earth has not been reported.
In this work, we have employed various analytical techniques to characterize the composition and structure of the Kaiyang phosphate sedimentary. The state of phosphate and rare earth elements in it has been analysed. Also a way to extract phosphate and rare earth elements has been develop.
Experimental
The feed used in this study was Guizhou Kaiyang medium grade carboneous siliceous phosphate. Chemical analysis was conducted by inductive coupled plasma spectroscopy (ICP) and ICP-MS.
The crystalline structure and phase component of the samples were determined by X-ray diffraction (XRD) using a Philips X-PeRT Pro Alpha 1 diffractometer operating with Cu Kα radiation (λ=1.5406 Å) at a tube current of 40 mA and a voltage of 45 kV. Data were collected over 2θ values from 20° to 80° at a speed of 1°/min.
The X-ray photoelectron spectroscopy (XPS) measurements were carried out using a Perkin-Elmer RBD upgraded PHI-5000C ESCA system with monochromatic Mg Kα excitation and a charge neutralizer. All bonding energies were calibrated to the C 1s peak at 284.8 eV of the surface adventitious carbon.
Fourier transform infra-red (FTIR) spectra were carried out by diffused reflectance using a Bruker Vertex-70. The geometry and morphology of the phosphate rock at different stages were investigated by JSM840F scanning electron microscopy (SEM), and the element analysis were determined by energy dispersive X-ray (EDX) in the scanning SEM mode.
A 40 mL hanging cell floatation machine is applied to conduct pure phosphate rock flotation tests. The rock sample of each test is 1.5 g, adding 25 ml distilled water, the action times of modifier and collector are kept for 1 minute in both cases, the floated sample is collected, filtered and weighed, and the recovery yield is calculated
Results and Discussion
The Kaiyang phosphate rock sedimentary is generally low to medium grade reserve. In this research, 8 medium grade phosphate rock samples have been collected from different points and mixed evenly. The composition of the samples is given in Table 1.
| Element | P2O5 | CaO | SiO2 | MgO | Al2O3 | FeOx | F | total Re2O3 |
| Content wt% | 21.4 | 53.1 | 9.2 | 8.36 | 6.1 | 2.51 | 2.1 | 0.089 |
Table 1: the composition of Kaiyang Phosphate Rock.
It is seen that the Kaiyang phosphate reserve contains about 21.4 wt% of P2O5, it also contains significant amounts of Ca, Mg, MgO and Al as the metal components. There is a small amount of F, and the total rare earth metal oxide content is about 0.089 wt%.
It is seen that the Kaiyang phosphate reserve contains about 21.4 wt% of P2O5, it also contains significant amounts of Ca, Mg, MgO and Al as the metal components. There is a small amount of F, and the total rare earth metal oxide content is about 0.089 wt%.
Each of the rare earth elements in the Kaiyang phosphate is measured in the raw rock and the results of the metal are shown in Table 2. The low non-detectable rare earth elements are not included. The high content of rare earth elements in Kaiyang reserve are Y, and La, Ce, the level of Nd is medium, and some Sm, Dy and Gd are also detected, although the main rare earth elements are light ones. Overall, the total rare earth content is high, in agreement with the literature.
| Rare-Earth Element | La | Ce | Nd | Pr | Sm | Dy | Gd | Y |
| In the raw rock:wt% x1000 | 0.24 | 0.205 | 0.12 | 0.036 | 0.032 | 0.036 | 0.031 | 4.04 |
*The data are from ICP analysis
Table 2: Rare earth element* content from raw phosphate rock.
