Study of Serum N-Terminal-Pro C-Type Natriuretic Peptide and its Relation to the Risk of Variceal Bleeding in Cirrhotic Hepatitis-C Virus Patients
Received: 16-Mar-2016 / Accepted Date: 26-Mar-2018 / Published Date: 02-Apr-2018 DOI: 10.4172/2161-069X.1000556
Abstract
The current guidelines recommend that all cirrhotic patients should undergo screening endoscopy at diagnosis to identify patients with risky varices who will benefit from primary prophylaxis. This leads to a heavy burden on endoscopy units and affects patient compliance. Noninvasive identification of risky patients would limit performing endoscopy to those most likely to benefit. Upper GIT endoscopy is the gold standard against which other tests are compared, but is not without its limitations. Some tests are clearly preferable to patients but are not as accurate as upper GIT endoscopy in the diagnosis of esophageal varices. The aim of this work is to study the relation of serum NT pro-CNP to severity of cirrhosis and the presence of esophageal varices and the risk of their bleeding. The study was carried-out on 80 subjects divided into 4 groups: 20 cirrhotic patients with esophageal varices which have previously bled, 20 cirrhotic patients with esophageal varices which have not yet bled, 20 cirrhotic patients without esophageal varices and 20 normal healthy control subjects. Serum NT pro-CNP level was significantly elevated in cirrhotic patients with esophageal varices compared to cirrhotic patients without esophageal varices, but not significantly elevated in bleeders than in nonbleeders. Also spleen and portal vein diameters, Child’s score, Fib-4 score, serum bilirubin and AST levels and prothrombin activity were significantly elevated in the same group. Therefore, serum NT pro-CNP is a promising noninvasive marker for predicting severity of cirrhosis and presence of esophageal varices and not the risk of variceal bleeding in HCV-related liver cirrhosis patients.
Keywords: Esophageal and gastric varices; Natriuretic peptide; C-type; Gastrointestinal hemorrhage; Liver cirrhosis; Hepatitis C
Introduction
For more than a decade, Egypt has been widely classified as having an epidemic of hepatitis-C virus (HCV), with the highest recorded HCV-related liver diseases prevalence in the world, particularly cirrhosis and hepatocellular carcinoma [1]. Liver cirrhosis is a diffuse hepatic process characterized by fibrosis and conversion of normal hepatic architecture into structurally abnormal nodules, leading to deterioration of hepatic synthetic function [2].
Cirrhotic patients vary clinically from totally asymptomatic to a multitude of the most severe symptoms of end-stage liver disease. The most common manifestations include jaundice, ascites, hepatic encephalopathy and variceal bleeding. Portal hypertension (PHT) is considered one of the most serious complications of liver cirrhosis. Hepatic venous pressure gradient (HVPG) of 12 mm Hg is the threshold for the potential formation of varices [3]. The incidence of esophageal varices (EV) in patients with cirrhosis increases about 5-8% per year [4].
Esophagogastroduodenoscopy (EGD) is considered the gold standard tool for all cirrhotic patients to evaluate the risk of variceal bleeding. Three factors identify patients suffering from an increased risk of variceal bleeding: large variceal size, red color signs on the varices, and advanced liver disease (Child-Pugh scoring system class B or C) [5]. Several biochemical parameters have been identified as noninvasive predictors of variceal presence and risk of variceal bleeding, such as a low platelet count, hypoalbuminemia, and low prothrombin activity. However, EGD remains the best tool to confirm the presence of varices and their degree [6]. Several biomarkers have been evaluated in cirrhotic patients in relation to fibrosis and cirrhosis [7]. One of the recently studied biomarkers in cirrhotic patients is the amino (N)- terminal-fragment of pro C-type natriuretic peptide (NT-pro CNP) [8].
The C-type natriuretic peptide (CNP) is a paracrine molecule which is mainly synthesized in the human vascular endothelium causing vasorelaxation and vascular remodelling. It is synthesized from the precursor pre-pro-CNP protein which is converted to pro-CNP protein that is further cleaved to the biologically active hormone CNP by the intracellular endoprotease furin, releasing the NT-pro-CNP that circulates in equimolar amounts with CNP in human plasma, thus considered to be a more reliable marker of the extent of CNP biosynthesis being a larger molecule with a longer half-life compared to the active CNP hormone [9].
The pro-CNP exerts limited diuretic and natriuretic actions, but counteracts Angiotensin II and Endothelin-1 induced vasoconstriction, and complements actions of other endothelial vasorelaxant mediators such as nitric oxide and prostacyclin [10,11]. From experimental models and clinical data, NT-pro-CNP has been suggested as an important mediator of vasodilatation as well as a regulator of fluids and sodium balance in liver cirrhosis [12]. Serum NT-pro-CNP concentrations were reported to be significantly elevated in patients with liver diseases, particularly cirrhotic ones, compared to healthy controls [13,14]. The relation of serum NT-pro-CNP to the presence of varices and/or its risk of bleeding in cirrhotic patients has not yet been established, so it was noteworthy to study its serum level in such cases.
