Research Article Open Access
Study on Catalytic Ozone Oxidation with Nano-TiO2 Modified Membrane for Treatment of Municipal Wastewater
Yueqi Zhu1, Hongwei Zhang1,2* and Xuehua Zhang1,21School of Environmental Science and Engineering, Tianjin University, 300072, China
2School of Economics, Tianjin Polytechnic University, 300160, China
- Corresponding Author:
- Hongwei Zhang
School of Environmental Science and Engineering
Tianjin University, 300072, China
Tel: +86 13821653658
Fax: +86 022 23121662
E-mail: hunanzhilv@163.com
Received date August 16, 2013; Accepted date October 10, 2013; Published date October 17, 2013
Citation: Zhu Y, Zhang H, Zhang X (2013) Study on Catalytic Ozone Oxidation with Nano-TiO2 Modified Membrane for Treatment of Municipal Wastewater. J Biomim Biomater Tissue Eng 18:113. doi:10.4172/1662-100X.1000113
Copyright: © 2013 Zhu Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
In this paper, we studied the application of Nano-TiO2 Modified membrane technology in municipal wastewater treatment, which has not been previously reported to exhibit in this area. Ozone (O3) aeration can produce a large number of high oxidizing free radicals (hydroxyl radical HO•) to degrade organic matters when ozone contacts with nano- TiO2 Modified membrane, which process is called catalytic ozone oxidation process. This approach is a new frontier of municipal wastewater treatment, which has excellent reactive activity and degradation of organic compounds without the need of catalyst recycling. Our purpose is to remove organic matters by ozone aeration pre-treatment and nano-TiO2 modified membrane. This method is more efficient since it combines both chemical oxidation and physical filtration. In this study, municipal sewage is called raw water. Polyvinylidene fluoride (PVDF) and the nano-TiO2 modified PVDF ultrafiltration membranes are selected as experimental materials. Results suggest that the removal rate of organic matters with this new method (TiO2+O3) is 66.4%, which is 13.3% higher than original membrane with O3 (PVDF+O3) under the same condition. Therefore we can extract that, this part of contribution belongs to catalytic ozone oxidation process on TiO2 modified membrane. Compared with traditional PVDF membrane filtration, whose removal rate is 38.7%, this novel approach has a removal rate 27.7% higher.
Keywords
Catalytic ozone oxidation; Modified ultra-filtration membrane; Nano-TiO2; Treatment of municipal wastewater; Membrane fouling
Introduction
In recent decades, membrane separation technology as one of new high-efficiency separation techniques has developed rapidly. Compared with traditional separation techniques, membrane separation is highly efficient, energy-saving and easy to operate. Membrane separation technology has been widespread used and considered to be “one of the most promising high-techs from 20th century to the 21st century medium-term” [1].
In the past decades, plenty of reports indicated that ozone could be used as pretreatment of municipal wastewater treatment, because of its strong oxidizing property [2], but the process is expensive and hard to control [3]. Currently, several studies showed that the using of catalytic ozone oxidizing with metal oxides catalysts for organics degradation of waste, and it becomes a new trend of advanced oxidation technology [4].
Polyvinylidene Fluoride (PVDF) ultra-filtration membrane has good chemical stability and fiber durability, and it is widely used in membrane separation. Nano-TiO2 modified membrane is a new membrane with Nano-TiO2 coating on constituent particles of the PVDF membrane through micro-electrophoresis, which can catalyze ozone to produce a large number of high oxidizing free radicals (hydroxyl radical HO•) [5,6] to degrade organic matters inside/on the surface of membrane. In addition, the product of ozone oxidation is oxygen; accordingly catalytic ozone oxidation reaction is highly active without secondary pollution oxidants [7]. In a word, this experimental application of catalytic ozone oxidation in water treatment is mainly based on characteristics such as strong oxidizing property and non secondary pollution.
In this paper, the brand-new method of catalytic ozone oxidation with nano-TiO2 modified PVDF membrane is used for treatment of municipal wastewater (raw water). This study put emphases on two aspects: 1. Analyze molecular weight distribution of organic matters in different water samples by molecular sieving membrane and compare efforts of removal rate of catalytic ozone oxidation approach with traditional ones; 2. Study the mechanism of catalytic ozone oxidation by molecular weight distribution.