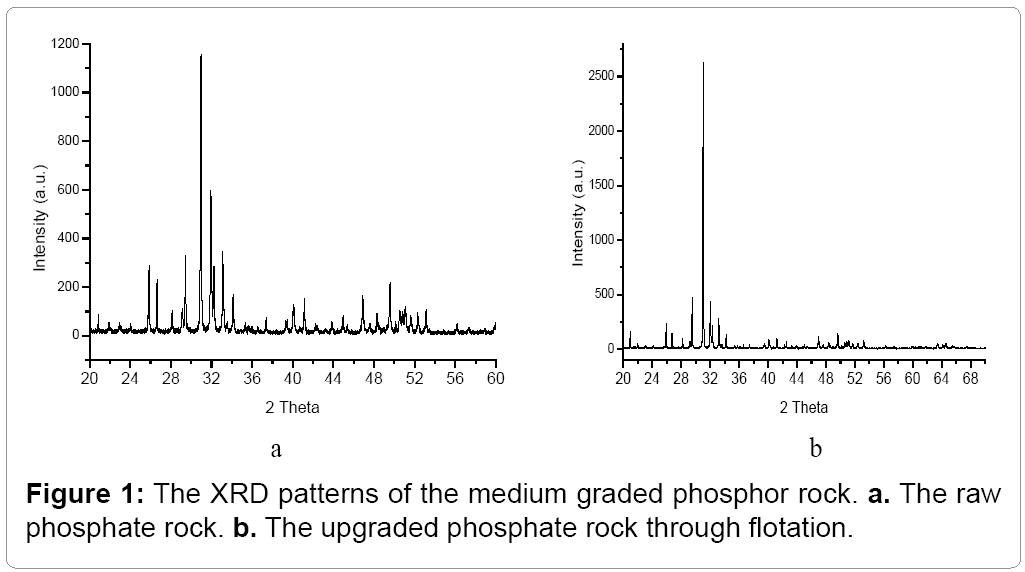
The XRD patterns of the Kaiyang phosphate rock are shown in Figure 1. The crystalline phases in the Kaiyang phosphate rock include quartz, fluorapatite which may contain La and Y even Gd in the crystallites. However the main phosphate crystalline phase is fluorapatite, which accounts for 90% of the phosphate in the rock. The gangue in the Kaiyang phosphate rock are dolomite and calcite, which are not very high, however, pure CaF2 phase is not detected which suggests that the F is mainly in fluorapatite [11-14].
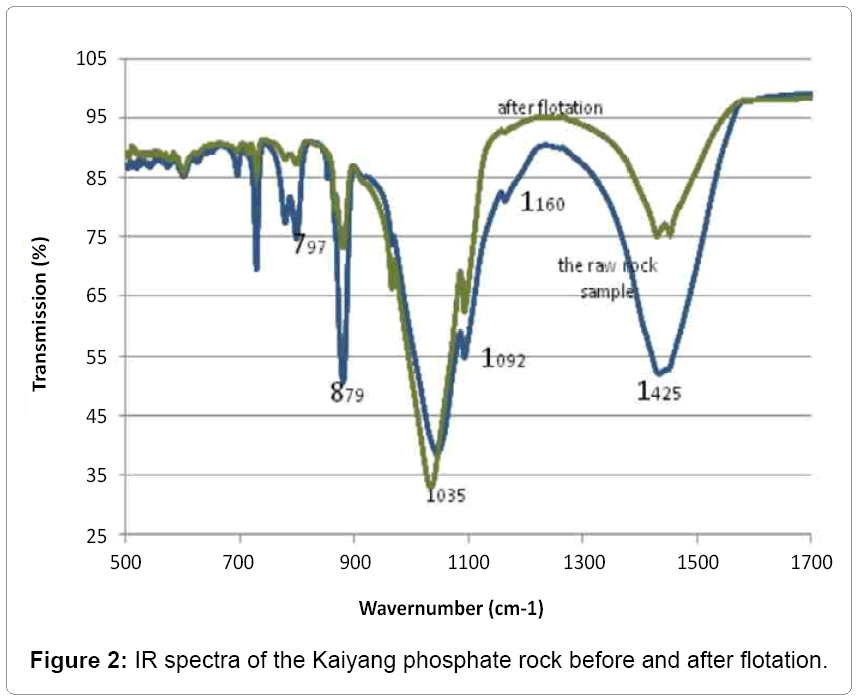
The infrared spectra of the raw Kaiyang phosphate rock are shown in Figure 2. The raw phosphate rock has the main peaks at around 1035, 602 and 501 cm-1 are ascribed to the apatite, maybe due to the rare earth existence in the lattice of the fluorapatite phosphate, the frequencies of the P=O vibrations tends to be lower than the ones containing no rare earth [13-15].
The bands at 1457.38, 1430.38 and 879.2 cm−1 are due to the vibrations of C=O in the calcite [12,14-16] the peaks are strong, which suggests that there is a significant amount of calcite in the raw phosphate rock. The peaks at 1092, 797.63, 692.89 and 516.27 cm−1 are from Si–O stretching attributed to quartz mineral [12]. The IR results are generally in agreement with the XRD results, which also revealed that these phases are present in the Kaiyang phosphate rock.
The X-ray photoelectron spectroscope (XPS) is a powerful tool to analyse the states of the elements and also their surface composition in the materials. In this work we used XPS to analyse the Kaiyang phosphate sample, and the results are shown in Table 3.
| The raw phosphite rock | The floated sample | ||||
|---|---|---|---|---|---|
| Name | Position BE | %At Conc (Atomic Concentrations) |
Position BE | %At Conc (Atomic Concentrations) |
|
| P 2p | 134.7 | 4.864 | 135.03 | 6.428 | Na3PO4 (132 eV) |
| O 1s | 531.8 | 49.661 | 531.95 | 43.721 | |
| Ca 2p 1/2 | 351.56 | 4.347 | 352.03 | 3.588 | |
| Ca 2p 3/2 | 348.1 | 6.508 | 348.41 | 5.414 | like CaF2at 347.8 eV, or CaCO3 (346.7 eV) |
| C 1s | 285.54 | 28.018 | 285.88 | 28.478 | |
| F 1s | 684.51 | 2.411 | 684.92 | 2.979 | Metal-F |
| Si 2p | 104.07 | 3.529 | 103.78 | 3.12 | Quartz |
| Y 3d | 154.81 | 0.661 | 154.88 | 0.692 | In forms of oxides, like Y2O3, not YF3 |
Calibration to O1s at 532 eV, metal oxides
Table 3: The XPS analysis results of the Kaiyang raw phosphate rock and floated sample.
It is seen that the surface of the raw Kaiyang phosphate rock is mainly covered with oxygen element, which can be understood as the phosphate, quartz, calcite and even dolomite all contain oxygen. The surface phosphorus content is only 4.68 at%, which is in the form of PO3-, as confirmed by XRD (Figure 1), which showed that the phosphorus is phosphite and not phosphate group. The carbon may come from calcite or the surface contained carbon. Silica content in the surface is about 3.52 at%. It is interesting to see that although there are La, Ce as evidenced by ICP-MS and XRD, the surface of the raw phosphate sample has Y, whose content is about 0.661 at%. The F is also in the form of metal fluoride attach to the Ca, but no separate CaF2 detected in the XRD, suggesting that the F and Y may co-exist with the phosphate.
The physical floated sample to upgrade the phosphate rock
To remove the gangue in the rock, the raw phosphate rock was floated with a water solution of pH at 5.0 with fatty acid as the collector. The P2O5 content can be upgraded to 33.85 wt% with recovery rate of 60%.
The composition of the floated sample is given in Table 4. It is seen that the P2O5 increases with F and rare earth oxides while the MgO, SiO2 and Al2O3 decrease significantly, this further supports the XRD results, which show that the phosphate, F and rare earth elements coexist in the rock. The less significant change of CaO content is due to the Ca present in both the apatite and also dolomite and calcite.
| P2O5 | CaO | MgO | SiO2 | Al2O3 | TFe2O3 | F | Re2O3 |
| 33.85 | 45.04 | 4.24 | 3.7 | 4.75 | 1.65 | 3.47 | 0.162 |
Table 4: Multi-element analysis of the floated pphosphate rock.
The XRD pattern of the floated phosphate rock is shown in Figure 1b, it is seen that the gangue phase such as SiO2 and CaCO3 decreases significantly while the main phase, the rare earth containing calcium fluor phosphate increase in intensity, which is in agreement with the elemental analysis results.
The IR spectrum of the refined phosphate rock is also shown in Figure 2. These intensities of the bands correspond to SiO2 and CaCO3 drop while the ones arising from PO3 - increase, in agreement with the XRD results.
The XRD and IR results give the bulk composition of the floated phosphate sample, the surface composition of the sample were analysed using XPS, and the results are shown in Table 2. It is seen that the states of the elements e.g., the binding energy position, of the P, Ca, Si, F and Re do not change compared to the raw sample, suggesting that the flotation does not change the surface property. However the composition of the element content changes. The P, F and Y content increases while the Ca and Si decrease. It is interesting that no Al or Mg is detected in XPS results, which may be covered by the phosphate, and rare earth metals.
The rare earth metal content in the residue of the acid treated floated phosphate
Phosphate rock is most often used for fertilizer or phosphoric acid production using sulfuric acid extraction into the process of phosphoric acid production. Phosphoric acid is normally produced by a wetprocess, in which the phosphate rock reacts with a mixture of sulphuric acid. Calcium sulphate is the solid precipitation and aide products such as phosphogypsum and can be filtered away. In this way, the rare earth can easily get lost in the filtered precipitation. The sulfuric acid extract process is shown as follows:
Ca5F(PO4)3+5H2SO4+nH2O=5CaSO4. nH2O+3H3PO4+HF
The rare earth elements may react with sulfuric acid to give Re2(SO4)3, however, the reaction may not be favored as the RePO4 is much more stable than Re2(SO4)3.
2RE PO4+H2SO4=2RE (SO4)3+2H3PO4
The rare earth element content in the residue of the H2SO4 treated phosphate rock is mainly gone with the phophogysum, where the rare earth element is analysed and the result is shown in Table 5.
| Rare-Earth Element | La | Ce | Nd | Pr | Sm | Y |
| In the raw rock:wt% x1000 | 0.32 | 0.28 | 0.25 | 0.046 | 0.032 | 20.7 |
Table 5: Rare earth element* content from the residue of the H2SO4 treated phosphate rock.
It is seen that there is not a big difference between the rare earth element in the residue and the one in the raw phosphate rock, although the heavy re like Y is significantly concentrated in the residue. This is that the heavy Re sultate is less soluble in the the extract system.
Here we tried to use 50% H3PO4 to treat floated phosphate rock at the ratio of the rock to acid ratio 1:4, we found that most rock was dissolved in the solution, which can be expressed as follows:
Ca5F(PO4)3+8H3PO4+ nH2O=5Ca(H2PO4)2+nH2O+HF
This is a soluble solution, while the RePO4 is insoluble in the aqueous system, which is highly concentrated with the gangue such as SiO2 and clay.
The elemental analysis of the residue in the H3PO4 treated system has been analysed for the rare earth content and the result is shown in Table 6.
| Rare-Earth Element | La | Ce | Nd | Pr | Sm | Dy | Gd | Y | Yb |
| in the H3PO4 treated residue wt% x1000 | 213.5 | 298.4 | 76.3 | 20.6 | 12.1 | 9.3 | 10.4 | 698.6 | 8.1 |
Table 6: Rare earth element content from the H3PO4 treated phosphate rock residue.
It is noted that a significant concentration of rare earth has been achieved in the residue of the H3PO4 extracted system. The Y content is as high as 0.698 wt% in the residue, which can be further refined for rare earth extraction.
Conclusion
The Kaiyang phosphate rock samples have been analyzed using elemental analysis, XRD, IR and XPS for its composition and the rare earth content. It is seen that the P2O5 in the phosphate rock is about 25 wt%, which is mainly in fluoapatite form, while there is quartz and calcite and dolomite present in the raw rock. The rare earth elements include light, such as La, Ce and heavy rare earth like Y.
The rare earth and F co-exist in the lattice of the calcium rare fluroapatite, while the surface of the raw phosphate rock is mainly covered with Ca, O, F, P, and interestingly Y, which are mostly present in oxide form.
The flotation process can upgrade the phosphate rock and increase the P2O5 content to 33 wt% with more than 60% of recovery rate. The process increases the content of P, Ca, F and rare earth in the upgraded sample, suggesting that these elements exist in the same phase. The flotation removes mostly MgO, some quartz and calcite, but did not change the surface status of the elements.
The extraction of the phosphoric acid with sulfuric acid can give pure phosphoric acid, but also dissolves the rare earth elements in the phosphoric acid and the calcium lead to phosphogypsum. The rare earth element has a relatively small increase in the residue; however, extracting the calcium dihydro phosphate using H3PO4 can significantly increase the rare earth content in the residue which may be a way to enrich the rare earth elements.
We would like to thank the Ministry of Science and Technology of China for the financial support for the international collaboration between Guizhou University and Dr Tiancun Xiao from Oxford University.
References
- Zhang Q, Long Z, Mao S, Feng Y, He F, et al. (2010) Study on pure mineral floatation tests of low-grade phosphate ore in Zhijin, Guizhou province, China. Publ. Australas. Inst. Min. Metall.
- Zhao W, Zhang W, Chen K, Wu Q, Linhu C, et al. (2010) Study on the interaction mechanism of flotation reagents and rare-earths-bearing phosphate ore. International Mineral Processing Congress (IMPC).
- Huixin J, Junqi L, Fuzhong W (2011) Kinetics and three-dimensional graph characterization on flotation of Zhijin Xinhua REE bearing phosphorite ores. Journal of the Chinese Rare Earth Society, 29:239-247.
- Liang LI, Qin Z, Song-wei H (2010) Development and application of automatic adding-reagent system in continuous pilot flotation experiment of phosphate rock containing rare-earth elements. Industrial Minerals and Processing, 39: 10-12.
- YunL (2009) Preliminary study on the grinding of medium and low grade rare-earth containing phosphate ore from Zhijin, Guizhou. JinshuKuangshan, 3:71-72,76.
- Betz, EW (1981)Beneficiation of Brazilian phosphates. Dev Miner Process.
- Hui-xin J,Hua W, Li Jun-q, x Xiao-hao M, Ping-yuan Zet al. (2008) Experimental study on flotation of Xinhua rare earths-bearing phosphorite ore. GuochengGongchengXuebao, 8: 453-459.
- Gong WQ, Warren LJ (1991) Research on recovery of phosphate minerals from the Mount Weld deposit by froth flotation. AusIMMExtrMetall Conf.
- Harbi HM, Eldougdoug AA, El-Shahawi MS (2011) Mineral processing and extraction of rare earth elements from the WadiKhamalNelsonite Ore, Arabian Journal of Geosciences,4: 353-363.
- Zhang, X-m (2004) Study on the beneficiation process of phosphate rock containing rare earth elements. HuagongKuangwu Yu Jiagong, 33: 12-13.
- Born H, Lenharo SLR, KahnH (1996) Mineralogical characterization of apatites from Brazilian phosphate deposits with reference to flotation behavior. TransInst Min Metall, Sect. B, 105: 117-126.
- Knubovets, YurkovaRG LA, (1981) Use of physical methods for the evaluation of the results of the beneficiation of Toolsephosphorites. ObogashchRud, 26: 27-29.
- MozheikoFF(2002) Composition of phosphorites of the Mstislavl deposit. VestsiNats. Akad. NavukBelarusi, Ser. Khim. Navuk.
- Uçuruma M, Toramana ÖY, Depcib T , Yogurtçuoglua E, et al. (2011)A Study on Characterization and Use of Flotation to Separate Unburned Carbon in Bottom Ash from Cayirhan Power Plant. Energy Sources, Part A, 33: 562-574.
- Kholomyanskii IY (1971) Effect of roasting on the properties of phosphorites. Khim, 47: 111-112.
- Jorjani E, Amir Hossein B, Rezai B (2007) Determination of rare earth elements in products of Chadormalu Iron Ore Concentrator Plant (Iran) from beneficiation point of view. JChemChemEng, 26: 11-18.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 15616
- [From(publication date):
December-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10866
- PDF downloads : 4750