Material and Methods
All subjects are subjected to:
• Thorough history taking: including history of gastro-intestinal (GI) bleeding.
• Complete physical examination: to detect signs of hepatic dysfunction.
• Echocardiography: to exclude heart failure.
• Abdominal ultrasound: to confirm diagnosis of cirrhosis, detect ascites, measure spleen and portal vein diameters and exclude presence of focal lesions.
• Upper GI endoscopy: for detection and grading of EV according to North Italian Endoscopic Club (NIEC) index, detection of gastric varices (GV) and detection and grading of portal hypertensive gastropathy.
• Child-Pugh classification and score.
• Parameters predicting presence of EV: Fib-4 score and platelet count/spleen diameter.
• Laboratory investigations: Blood urea, serum creatinine, ALT, AST, serum bilirubin (total and direct fractions), prothrombin time and INR, HCV-Ab, HBs-Ag, HBc-Ab, anti-Bilharzial Ab, ANA, ASMA, LKMA and serum NT-pro-CNP levels (using ELISA immunoassay).
Results
This study was conducted on 80 subjects, divided equally (20 subjects in each group) into 4 groups:
Group I: Chronic HCV-related cirrhotic patients with EV, with history of gastro-intestinal bleeding (hematemesis and/or melena).
Group II: Chronic HCV-related cirrhotic patients with EV, but without history of gastro-intestinal bleeding.
Group III: Chronic HCV-related cirrhotic patients without EV.
Group IV: An age and gender matched apparently healthy volunteers (control group).
Inclusion criteria
Chronic HCV-related liver cirrhosis: diagnosis was based on clinical examination, biochemical investigations and ultrasonographic criteria.
Exclusion criteria
Any patient with bilharzial hepatic fibrosis, auto-immune hepatitis, serum hepatitis B surface antigen (HBs-Ag) positive, serum hepatitis B core antibody (HBc-Ab) positive, hepato-cellular carcinoma, hepatorenal syndrome (patients with creatinine more than 1.5 mg/dl) or heart failure were excluded from this study. All selected patients provided written informed consents before enrollment in the study. The study was approved by the ethics committee of Alexandria faculty of medicine. In our study male gender, 50-60 years age-group and living in rural areas were the highest percentages among patients groups.
Regarding serum albumin levels, there were statistically significant differences between groups I and II (HCV-cirrhotic patients with EV) and group III (HCV-cirrhotic patients without EV). Regarding serum AST levels, there were statistically significant differences between groups I and II (HCV-cirrhotic patients with EV) and group III (HCVcirrhotic patients without EV). While regarding blood urea, serum creatinine, serum ALT, serum bilirubin (total and direct fractions), pro-thrombin time, hemoglobin levels, white cells count and platelets count, there were no statistically significant difference between different studied groups.
Regarding spleen diameters, there were statistically significant differences between groups I and II (HCV-cirrhotic patients with EV) and group III (HCV-cirrhotic patients without EV) (p=0.017). While on the other hand, there were no statistically significant differences between different studied groups regarding portal vein (PV) diameters. Regarding NIEC scores, there were no statistically significant differences between different studied groups. There were 3 patients in group I and 5 patients in group II with gastric varices.
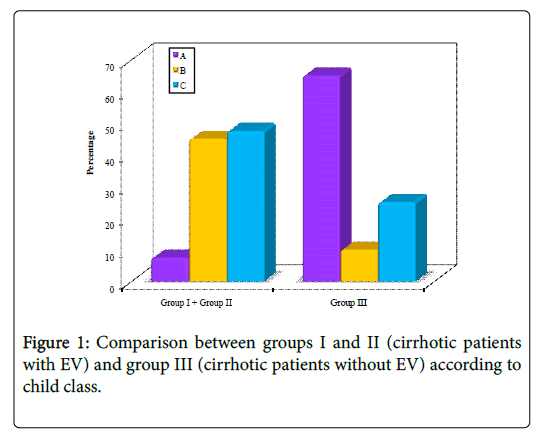
Regarding portal hypertensive gastropathy (PHG), 15% (3 patients) of cases in group I vs. 10% (2 patients) in group II and 50% (10 patients) in group III had no PHG. Mild PHG was detected in 35% (7 patients) of cases in group I vs. 25% (5 patients) in group II and 20% (4 patients) in group III respectively. Severe PHG was detected in 50% (10 patients) of cases in group I vs. 65% (13 patients) in group II and 30% (6 patients) in group III respectively. Regarding Child-Pugh class and score, there were statistically significant differences between groups I and II (HCV-cirrhotic patients with EV) and group III (HCV-cirrhotic patients without EV) (p<0.001) (Table 1, Figure 1).