Ozone is a strong oxidant, research shows that ozone has strong oxidizing property and fast reaction ability, in the treatment of industrial wastewater, ozone oxidation is available for a variety of organic compounds. Why dose ozone have such a strong oxidizing property? Because the oxygen atom in the molecule has strong electrophilicity or protophilia and the nascent oxygen atom of the ozone decomposition also has high oxidation activity [8]. In aqueous solutions, ozone reacts with organic compounds in two ways [9]: ozone molecules direct attack and the free radical reactions by decomposition of ozone.
The stability of ozone is related pH, UV, ozone concentrations and the capture concentration of free radicals. The formation of free radicals depends on the ozone decomposition which causes free radical reactions [10].
Ozone decomposition reaction is carried out in a chain reaction, involving the following basic steps. Free radicals reaction is the ratelimiting step. In addition, the steps of producing ·O2− and ·HO2 are also important, because these substances can increase the stability of ozone in the water. This machine includes a two-electron transfer process and one oxygen atom transfers from the ozone molecule to hydrogen peroxide ion.
Experimental Materials and Methods
Experimental materials
The raw water used in this study was obtained from a wastewater treatment plant in China. The water qualities are shown in Table 1.
| Parameters | Values |
|---|---|
| pH | 8.04-8.35 |
| Turbidity, NTU | 11.39-18.61 |
| DOC, mg/ L | 95.27-106.31 |
| CODMn, mg/ L | 92.62-104.64 |
| UV254, cm├ó┬?┬?1 | 2.7-11.8 |
Table 1: Raw water quality parameters.
The PVDF with nano-sized TiO2 sol was prepared by sol-gel method. The structure and performance of the membrane were investigated by X-ray diffraction (XRD) scanning electron microscopy (SEM).
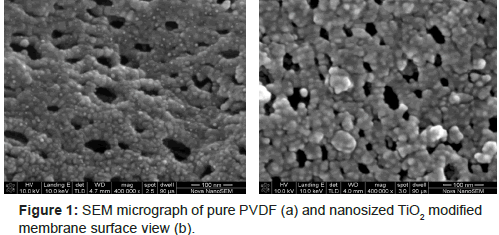
The synthesis method of the nano-TiO2 modified PVDF membrane is as follows: add 10 mL dibutyl phthalate (DBP, A.R., Tianjin Fuchen Chemical Reagents Factory, China) into a certain amount of anhydrous ethanol (EtOH, A.R., Tianjin University Chemical Reagents Factory, China), and then drop dimethyl acetamide (DMAc, A.R., Tianjin Chemical Reagents Factory, China) containing nitric acid and distilled water into the solution under stirring, maintain this reaction for 24 hr at room temperature (25°C), thus gain transparent TiO2 sol; with 0.10% of the mass fraction, put the sol into DMAc solution containing 7% polyvinylpyrrolidone (PVP, A.R., Tianjin Fuchen Chemical Reagents Factory, China) and 18% polyvinylidene fluoride (PVDF, A.R., Shanghai 3F New Material Co., Ltd., China), continuously stir for 24 hr, consequently get homogeneous hybrid casting solution; after static defoaming, pour the solution onto glass plate for knifing, after staying a while in the air, put it into the coagulating bath for film formation, as a result, the nano-TiO2 modified PVDF membrane with average thickness d of 0.2 mm can be produced [11,12]. In this study, PVDF ultra-filtration membrane (Tianjin MOTIANMO Co., Ltd, China) and nanosized TiO2 modified ultra-filtration membrane (0.10 w%) were used as experimental materials (Figure 1).
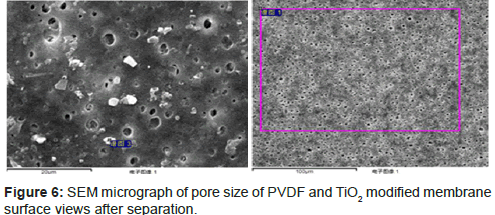
It can be seen from Figure 1 that nanosized TiO2 modified ultrafiltration membrane has the advantage of high porosity. Compare Figure 1a with Figure 1b, because of the great adsorption process between PVDF and nanosized TiO2, a large number of nanosized TiO2 particles have been adsorbed and embedded in the membrane surface and in intra-pore. In the meanwhile, a significant increase in the profile layer can be observed as well as a decline in finger holes of sub-planes.
