Study of Plasma Endostatin Level in Patients with Acute Myeloid Leukemia
Received: 08-Mar-2016 / Accepted Date: 26-Apr-2016 / Published Date: 06-May-2016
Abstract
Background: Endostatin is a broad spectrum angiogenesis inhibitor and may interfere with the proangiogenic action of growth factors.
Methods: To evaluate the prognostic value of endostatin serum level in acute myeloid leukemia (AML), sixty subjects were included in this study, 30 apparently healthy adult subjects as control (group I) and 30 adult patients with newly diagnosed de novo AML (group II). Estimation of serum endostatin level by ELIZA, at the onset of the disease and after 1 month of induction treatment.
Results: Serum endostatin levels varied widely from 2.1 ng/mL to 7.1 ng/mL (median, 4.1 ng/mL), and the median level was higher in patients with AML compared with patients in the control group (2.6 ng/mL; P<0.001). No significant difference was found between pre-and post-treatment in group II (0.23). The overall survival of patients who had pre-treatment endostatin level ≤50% percentile was significantly longer than patients with pre-treatment endostatin level >50% percentile (p=0.007).According to change of endosatin levels, the overall survival of patients with increased endostatin level was significantly longer than patients with decreased endostatin (p=0.03).
Summary/Conclusion: Inhibition of the angiogenesis may have a role in remission of AML patients.
Keywords: Acute myeloid leukemia; Endostatin; Prognosis; Angiogenesis
77235Introduction
Acute Myeloid Leukemia (AML) is an aggressive hematologic malignancy characterized by accumulation of immature malignant myeloid cells in the bone marrow and blood due to their clonal proliferation without substantial maturation [1].
Angiogenesis is the formation of new vessels from an existing network of vasculature [2]. Irrespective of cellular origin, induction of angiogenesis requires a shift/switch towards activation/upregulation of inducers of angiogenesis over suppression of angiogenic inhibitors (hereafter AI). Some key angiogenic activators include vascular endothelial growth factor A hereafter VEGF (VEGF-A) [3], Matrix Metalloproteinases (MMPs), Placenta Growth Factor (PlGF), Fibroblast Growth Factor (FGF) and Hepatocyte Growth Factor (HGF) [4]. Endogenous inhibitors of angiogenesis include Thrombospondins (THSBs) endostatin, angiostatin and cytokines such as interleukin-12 [5].
The crucial role of angiogenesis in the growth, persistence, and metastases of solid tumors has been indicated in many studies [6,7]. There is mounting evidence that angiogenesis is also significant in leukemia [8].
Endostatin, C-terminal fragment of collagen XVIII, is one of the most potent and specific inhibitors of angiogenesis. Endostatin, originally isolated from medium of hemangioendothelioma, is generated from collagen XVIII through cleavage of an Ala-His linkage. On the cellular level, endostatin was shown to inhibit endothelial cell proliferation and migration and to induce apoptosis of endothelial cells [9,10].
Higher levels of serum endostatin have been associated with poor prognosis in patients with non-small cell lung carcinoma [11], and Non Hodgkin Lymphoma [12]. The results are not parallel to those in acute leukemia in which a limited number of the studies [13].
The aim of the study is to evaluate the prognostic role of endostatin in AML patients.
Subjects and Methods
This study was conducted in Medical Oncology and Clinical Pathology Departments, Faculty of Medicine, Zagazig University during the period between January 2013 and February 2014.
It comprised 60 patients (28 women and 32 men); they were classified into 2 groups, Group I: Included 30 apparently healthy adult subjects (15 males, 15 females) with a mean age 35.8 ± 13.5 years. They matched well with patients in terms of age and sex. Group II: Included 30 adult patients with newly diagnosed de novo AML (17 males, 13 females) with a mean age 38 ± 16.2 years. Patients and controls were subjected to the following:
(1) Complete history taking and thorough clinical examination particularly for pallor, petechiae, bruising, gum swelling, lymph node swelling and spleenomegaly.
(2) Routine laboratory Investigations.
- Complete blood count (CBC): by automated cell counter “Advia 120”, together with examination of Leishman stained peripheral blood smears for differential leucocytic count.
- Liver, kidney functions tests and Lactate dehydrogenase using automated analyzer “Dimension RxL Max”.
(3) Bone marrow Aspiration for Patients group only: Bone marrow smears were stained by Leishman and peroxidase stains and prepared for Immunophenotyping by flow cytometry: using Bectron Dickenson FacsCalibar device to detect the following markers (MPO, CD13, CD33, HLA-DR, TdT, CD14, CD64, CD34, CD 3, CD20 and CD22) for diagnosis of AML.