| Child Class | Group I + Group II | Group III | Test of sig. | P | ||
| (n=40) | (n=20) | |||||
| No. | % | No. | % | χ2=23.119* | <0.001* | |
| A | 3 | 7.5 | 13 | 65 | ||
| B | 18 | 45 | 2 | 10 | ||
| C | 19 | 47.5 | 5 | 25 | ||
| Child Score | - | - | t=4.054* | <0.001* | ||
| Min.-Max. | 5.0-13.0 | 5.0-12.0 | ||||
| Mean ± SD. | 9.37 ± 1.75 | 7.05 ± 2.67 | ||||
| Median | 9 | 6 | ||||
Table 1: Comparison between groups I and II (cirrhotic patients with EV) and group III (cirrhotic patients without EV) according to child class and score.
Regarding Fib-4 score, there were statistically significant differences between groups I and II (HCV-cirrhotic patients with EV) and group III (HCV-cirrhotic patients without EV) (p=0.002).
While on the other hand, there were no statistically significant differences between groups I and II (HCV-cirrhotic patients with EV) and group III (HCV-cirrhotic patients without EV) regarding platelet count to spleen diameter ratio.
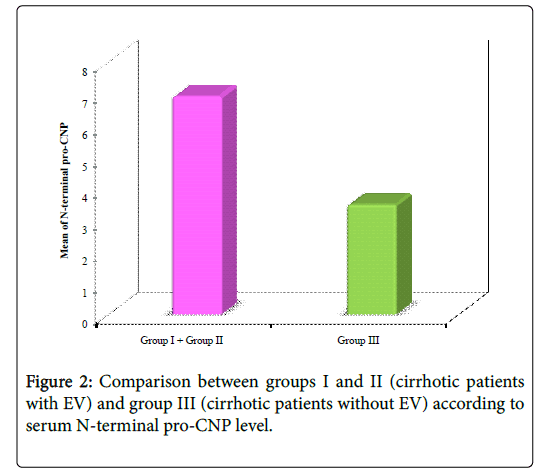
Regarding serum NT pro-CNP levels, there were statistically significant differences between groups I and II and group III (between cirrhotic patients with EV and cirrhotic patients without EV) (p=0.003) (Tables 2 and 3, Figures 2 and 3).
| NT pro-CNP | Group I+Group II | Group III | Z | p |
| (n=40) | (n=20) | |||
| Min-Max | 0.77-36.90 | 1.30-10.88 | 2.995* | 0.003* |
| Mean ± SD | 6.93 ± 7.11 | 3.50 ± 2.21 | ||
| Median | 4.44 | 2.85 |
Table 2: Comparison between groups I and II (cirrhotic patients with EV) and group III (cirrhotic patients without EV) according to serum NT-pro-CNP.
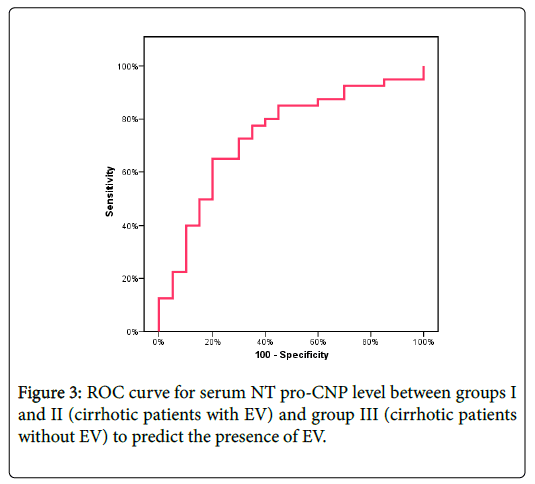
| N-terminal Pro-CNP | |||||||
|---|---|---|---|---|---|---|---|
| AUC | p-value | Cut off value | Sensitivity | Specificity | PPV | NPV | Accuracy |
| 0.739* | 0.003* | ≥3.95 | 65 | 75 | 83.87 | 51.7 | 68.33 |
Table 3: Agreement (sensitivity, specificity and accuracy) for NT pro- CNP between groups I and II (cirrhotic patients with EV) and group III (cirrhotic patients without EV).
Discussion
Liver cirrhosis is a diffuse hepatic process, characterized by fibrosis and conversion of normal liver architecture into structurally abnormal nodules, leading to loss of liver functions. PHT is the hemodynamic abnormality which develops in patients with liver cirrhosis. It results from increased splanchnic blood flow secondary to vaso-dilation within the splanchnic vascular bed, as well as increased resistance to the passage of blood through the liver. It is associated with the most severe complications of cirrhosis including ascites, hepatic encephalopathy and bleeding from gastro-esophageal varices.
Gastro-esophageal variceal bleeding is a major complication of portal hypertension resulting from cirrhosis. Variceal bleeding occurs in 25 to 35% of patients with cirrhosis and accounts for 80 to 90% of bleeding episodes in these patients. Up to 30% of initial bleeding episodes are fatal and a higher percent of survivors have recurrent bleeding after the first variceal bleeding. Therefore early detection of EV and timely introduction of beta-blockers and band ligation for primary prevention of bleeding decrease the morbidity and may improve quality of life in liver cirrhosis patients. For optimal management, it is important to understand which patients are most likely to have bleeding.
Our results denoted that there is a significant relation between serum levels of NT-pro-CNP and the severity of HCV-related liver cirrhosis and the presence of EV. This could be used in the future as a promising non-invasive biochemical marker for prediction of the presence of EV and the risk of variceal bleeding, decreasing the rate of their morbidites and mortalities.
Conclusion
From this study we can conclude that:
• Serum NT pro-CNP could be used as a new promising noninvasive marker for predicting the presence of EV in HCV-related liver cirrhosis patients. However, the results of this study were not able to prove its predictive power in the risk of bleeding from EV among such patients.
• Based on its relation with Child-Pugh score, serum NT pro-CNP could also be used as a non-invasive marker of severity of HCVrelated liver cirrhosis.
References
- Miller FD, Abu-Raddad LJ (2010) Evidence of intense ongoing endemic transmission of Hepatitis-C in Egypt. Proc Natl Acad Sci USA 107: 14757-14762.
- Iredale JP, Guha IN (2007) The evolution of cirrhosis. In: Rodés J, Benhamou JP, Blei AT, Reichen J, Rizzetto M (eds) Textbook of hepatology from basic science to clinical practice. Oxford: Blackwell Publishing. pp: 583-589.
- Kim MY, Choi H, Baik SK, Yea CJ, Won CS, et al (2010) Portal hypertensive gastropathy: correlation with portal hypertension and prognosis in cirrhosis. Diag Dis Sci 55: 3561-3567.
- D’Amico G, Garcia-Pagan JC (2003) Portalhypertension. In: Schiff ER, Sorrell MF, Maddrey WC (eds) Williams &Wilkins: Philadelphia. (9thedn), Lippincott. pp: 428-485.
- Christensen E (2004) Prognostic models including the Child-Pugh, MELD and Mayo risk scores-where are we and where should we go? J Hepatol 41: 344-350.
- Iredale JP (2003) Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ 327: 143-147.
- Adams LA (2011) Biomarkers of liver fibrosis. J Gastroenterol Hepatol 26(5): 802-809.
- Prickett TC, Yandle TG, Nicholls MG, Espiner EA, Richards AM (2001) Identification of amino-terminal pro-C-type natriuretic peptide in human plasma. Biochem Biophys Res Commun 286: 513-517.
- Gulberg V, Moller S, Henriksen J, Gerbes A (2000) Increased renal production of C-type natriuretic peptide (CNP) in patients with cirrhosis and functional renal failure. Gut 47: 852-857.
- Vlachopoulos C, Loakeimidis N, Terentes-Printzios D, Aznaouridis K, Baou K, et al (2010) Amino terminal pro-C type natruretic peptide is associated with arterial stiffness, endothelial function and early atherosclerosis. Atherosclerosis 211: 649-655.
- Wu C, Wu F, Pan J, Morser J, Wu Q (2003) Furin-mediated processing of Pro-C-type natriuretic peptide. J Biol chem 278: 25847-25852.
- Scotland RS, Ahluwalia A, Hobbs AJ (2005) C-type natriuretic peptide in vascular physiology and disease. Pharmacol Ther 105: 85-93.
- Simon A, Harrington EO, Liu GX, Koren G, Choudhary G (2009) Mechanism of C type natriuretic peptide-induced endothelial cell hyperpolarization. Am J Physiol Lung Cell Mol Physiol 296: L248-256.
- Koch A, Voigt S, Sanson E, Dückers H, Horn A, et al. (2011) Prognostic value of circulating amino-terminal pro-C-type natriuretic peptide in critically ill patients. Crit Care 15: R45.
Citation: Asser ML, Ooda SA, Zaki MA, Amin GA, El-Hassafi MY (2018) Study of Serum N-Terminal-Pro C-Type Natriuretic Peptide and its Relation to the Risk of Variceal Bleeding in Cirrhotic Hepatitis-C Virus Patients. J Gastrointest Dig Syst 8: 556. DOI: 10.4172/2161-069X.1000556
Copyright: © 2018 Asser ML, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4211
- [From(publication date): 0-2018 - Nov 24, 2024]
- Breakdown by view type
- HTML page views: 3545
- PDF downloads: 666