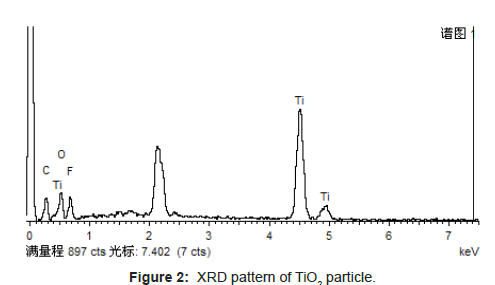
The white powder is nanosized TiO2 by XRD (Figure 2), which can be found directly that even smaller particles are successfully prepared. That means nanosized TiO2 particles have content deposited regularity in the membrane surface and in intra-pore.
Experimental design
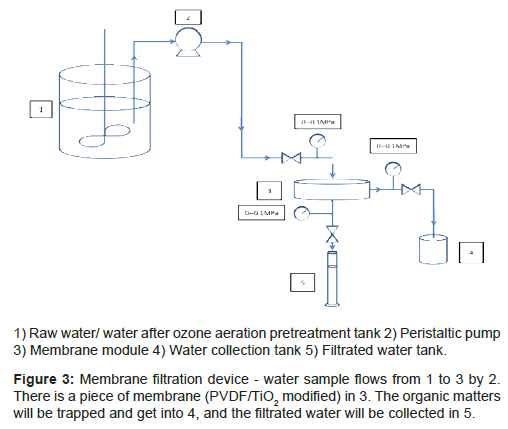
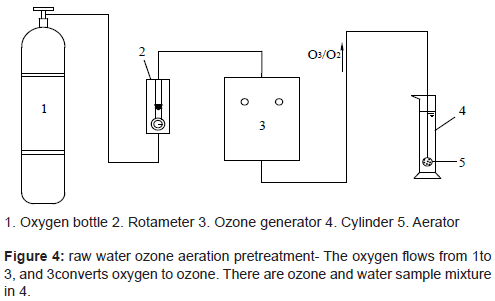
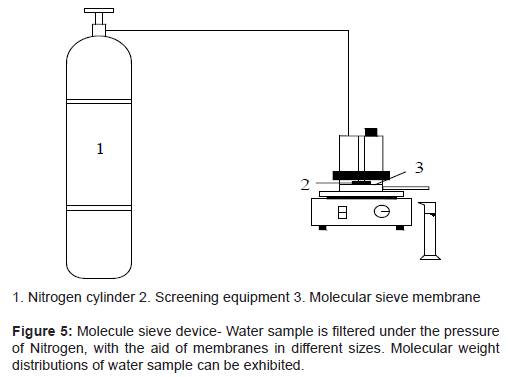
In the research, experimental devices are divided into three main groups, as shown in Figure 3 for membrane filtration, Figure 4 for ozone aeration pretreatment of raw water, and Figure 5 for molecular weight sieving respectively.
The experiment device in Figure 3 is used for raw water filtration and the water filtration after ozone aeration pretreatment.
Experimental device shown in Figure 4 is used for raw water ozone pretreatment. It can reach the target of ozone pretreatment to remove part organic matters. Meanwhile, extra ozone also can dissolve in the water and through the pipe. Catalytic ozone oxidation with nano-TiO2 modified membrane will remove organic matters that stick to the membrane (physical trapping and catalytic ozone oxidation).
Device shown in Figure 5 is mainly used for molecular fractionation of raw water and percolates after different membranes (PVDF and nano-TiO2 modified membranes). In the research, we use molecular sieve membranes of different apertures in order to trap organic matters of different molecular weights. In the end, molecular weight distributions are determined by CODMn [13].
We sieve different molecular weight organics by plate Ultrafiltration membranes in different pore sizes as demonstrated in Table 2.
| Membrane size | Material | Rage of molecular weight├»┬╝┬?Dalton |
|---|---|---|
| 0.45├?┬╝m | Cellulose Acetate | Diameter>0.45├?┬╝m |
| 100,000(100K) | Polysulfone | >100,000 |
| 30,000(30K) | Polysulfone | >30,000 |
| 10,000(10K) | Polysulfone | >10,000 |
| 3000(3K) | Polysulfone | >3000 |
| 1000(1K) | Polysulfone | >1000 |
Note: 1 Daltan (Da)=1.66├?┬?10-27 kg.
Table 2: Material and molecular weight cut-off of the membrane.
Analysis method of the molecular weight distribution of water
On the first stage, as shown in Figure 3, we filter raw water (water in wastewater treatment plant after primary treatment, meaning sinking sand and primary sedimentation) directly by PVDF membrane and TiO2 modified membrane until the membrane is blocked up, and then trap percolates through molecular sieve membranes of 1 kDa, 3 kDa, 10 kDa, 30 kDa and 100 kDa apertures respectively. After that, we determine CODMn of percolates. On the second stage, as shown in Figure 4, under the operating condition of 1.34 mg/L ozone dosage and after aeration of 5 min, we make the samples go through PVDF and TiO2 modified membranes. Then, repeat the first stage experiment after the ozone aeration pretreatment.
Identification of catalytic ozone oxidation function
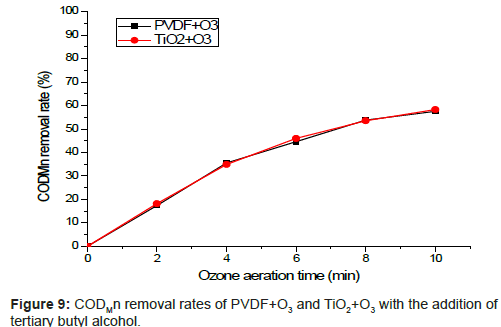
In order to confirm the effect of catalytic ozone oxidation, we performed comparison experiment on PVDF+O3 and (TiO2+O3) as shown in Figure 3 under the operating condition of 1.34 mg/L ozone dosage from 0 min to 10 min. The removal rate of CODMn is recorded every 2 min. Next, under the same condition, we added tertiary butyl alcohol (an inhibitor of HO·) to avoid the reaction of catalytic ozone oxidation. Catalytic ozone oxidation function can be verified from both comparison experiments.
Results and Discussion
Characterization of nano-TiO2 modified membrane
It is found that if appropriate nano-sized TiO2 sol is added the microstructure of PVDF membrane can be obviously improved. Meanwhile, the hydrophilic property and anti-fouling capability of the membrane can also be enhanced.
The surface views of PVDF and TiO2 modified membranes after filtration are shown in Figure 6. In the filtration by PVDF membrane, the flux dropped rapidly due to membrane fouling in Figure 6a. It suggests that the membrane fouling occurred in the initial filtration. In the filtration by TiO2 modified membrane, the flux kept well and there was no significant fouling in/on the membrane as shown in Figure 6b, also the flux recovered completely for each filtration cycle. We can conclude that organic matters inside/on the surface of membrane were degraded by high oxidizing free radicals (HO•).
The molecular weight distribution of organic matters
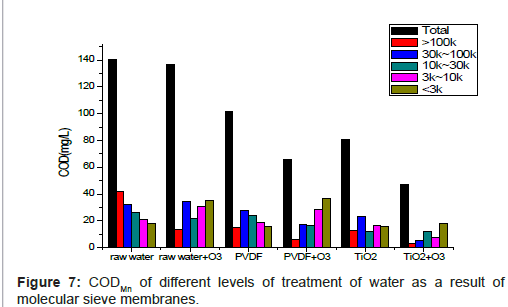
The molecular weight distribution of organic matters is shown in Figure 7.
According to Figure 7, in the raw water, the main organic matters are centered on >100k and 30k~100k scales, and COD of raw water is reduced by 2.5% with ozone aeration treatment. Therefore, we can infer that, after a short time, the ozone aeration does not reduce the COD significantly. As shown in Figure 7, the large organic matters reduces while the small ones get increased and the >100 kDa ones make the chief contribution to COD reduction. Meanwhile, the 3k~100k ones grow and others are not very obviously. That is mainly due to the strong selectivity of ozone reaction, this effort is only limited to unsaturated aromatic, aliphatic compounds and certain special groups. But ozone has little effect on low molecular weight organic acids such as acetic acid, and various types of compounds such as alkanes which almost have no response to ozone [14]. Thus, molecules of ozone play the main role in pre-treatment. This process is affected by ozone aeration time and concentration strongly.
After filtration of PVDF membrane, the reduction of COD in raw water is only 27.6%, but we can find the concentrations of macromolecular organic compounds (mainly in molecular weight between >100 kDa, 30 kDa~100 kDa organic compounds) reduce significantly. Therefore, traditional membrane filtering only has effect on large molecular weight organics. Also, after the soon followed membrane fouling, membrane pore is blocked. As a consequence PVDF membranes need to be cleaned or replaced frequently.
After ozone aeration pre-treatment, the removal of COD is obviously (above 50%). Organic compounds of molecular weight >100 kDa is reduced obviously (84.8%), the removal rate of smaller organic compounds (molecular weight 30 kDa~100 kDa) reaches 45.9%. Ozone makes macro-molecules organic compounds degrade into smaller ones. That is why the organic compounds of >100 kDa removal rate rises significantly. Additionally, organic compounds of 30 kDa~100 kDa and 10 kDa~30 kDa can be removed by PVDF membrane mostly. Micromolecules and macro-molecules degradation organic compounds (<10kDa) cannot be removed by PVDF membrane clearly.
After filtration of TiO2 modified membrane, COD of raw water totally fell by 42.4%. TiO2 modified membrane can almost remove organic matters of molecular weight above 10 kDa while has only a little effect on smaller ones (<10 kDa).
After ozone aeration pre-treatment and filtration of nano-TiO2 modified membrane, we can gain the best removal result of organic matters -- total removal rate reaches 66.4%. Compared with PVDF+O3, it rises by 13.3%. So we can extract that the contribution comes from catalytic ozone oxidation on TiO2 modified membrane, for the reason that, after ozone aeration, there is still some ozone dissolved in the water. When dissolved ozone goes through TiO2 modified membrane, catalytic ozone oxidation helps to degrade organic matters. Compared with molecular ozone oxidation, ozone catalytic oxidation is speedy, full and non-selective. Therefore, this process (TiO2+O3) is to some extent better than others (Raw Water+O3, PVDF, PVDF+O3, TiO2). There may be two processes existed in nano-TiO2 catalytic ozone oxidation process: 1) Nano-Organics adsorbed on the surface. Adsorptive organic molecules, due to the changes in the electron cloud distribution, are easily to be oxidated. 2) Nano-TiO2 catalytic ozone oxidation produces more HO·, which is useful to remove more organic matters than molecular ozone oxidation.
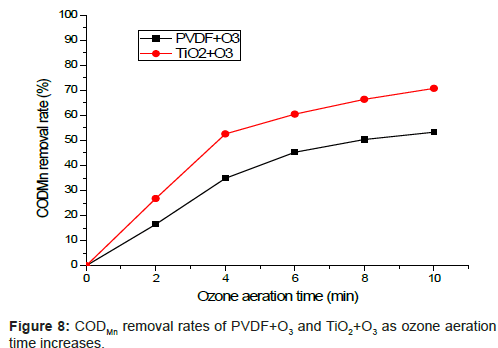
Figure 8 verifies the effect of catalytic ozone oxidation as follows: as the input of ozone aeration is increased, the removal rate of organic matters climbs up, which confirms that ozone can remove organic matters effectively. Meanwhile, process (TiO2+O3) is better than PVDF+O3 under the same condition which means that catalytic ozone oxidation has contributed that part.
Catalytic ozone oxidation function
TiO2 is good for the induction of ozone decomposition chain reaction and improve the generation of HO·. The reaction of HO· is non-selectively and the rate of the reaction is rapid, so the removal rate of TOC rises. If TiO2 loaded in the hybrid membrane indeed catalyzes ozone and produces HO·, then the reaction will be seriously affected by tert-butyl alcohol, so we can indirectly judge whether the reaction follows the mechanism of HO· or not by examining the impact of tertbutyl alcohol.
As Figure 9 shows, with the addition of tertiary butyl alcohol (10 mg/L), there is not a clear difference in CODMn removal rates between PVDF+O3 and TiO2+O3 approaches. Without free radical reaction of HO·, there is only reaction of molecular ozone in the water samples. This result proves the point that catalytic ozone oxidation process did take place and remove organic matters effectively.
As is showed in Figure 9, we can conclude that with the increasing of tert-butyl alcohol, the degradation of organics is strongly inhibited [15]. Compare Figure 9 with Figure 8, it is obviously that in the catalytic reactions of degradation of organics, HO· was dominant, and especially in the first six minutes, the inhibition capability of tert-butyl alcohol was obvious. Then the trend tended to become gentle because of the restriction of the concentration of catalyst.
According to Figure 9, due to the free radical reaction of HO· being inhibited by the addition of tert-butyl alcohol, there was no significant difference between the two organic removal rates. With the increasing of ozone reaction time, the CODMn concentrations of water samples after filtrations of original membrane and TiO2 modified membrane both dropped.
Conclusions
After ozone aeration pre-treatment and filtration of nano-TiO2 modified membrane, we gain the best available removal effect of organic matters - total removal rate reaches 66.4%. Compared with PVDF+O3, the total removal rate rises 13.3% under the same condition.
It is verified by comparison experiments that new method (TiO2+O3) can remove organic matters better than traditional membrane separation process. It is more efficient, combining catalytic ozone oxidation, ozone oxidation and physical filtration.
In order to verify the function of catalyzed ozonation, tertbutyl alcohol is used as quenching reagent to detect operation and contribution of HO·. The result proved that catalyzed ozonation is efficient in removing most of organic pollutants followed the HO· oxidation process.
Consequently, catalytic ozone oxidation joint ozone aeration pretreatment process can achieve better treatment effect than traditional process. Compared with traditional process (PVDF membrane filtration) whose removal rate is 38.7%, this novel approach is 27.7% higher. Meanwhile, ozone catalytic oxidation removes the organic matters sticking to the membrane, which is an effective way to prevent membrane fouling.
References
- Rivera-Utrilla J, Sanchez-Polo M (2004) Onation of 1, 3, 6-nap-htalenetrisulphonic acid catalysed by activated carbon in aqueous phase. Applied Catalysis 39: 319-329.
- Nissinen TK, Miettinen IT Martikainen PJ, Vartiainen T (2002) Disinfection by-products in Finnish drinking waters. Chemosphere 48: 9-20.
- Agrawal DK, Stubican VS (2006) Germanium modified cordierite ceramics with low thermal expansion. J Am Ceram Soc. 69: 847-851.
- Yang Y, Wang H, Li JX, He B,Wang T et al. (2012) Novel functionalized nano-TiO2 loading electro-catalytic membrane for oily wastewater treatment. Environ. Sci. Technol 46: 6815-6821.
- Ernst M, Lurot F, Schrotter JC (2009) Catalytic ozonation of refractory organic model compounds in aqueous solution by aluminum oxide. Applied Catalysis 47: 15-25.
- Thiruvenkatachari R, Shim WG, Lee JW, Aim RB, Moon H (2010) A novel method of powdered activated carbon (PAC) pre-coated microfiltration (MF) hollow fiber hybrid membrane for domestic wastewater treatment. Colloids Surf. A: Physicochem. Eng. Aspects 274: 24-33.
- Khan MMT, Lewandowski Z, Takizawa S, Yamada K, Katayama H, et al. (2011) Continuous and efficient removal of THMs from river water using MF membrane combined with high dose of PAC. Desalination 249: 713-720.
- Akal Solmaz SK, Ustun GE, Birgul A, Yonar T (2009) Advanced oxidation of textile dyeing effluents: Comparison of Fe+2/H2O2, Fe+3/H2O2, O3 and chemical coagulation processes. Fresenius Environmental Bulletin 18: 1424-1433.
- Droppo IG, Ongley ED (2010) The state of suspended sediment in the freshwater fluvial environment: a method of analysis. Water Res. 26: 65-72.
- Madaeni SS, Ghaemi N (2007) Characterization of self-cleaning RO membranes coated with TiO2 particles under UV irradiation. Membr Sci 303: 221-233.
- Cao XC, Ma J, Shi XH (2006) Effect of TiO2 nanoparticles size on the performance of PVDF membrane. Appl Surf Sci 253: 2003-2010.
- Chu CP, Lee DJ, Chang BV, You CS, Tay JH (2002) "Weak" ultrasonic pre-treatment on anaerobic digestion of flocculated activated biosolids. Water Res 36: 2681-2688.
- Chang CN (2000) Influence of molecular weight distribution of organic substances on the removal of DBPs in a conventional water treatment plant. Wat Sci Tech 41: 43-49.
- Tiehm A, Nickel K, Zellhorn M, Neis U (2001) Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Res 35: 2003-2009.
- Ma J, Graham NJD (2009) Degradation of atrazine by manganese-catalysed ozonation: Influence of humic substances. Water Res 33: 785-793.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 24268
- [From(publication date):
November-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 19435
- PDF downloads : 4833