(4) Specific laboratory Investigation: Plasma Human Endostatin level estimation by quantitative sandwich enzyme immunoassay technique (Boster Biological Technology Co., USA) Following manufacturer Instructions, sensitivity treatment.
(5) Treatment: Patients were treated by an induction regimen 3 and 7 regimen consisting of continuous infusion cytarabine (100 mg/m2) daily for 7 consecutive days combined with 3 days of doxorubicin (30 mg/m2) in addition to vesenoid in for patients with acute promyelocytic leukaemia (AML-M3). Patients with 60 years or poor performance status were treated by low dose cytarabine 10 mg/m2/12 hours for 14 days.
(6) Statistical analysis: The statistical analysis was performed on an IBM Statistical Package for Social Scientists (SPSS) program version 20 (Chicago, Illinois). Spearman’s correlation coefficient was used to evaluate the correlation between non parametric variables. Survival analysis was done according to Kaplan-Meier method, and compared by log-rank test. Differences were considered significant if P values were ≤ 0.05.
(7) Ethics: This research was approved by the Ethics Committee of the Zagazig University. All patients gave their informed consents.
Results
The demographic characteristics of group II is shown in Table 1, mean age was 38 ± 16.2 (range 15-64), 56.7% are males (17/30 patients), 80.0% had ECOG performance status (PS ≤ 1), and fever was a presentation sign in 26 patients (86.7%).
Subtypes (according to the French-American-British) classification were 1 (3.3%) M1, 7 (23.3%) M2, 8 (26.7%) M3, 12 (40.0%) M4 and 2 (6.7%) M5.
In group II, 12 patients (40%) achieved CR to induction treatment while 18 patients (60%) didn’t achieve CR.
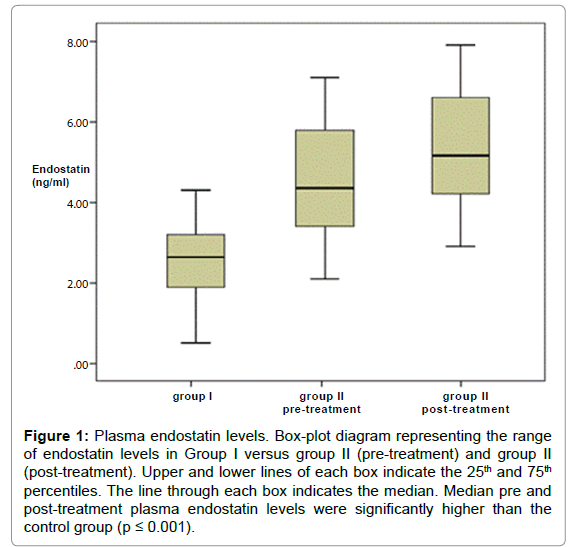
Plasma endostatin levels in Patients with AML and Control Participants. The Plasma Endostatin (PE) levels in the control group were lower compared with the levels in pre-treatment AML patients (median, 2.6 ng/mL; (range; 0.5-6.1 ng/mL) vs. 4.1 ng/mL (range; 2.1- 7.1 ng/mL); P<0.001).
The post-treatment PE level was statistically significantly higher than controls (4.5 ± 1.6 vs. 2.5 ± 1.1; P<0.001), but did not differ from pre-treatment levels (p=0.23; Table 2 and Figure 1).
No significant correlation was detected between basal PE level and the patients’ age, leukocyte counts, hemoglobin levels, platelet counts, LDH, peripheral and bone marrow blast cells percentage (Table 3).
| Variables | No. | Percentage % |
|---|---|---|
| Age | ||
| (15-64 years) (mean± SD: 38 ± 16.2 years) | 30 | 100 |
| Gender | ||
| Males | 17 | 56.7 |
| Females | 13 | 43.3 |
| Performance status (PS) | ||
| ≤1 | 24 | 80.0 |
| 2 | 6 | 20.0 |
| FAB classification | ||
| M1 | 1 | 3.3 |
| M2 | 7 | 23.3 |
| M3 | 8 | 26.7 |
| M4 | 12 | 40.0 |
| M5 | 2 | 6.7 |
| Cytogenetics | ||
| Normal | 14 | 46.7 |
| Favorable | 9 | 30 |
| Failed | 7 | 23.3 |
| Induction chemotherapy | ||
| 3+7 | 19 | 63.3 |
| 3+7 and ATRA | 6 | 20 |
| Low dose cytarabine | 5 | 16.7 |
| Treatment response | ||
| CR | 12 | 40 |
| Non-CR | 18 | 60 |
Table 1: Patient Characteristics.
| AML untreatedversus Control | TreatedAML versus Control | Untreatedversus treated AML | |
|---|---|---|---|
| Endostatin ng/ml | 4.7 ± 1.4 | 4.5 ± 1.6 | 4.7 ± 1.4 |
| v. | v. | v. | |
| 2.5 ± 1.1 | 2.5 ± 1.1 | 4.5 ± 1.6 | |
| P | <0.001 | <0.001 | 0.23 |
Table 2: Serum endostatin concentration in (untreated and treated) AML patients and healthy control.
Figure 1: Plasma endostatin levels. Box-plot diagram representing the range of endostatin levels in Group I versus group II (pre-treatment) and group II (post-treatment). Upper and lower lines of each box indicate the 25th and 75th percentiles. The line through each box indicates the median. Median pre and post-treatment plasma endostatin levels were significantly higher than the control group (p ≤ 0.001).
In group II, post-treatment endostatin levels were significantly higher than pre-treatment levels in patients with CR (mean ± SD: 5.7 ± 1.05 and 3.64 ± 0.92 ng/ml, respectively; P<0.01; Table 4).
Survival Analyses
In group II, after a median follow up of 14 (1-16) months, the median OS was 7 months and a median disease free survival of 8.5 months.
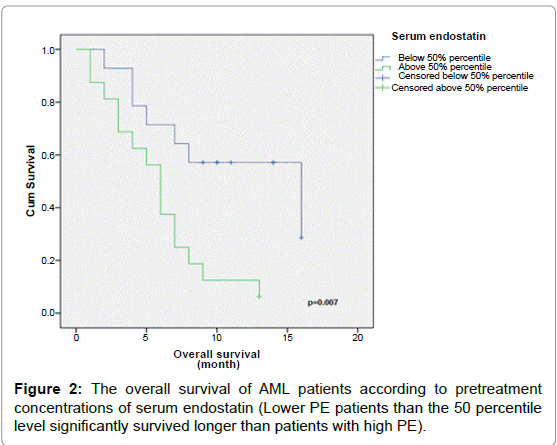
The prognostic value of PE was then evaluated by dividing AML patients into low and high PE groups using the 50 percentile level of AML group (4.4 ng/mL) as the cut-off. Low PE patients survived for a significantly longer time than high PE patients (Table 5, and Figure 2, P=0.007).
Similarly, a significant relation between the overall survival of group II and age. While there were no significant relation between the overall survival and gender, performance status, TLC and FAB classification, BM blast cells and platelet count (Table 5).
Subgroup analysis according to change of PE levels, the overall survival of patients with increased endostatin level after induction treatment was significantly longer than patients with decreased PE (p=0.03). Also there were significant association between change of PE level and age, performance status, cytogenetics, response to treatment (P=0.03, 0.02, 0.04, and <0.001, respectively).
| Factor | Endostatin levelgroup II (No.=30) | |
|---|---|---|
| r | P | |
| Age | 0.11 | 0.08 |
| TLC | -0.10 | 0.58 |
| HB | 0.27 | 0.13 |
| PLT | -0.10 | 0.57 |
| LDH | -0.19 | 0.30 |
| Peripheral blast % | 0.11 | 0.08 |
| Bone Marrow blast % | 0.03 | 0.87 |
Table 3: Correlation between baseline endostatin levels and some clinical and laboratory parametersin group II.
| Response to treatment | No. | Endostatin levelgroup II (No.=30) | P | |||
|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | |||||
| Mean | SD | Mean | SD | |||
| CR | 12 | 3.64 | 0.92 | 5.74 | 1.05 | 0.0008 |
| Non-CR | 18 | 4.83 | 1.41 | 3.63 | 0.72 | 0.001 |
Table 4: Comparison between endostatin levels of group II (pre-treatment and post-treatment) as regards response to treatment.
| Factor | No. ofpatients | Median OS (month) | 1-yrSurvival (%) | P value |
|---|---|---|---|---|
| Age | ||||
| <60 | 27 | 7 | 37 | <0.001 |
| ≥60 | 3 | 2 | 0 | |
| Gender | ||||
| Males | 17 | 7 | 41 | 0.42 |
| Females | 13 | 7 | 23 | |
| Performance status | ||||
| <2 | 24 | 7 | 42 | 0.05 |
| ≥ 2 | 6 | 4 | 0 | |
| WBCs (×103) | ||||
| ≤ 50 | 18 | 7 | 39 | 0.3 |
| >50 | 12 | 5 | 25 | |
| Blast Cells (%) | ||||
| ≤ 75 | 14 | 8 | 43 | 0.27 |
| >75 | 16 | 6 | 25 | |
| Platelets ( ×103) | ||||
| >60 | 25 | 7 | 32 | 0.8 |
| <60 | 5 | 7 | 40 | |
| FAB | ||||
| M1-2 | 8 | 8 | 37 | |
| M3 | 8 | 9 | 30 | 0.22 |
| M4 | 14 | 5 | 21 | |
| Pre-treatment Endostatin | ||||
| >50% | 16 | 6 | 13 | 0.007 |
| ≤ 50% | 14 | 16 | 57 | |
| Splenomegally | ||||
| Absent | 27 | 7 | 33 | 0.9 |
| Present | 3 | 6 | 33 | |
Table 5: Prognostic factors in univariate analyses.
Discussion
Acute myeloid leukemia is a heterogeneous group of diseases characterized by uncontrolled proliferation of myeloid progenitor cells that gradually replace normal hematopoiesis in the bone marrow [14].
Whereas cancer angiogenesis is classically thought of in context to solid tumors, there is mounting evidence that angiogenesis is also significant in leukemia [8].
Angiogenesis plays an important role in progression of the premalignant lesions to malignancies, tumor development, entering tumor cells into circulation and transforming the micro-metastases to obvious metastatic lesions [15].
Several studies have demonstrated that there was an increased angiogenesis in the bone marrow of AML patients [16-18]; while, when the patients achieved a CR after induction chemotherapy, histological analysis revealed a decreased microvessel density in the bone marrow [19,20].
Angiogenesis is a highly regulated process under the tight control of activators and inhibitors. Endostatin is an anti-angiogenic agent that blocks endothelial cell proliferation, tumor growth, and metastasis [21].
In the current study, we found higher mean p-endostatin levels in pretreatment period in group II patients than those control participants in group I. These results were similar to results of studies performed by Glenjen et al. [22], Wrobel et al. [23], and Simten et al. [13]. The elevated levels of endostatin in patients with acute leukemia at the time diagnosis are a supporting finding for increasing angiogenesis in bone marrow, this is associated with increased of both pro and antiangiogenic factors.
We reported that post-treatment endostatin levels were significantly higher than pre-treatment levels in patients with CR. This elevation of endostatin levels at AML remission might represent a rebound effect after reduction of the tumor mass and subsequently reduction in the production of the secretion of proangiogenic factors while the production of inhibitors continue.
On other hand, Lai et al. [24] found the significant lower levels of endostatin in patients with CR in comparison to patients without complete remission; they explained this finding by decreased bone marrow vascularity with treatment thus lowering endostatin level.
However, they also reported the limitation of their study that the endostatin assay could not detect the modified or degraded forms of endostatin and it may react differently with various glycosylated forms of endostatin, which may explain why our results are different from these results.
No significant correlation was detected between the endostatin level in group II (pretreatment) and age, TLC, hemoglobin concentration, platelets count, LDH level, peripheral and B.M. blast cells percent. These results coincide with the results reported by Simten et al. [13], Wrobel et al. [23] and Aref et al. [25], but inconsistent with Lai et al. [25] found significant correlation between pretreatment PE levels and age.
In the current series, high pretreatment endostatin levels were associated with unfavorable outcome in univariate survival analyses of patients with AML. This can be explained by elevation of endostatin might be a result of increased angiogenesis of the tumor reflecting the tumor burden.
Similarly, Lai et al. [24] found that when the mean endostatin value of control cases was considered as the cut-off value, the survival of the AML patients with endostatin values higher than the cut-off is significantly shorter.
This finding is different from the ones that have been reported by Aref et al. [25] reported this prognostic value of PE as they divided AML patients into two groups using the 75 percentile endostatin levels of the patients group as cut-off value. They found that high PE levels were correlated with good clinical outcome. Their explanation was that it might be attributed to the hindering effect of PE on angiogenesis.
It must be mentioned that the main difference between these two studies is, that in the present work, endostatin was quantified in plasma, whereas in Aref et al. [25], endostatin was analysed in serum.
Simten et al. [13] also reported that endostatin levels could not be shown to have a prognostic effect in patients with AML, as no significant correlation was found between the endostatin levels and the overall survival or disease free survival.
Conclusion
Significant higher PE levels in patients with AML during CR period indicate that chemotherapy and angiogenesis inhibitors could modulate the regulation of angiogenesis in AML patients.
Levels of endostatin in patients with AML may be effective in predicting the survival. Wide scale studies are recommended in order to establish the mechanism underlying the association between high PE and poor clinical outcome.
Conflict-of-Interest Disclosure
The authors declare no competing financial interests.
References
- Lowenberg B, Downing JR, Burnett A (1999) Acute myeloid leukemia. N Engl J Med 341: 1051-1062.
- Ferrara N, Henzel WJ (1989) Pituitary follicular cells secrete a novel heparin binding growth factor specific for vascular endothelial cells. BiochemBiophys Res Commun 161:851-858
- Khoury CC, Ziyadeh FN (2011)Angiogenic factors. ContribNephrol 170:83-92.
- Taraboletti G, Rusnati M, Ragona L, Colombo G (2010) Targeting tumor angiogenesis with TSP-1-based compounds: rational design of antiangiogenicmimetics of endogenous inhibitors.Oncotarget1:662-673.
- Smith M, Barnett M, Bassan R, Gatta G, Tondini C, et al. (2004) Adult acute myeloid leukaemia. Crit Rev OncolHematol 50:197-222.
- Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. SeminOncol 29:15-18.
- Trujillo A, McGee C, Cogle CR (2012) Angiogenesis in acute myeloid leukemia and opportunities for novel therapies. J Oncol 1-9.
- Schuchg, Heymachjv, Nomi M, Machlufm, Force J, et al. (2003)Endostatin inhibits the vascular endothelial growth factor-induced mobilization of endothelial progenitor cells. Cancer Res 63: 8345-8350.
- O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, et al. (1997)Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88: 1-20.
- Suzuki M, Iizasa T, Ko E, Baba M, Saitoh Y, et al. (2002) Serum endostatin correlates with progression and prognosis of non-small cell lung cancer. Lung Cancer 35: 29-34.
- Bono P, Teerenhovi L, Joensuu H (2003) Elevated serum endostatinis associated with poor outcome in patients with non-hodgkin lymphoma. Cancer 97: 2767-2775.
- Simten D, Gulsum O, Mesude Y, Murat A, Funda C (2011) Assessment of Plasma Endostatin Levels in Patients with Acute Myeloblastic and Lymphoblastic Leukemia. Int J Hematol Oncol 4: 210-216.
- Fang Y, Vera M (2013) Acute myeloid leukemia. In: The Bethesda Handbook of Clinical Hematology. GriffinP and Neal S.Lippincott Williams and Wilkins137.
- Liu Y, Deisseroth A (2006) Tumor vascular targeting therapy with vectors.Blood107:3027-3033.
- Hussong JW, Rodgers GM, Shami PJ (2000) Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood 95:309-313.
- Mesters RM, Padró T, Steins M, Bieker R, Retzlaff S, et al. (2000) Increased angiogenesis in the bone marrow f patients with acute myeloid leukemia. Blood 95:2637-2644.
- Aguayo A, Kantarjian H, Manshouri T, Gidel C, Estey E, et al. (2000) Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood 96:2240-2245.
- Aguayo A, Estey E, Kantarjian H, Mansouri T, Gidel C, et al. (1999) Cellular vascular endothelial growth factor is a predictor of outcome in patients with acute myeloid leukemia. Blood 94:3717-3721.
- Kuzu I, Beksac M, Arat M, Celebi H, Elhan AH, et al. (2004) Bone marrow Microvessel Density (MVD) in adult Acute Myeloid Leukemia (AML): Therapy induced changes and effects on survival. Leuk Lymphoma 45:1185-1190.
- Ricard-Blum S (2010) The First Draft of the Interaction Network of Endostatin, an Inhibitor of Angiogenesis. J Biomol Tech 21: S4.
- Glenjen N, Mosevoll K, Bruserud O (2002) Serum levels of angiogenin, basic fibroblast growth factor and endostatin in patients receiving intensive chemotherapy for acute myelogenous leukemia. Int J Cancer 101: 86-94.
- Wrobel T, Mazur G, Kapelko K, Kuliczkowski K (2005)Â Endostatin serum level in acute myeloid leukemia. Neoplasma 52: 182-184.
- Lai R, Estey E, Shen Y, Despa S, Kantarjian H, et al. (2002) Clinical significance of plasma endostatin in acute myeloid leukemia/myelodysplastic syndrome. Cancer 94: 14-17.
- Aref S, El-Sherbiny M, Azmy E, Goda T, Selim T, et al. (2008) Elevated serum endostatin levels are associated with favorable outcome in acute myeloid leukemia. