Study on Factors Affecting Iron Removal from Kerf Loss Slurry Waste Using Acid Leaching
Received: 02-May-2017 / Accepted Date: 07-May-2017 / Published Date: 16-May-2017 DOI: 10.4172/2168-9806.1000167
Abstract
It is significantly valuable to recover silicon and silicon carbide powder from kerf loss slurry. Iron fragments undermine both the performance of cutting efficiency and the quality of recycled powder, thus requires to be removed. The current study is to shed lights on the effect of the several physico-chemical parameters on the efficiency of iron removal during acid leaching. The significance of each parameter is studied using the method of analysis of variance. The results show that the acid leaching is an effective technique to remove iron by investigating the theory and practice. Leaching duration is a dominant parameter to determine the leaching rate, followed by the effect of temperature and acid concentration.
Keywords: Kerf loss slurry; Acid leaching; Silicon powder; Analysis of variance
95091Introduction
Worldwide growth of photovoltaics has been increasing exponentially for the last two decades because of the increasing global demand for renewable and clean energy. By 2050, solar power is anticipated to become the world’s largest source of electricity, with solar photovoltaics and concentrated solar power contributing 16% and 11% respectively [1]. The crystalline silicon solar cell has been dominating the photovoltaic industry (accounting for more than 90%) for the advantages of cost-competitive, producing process and production quality, which has been experiencing dramatic development as well. During the manufacturing of silicon wafer, solar grade silicon is melted and solidified into either single-crystal or polycrystalline silicon ingots. The ingots are sliced by wire sawing using abrasive slurry comprising silicon carbide particles in a glycol-based solution. 25-50% of the silicon ingot is wasted to the cutting slurry to form kerf loss slurry together with the abrasive solution [2]. The kerf loss slurry is typically composed of SiC and Si particulates, solvents and metal impurities. Considerable attempts have been made to recover this waste for the benefits of environment and economic values [3-6]. The liquid solvents can be easily separated using a variety of techniques, such as filtering and distilling. The Si cut-off and abrasive SiC particles, especially the Si particulates, are considered to be the most valuable in the slurry waste, which have drawn particular attention for reutilization. The recovering strategies for Si cut-off and SiC fall into three categories: 1) recycling the Si particles as the feedstock for silicon ingot production using a series of physical and chemical techniques, which was reviewed in detail by Drouiche et al. [3]; 2) recycling the SiC particles as abrasive agent or recovering the SiC for other applications [5,7]; and 3) directly reuse the Si and SiC without separation, for example, used for the fabrication of ceramic materials [7]. In addition to the Si and SiC, there are about 5 wt% metal impurities, mostly iron from the steel cutting wires in the kerf loss slurry. To be specific, metal impurities need to be removed before the following separation of Si and SiC techniques, such as sedimentation [5] and centrifugation [8]. These impurities usually need to be removed, as they are detrimental to the recovering process and final products. Iron is known as harmful “lifetime killer” impurities in solar silicon, which shortens the lifetime of excited carriers in silicon solar cell and disturb power generation [9]; iron fragments decrease the cutting efficiency of recycled SiC particles [2]. The anticorrosion ability of SiC ceramics is also demonstrated to be reduced with iron impurities [10].
The techniques reported in literatures to remove the metal impurities, mainly the Fe in the kerf loss slurry, are summarized in Table 1. The major technique is acid leaching, which exhibits high efficiency and is quite popular in the recovery or removal of metals in hydrometallurgy area. The drawbacks of acid leaching lie in the possible hazards to the environment resulted from using non-eco-friendly acid solutions, but can be largely relieved with proper recycling and posttreatments. Electrokinetic separation and magnetic sedimentation treatments have been tested on a laboratory scale to test their viability. However, these techniques are still suffering the issue of time cost and yield, and remain to be developed for the industry application. For instance, the duration of electrokenetic and magnetic treatment can be as long as more than 1 day for 15 g slurry [2,11]. The attained iron removal rate was as high as 99.98% on the cost of high solvent/ solid volume ratio (nearly 1000:1) for 15 g samples in the magnetic sedimentation process [11-13]. Thus, the acid leaching is still the most promising technique to be applied in industry. However, there is still no sufficient understanding of the effect of various parameters on the leaching efficiency. The present study aims at investigating the effects and significances of different physico-chemical parameters, including temperature, time, stirring speed and acid concentration, on iron removal from kerf loss slurry in a stirred tank reactor. The significance level of these parameters is evaluated using analysis of variance (ANOVA). The waste powder, before and after leaching, are characterized.
| Separation techniques | Iron removal fraction (wt%) | Separation duration (h) |
|---|---|---|
| Acid leaching [2] | 89.4 | 40 min |
| Electrokinetic[2,12] | 90.2 | 30-120 h |
| Hydrobromination[6] | 94.86 | >2h |
| Sedimentation [11] | 14.4 | 24 h |
| Magnetic sedimentation [11] | ~99.98 | 24h |
| Acid leaching [13] | 87.7 | >1h |
| Acid leaching+high temperature [13] | 97.5 | -- |
Table 1: Reported available iron removal techniques.
Experimental
Materials and leaching procedure
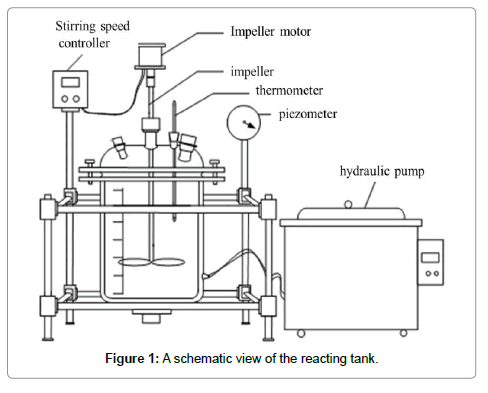
The studied kerf loss slurry waste was provided by the company of REC Solar Norway, of which the polyethylene glycol and large SiC particles (>20 μm) had already been recovered. The leaching apparatus, a stirred tank reactor, was employed to remove the iron fragments. A schematic view of the reactor is shown in Figure 1 which is capable of realizing reaction, stirring, water supplying and draining, and temperature control. In the experiments, 200 g of the slurry waste was added into the tank for leaching, under the parameters of certain temperatures (30-70°C), reaction duration (1.5-3.5 h), hydrochloric acid concentration (10%-23%, PH), and liquid-solid ratio (3.5:1 to 5.5:1). When the leaching was finished, the acid solution was recycled; the solid particles are washed, and dried in an oven at 100?. The achieved powder was then subjected to weighting and measuring.
Characterization and measuring techniques
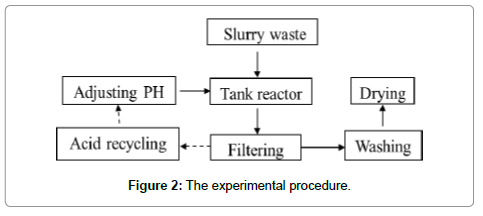
The as-received waste was characterized using XRF (X-Ray Fluorescence) to determine the composition of the powder, in company with potassium fluosilicate titration [14] to test the quantity of free Silicon. Matersizer 2000 was used to measure the particle size distribution of the powder. Phase analysis was carried out using XRD (X-Ray Diffraction) technique and the morphology of the powder was visualized with the assistance of SEM (Secondary Electron Microscopy). TEM (Transmission Electron Microscopy) was utilized to show the morphology of small particles. The content of iron was measured accurately using orthophenanthroline photometric method (YS/T 575.5-2007) and XRF (X-Ray Fluorescence) method (Figure 2).
Results and Discussion
Characterization of the Kerf loss slurry
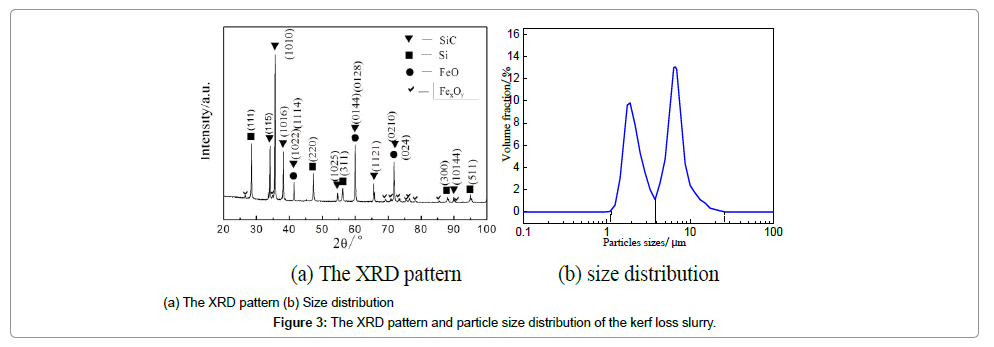
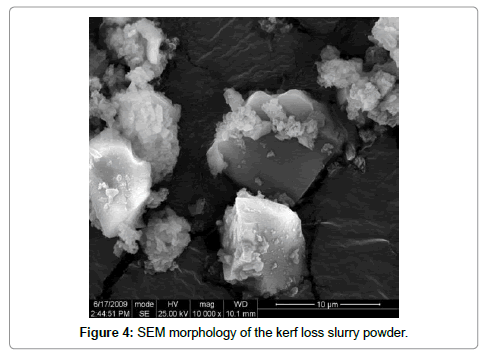
The XRF results of the impurities are summarized in Table 2. The contents of Si and SiC particles were measured to be 36.00% and 58.19 wt% respectively. The reported mass ratio of Si was nearly 10% in the review [3] in that the large SiC particles were recovered in the REC Solar company. Fe, existing in the form of oxide, is the major element of the inclusions which derives from sawing wires, and copper fragments are from the coating materials of the sawing wires. The particle size distribution is shown in Figure 3. There are two peaks (at 1.9 and 6.6 μm respectively) in the distribution curve, quite similar to the results of reference [13]. The volume fraction of particles with the size of 1.0-3.8 μm is about 40%, while particles with the size of 3.8-26.0 μm are around 60%. It has been demonstrated that the small particles are mainly Si, and large particles SiC in our and others’ previous studies [5,13]. The morphology of powder is shown in Figure 4. It can be observed that the irregular-shape SiC particles are nearly 10 μm and are attached with many small particles, which validate the particle size distribution cure as well.
| Element | Si | SiC | Fe | Cu | Na | Ca | Al | Ti | Zn | Mg |
|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 36.00 | 58.19 | 4.17 | 0.79 | 0.37 | 0.11 | 0.06 | 0.05 | 0.04 | 0.04 |
Table 2: The XRF results of the impurities in the kerf loss slurry.
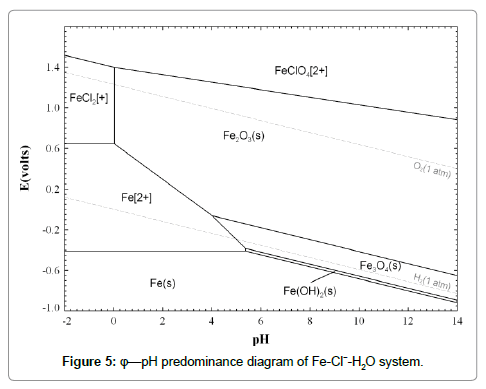
Thermodynamic analysis for the leaching process
The state and stability of metal ions are largely associated with the potential and pH value in aqueous solutions. The potential-pH predominance diagram is a powerful tool to analyze thermodynamic conditions of leaching in hydrometallurgy. The potential-pH of Fe-Cl--H2O system was calculated using Fact Sage software package and is depicted in Figure 5. It is shown that all of Fe, FeO, Fe3O4 and Fe2O3 can react with HCl solution. The state of iron ion is dependent on the relative content of Fe and Fe3+. The main reactions are listed as follows:
Fe+HCl=H2+Fe2++2Cl- (1)
FeO+2HCl=H2O+Fe2++2Cl- (2)
Fe3O4+8HCl=4H2O+Fe2++4Cl-+2FeCl+ (3)
Fe2O3+6HCl=3H2O+2FeCl++2Cl- (4)
Fe+2FeCl+=3Fe2++4Cl- (5)
2FeCl++H2=2HCl+2Fe2++2Cl- (6)
Effect of temperature and time
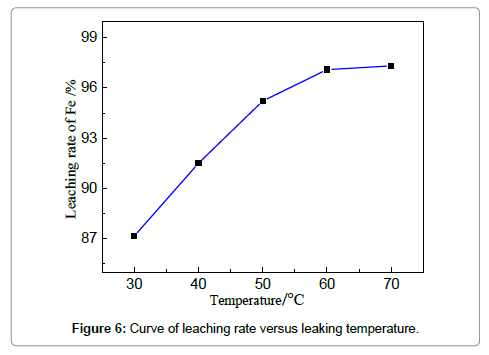
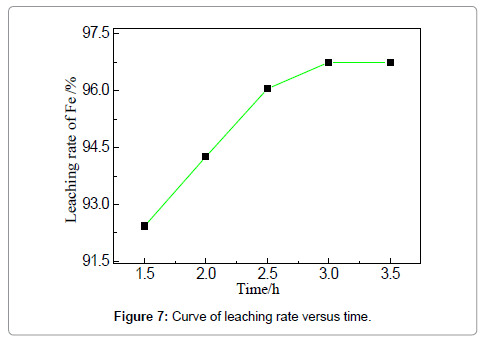
The efficiency of iron removal by acid leaching is closely related to the parameters of the stirred tank reactor, which involves temperature, time, stirring speed and solution/solid ratio. The influences of these variables are discussed in the following sections to have a good insight of the leaching process. The effect of leaching temperature on iron removal is investigated under the fixed condition: HCl concentration 19 wt%, solid-liquid ration 4:1, reaction duration of 3 h and stirring speed 150 r/min. It is known that the increase of temperature improves both thermodynamic and kinetic condition to favor the reaction between acid solution and iron-based oxides (or iron). Figure 6 shows that the removal rate of iron increases markedly from 87.2 to 97.8% when the temperature is elevated from 30 to 60?. However, the increment of removal rate is unapparent when the reaction temperature is increased from 60 to 70?. 97.1% at 60? is already relatively high; further increase of temperature cannot dramatically encourage the reaction; the leaching efficiency can also be limited by other parameters, such as HCl concentrations. Compared with the reported leaching rate of iron in Table 1, the efficiency is relatively high owing to the favorable mass diffusion and reaction in this reactor. The leaching duration is a key factor that should be considered for both experimental and industrial practices.When the concentration of HCl is 19 wt%, liquid-solid ratio 4:1, temperature 60?, stirring speed 150 r/min, the experiments for studying the effect of leaching time on iron removal were carried out and the results are shown in Figures 7and 8. Similar trend can be observed compared with Figure 7, showing that the removal rate increases apparently from 1.5 to 2.5 h; however, the trend is relatively smooth after 2.5 h.
Effect of HCl concentration and stirring speed
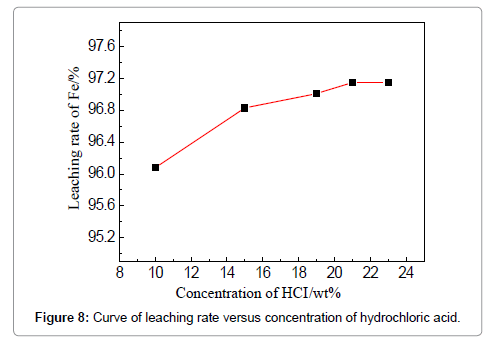
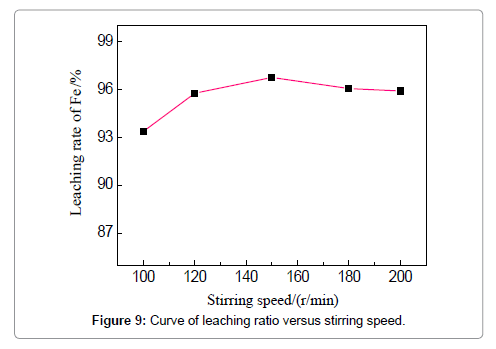
The concentration of HCl determines the quantity of required leaching agent for the iron removal, which should be a tradeoff between the leaching efficiency and acid consumption. To examine the effect of HCl concentration, the temperature, liquid-solid ratio, duration and stirring speed are fixed to be 60?, 4:1, 3 h and 150 r/min. The removal rate increases from 96.08 to 96.83% when the acid concentration is raised from 10 to 15 wt%, and increases slightly when the concentration is more than 15 wt%. However, the effect of the HCl concentration is not apparent in the current experiments. The effect of stirring speed can be visualized in Figure 9, when the HCl concentration is 19 wt%, leaching duration 3 h, and temperature 60?. An increase of iron removal rate is observed when raising the stirring speed from 100 to 150 r/min, and the leaching rate reaches 96.75%. Stirring is commonly believed to favor the updating of the liquid around the iron fragments and thinning the diffusion layer. However, the removal rate slightly decreases when the stirring speed is continuously increased to 180 and 200 r/min which means lower reaction efficiency is brought about. The possible reason could be that intensified stirring results into the entire rotation of both liquid and particles, instead, discouraging the updating and diffusion of the liquid around the particles; meanwhile, strong stirring can lead to the absorption and entrapment of bubbles which tend to attach on the particle surfaces to deter the reactions. These phenomena were reported in other mechanically stirred systems as well [15,16]. Thus, 150 r/min is suggested for the stirring speed to facilitate the removing of iron.
Significance study and optimization of physico-chemical parameters
In the light of the above one variance analysis, it can be clearly seen that the favorable stirring rate is demonstrated to be 150 r/min, and all of the temperature, time and HCl concentration encourage the removal of iron. Therefore, orthogonal experiments are carried out to optimize the significance of these factors.
Significance investigation and variables optimization: ANOVA is a collection of mathematical models used to test three or more variables for statistical significance. An orthogonal array of the factors is representative and identifies the significance of each factor through analyzing the results. Three levels for each three factors were studied in the current experiments, which are listed in Table 3, an array of L9 (33) (Tables 3 and 4). The calculation of square sum of the factor variance, degree of freedom and significance index (F-value) can be seen in reference [17], the results of which are listed in Table 5. It can be shown that leaching duration is the dominant factor (F-value is 81) to determine the leaching rate, followed by the effect of temperature. Concentration of HCl is not significant in the studied range, as the pH is selected to be relatively low to benefit the leaching. As a higher removal rate is encouraged, the parameters with higher significance, time and temperature, are recommended to choose the highest level (time 3 h, temperature 70?) to facilitate the leaching process. The HCl concentration is determined to be 15 wt% (Table 6). Three experiments were repeated to validate the optimized parameters, and the results are shown in Table 6. The removal rates are stabilized as high as more than 97% and the mean mass fractions of residual iron reaches 0.21 wt%. Meanwhile, the fractions of other elements are listed in Table 7. The Cu is dramatically decreased from 0.796 to 0.0073 wt% and other impurities are removed to trace level as well. The impurities are effectively removed and the content of Si and SiC reach a purity degree of more than 99.79%, being mostly sufficient to be reutilized or subjected to the subsequent separation.
| Parameters | A (HCl concentration)/wt% | B (leaching duration)/h | C (Temperature)/? |
|---|---|---|---|
| Level 1 | 15 | 2 | 50 |
| Level 2 | 19 | 2.5 | 60 |
| Level 3 | 23 | 3 | 70 |
Table 3: Factors and levels in orthogonal experiments of the leaching.
| Group | A/wt% | B/h | C/? | Leaching rate/wt% |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 88 |
| 2 | 1 | 2 | 2 | 94 |
| 3 | 1 | 3 | 3 | 97 |
| 4 | 2 | 1 | 2 | 89 |
| 5 | 2 | 2 | 3 | 93 |
| 6 | 2 | 3 | 1 | 93 |
| 7 | 3 | 1 | 3 | 91 |
| 8 | 3 | 2 | 1 | 92 |
| 9 | 3 | 3 | 2 | 97 |
Table 4: Results of orthogonal experiments in the leaching.
| Factor | Sum of the factor variance | Degree of freedom | Mean sum of square | F-value |
|---|---|---|---|---|
| A | 6.8 | 2 | 3.4 | 8 |
| B | 72.9 | 2 | 36.5 | 81 |
| C | 12.8 | 2 | 6.4 | 14 |
| Error | 0.9 | 2 | 0.5 | - |
Table 5: Variance analysis for leaching rate.
| Item | Temperature/°C | Duration/h | HCl concentration/wt% | Stirring speed/(r/min) | Removal rate/% | Residual iron/wt% |
|---|---|---|---|---|---|---|
| 1 | 70 | 3 | 15 | 150 | 97.08 | 0.22 |
| 2 | 70 | 3 | 15 | 150 | 97.43 | 0.19 |
| 3 | 70 | 3 | 15 | 150 | 97.01 | 0.21 |
Table 6: Results of the optimized leaching.
| Item | Si | SiC | Fe | Cu | Ca | Ti | Zn | Mg |
|---|---|---|---|---|---|---|---|---|
| wt% | 39.5610 | 60.2040 | 0.2101 | 0.0073 | 0.0078 | 0.0041 | 0.0034 | 0.0023 |
Table 7: XRF results of purified powder.
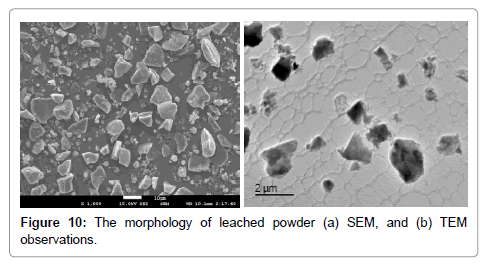
Characterization of leached products: The morphology of leached powder, observed by SEM and TEM, is shown in Figure 10. The main components, Si and SiC, are effectively recycled. The small particles, silicon, can be clearly viewed by TEM in Figure 10b. It can be seen that the Silicon powders are featured with polycrystalline and laminar shape.
Summary
Several physico-chemical variables have been considered to investigate their effects on iron removal during acid leaching process of kerf loss waste. Analysis of variance (ANOVA) is employed to study the significance level of these parameters. The results show that leaching duration is the main factor to determine the leaching rate, followed by the effect of temperature and HCl concentration. After leaching, the SiC and Si powders are well recycled.
References
- Tsai TH (2009) Pretreatment of recycling wiresaw slurries-Iron removal using acid treatment and electrokinetic separation. Separation and Purification Technology 68:24-29.
- Drouiche N, Cuellar P,Kerkar F, Medjahed S, BoutouchentGN, et al. (2014) Recovery of solar grade silicon from kerf loss slurry waste. Renewable and Sustainable Energy Reviews 32: 936-943.
- Li J, Huang K, Zhu H(2015)Phase separation of a micro sized powder mixture of Si and SiC by Cu-Si alloying. Chemical Engineering Science127: 25-30.
- Xing P, Guo J, Zhuang Y, Li F, Tu G(2013) Rapid recovery of polycrystalline silicon from kerf loss slurry using double-layer organic solvent sedimentation method. International Journal of Minerals, Metallurgy, and Materials20: 947-952.
- Tomono K, Furuya H, Miyamoto S, Okamura Y, Sumimoto M, et al. (2013) Investigations on hydrobromination of silicon in the presence of silicon carbide abrasives as a purification route of kerf loss waste. Separation and Purification Technology 103: 109-113.
- Yang X, Lv D, Pan Y(2015)Application advance on crystalline silicon cutting waste in silicon carbide and its composite.Matererials Review 29: 49-54.
- Lin Y, Wang T,Lan C, Tai C (2010) Recovery of silicon powder from kerf loss slurry by centrifugation. Powder Technonogy 200: 216-223.
- Miki T, Morita K, Sano N (1997) Thermodynamic properties of titanium and iron in molten silicon. Metallurgical and Materials Transactions B 28: 861-867.
- Munro R, Dapkunas S (1993) Corrosion characteristics of silicon carbide and silicon nitride. J Res NatlInst Stand Technol98: 607-631.
- Tsai T(2011) Iron Removal during Recovery of Silicon from sawing waste by applying magnetic field. Separation and Purification Technology 46: 702-707.
- Tsai T, Huang J(2009) Metal removal from silicon sawing waste using the electrokinetic method. Journal of the Taiwan Institute of Chemical Engineers 40: 1-5.
- Wang T, Lin, Y, Tai C, Sivakumar R, Rai D, et al. (2008) A novel approach for recycling of kerf loss silicon from cutting slurry waste for solar cell applications. Journal of Crystal Growth 310:3403-3406.
- Kalocsai G, Hockley J (1972) Titrimetric analysis for silicon. Mineral Magazine 38: 618-625.
- Naher S, Brabazon D, Looney L (2003) Simulation of the stir casting process. Journal of Materials Processing Technology 143: 567-571.
- Hamedan A, Shahmiri M(2012) Production of A356-1.0wt% SiC nanocomposite by the modified stir casting method. Materials and Science Engineering A 556: 921-926.
- Brown MB, Forsythe AB(1974) The ANOVA and multiple comparisons for data with heterogeneous variances. Biometrics 30: 719-724.
Citation: Jiangshan Z, Zhengyi J, Jing G, Jingwei Z, Zhixin C (2017) Study on Factors Affecting Iron Removal from Kerf Loss Slurry Waste Using Acid Leaching. J Powder Metall Min 6: 167. DOI: 10.4172/2168-9806.1000167
Copyright: © 2017 Jiangshan Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4951
- [From(publication date): 1-2017 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 4063
- PDF downloads: 888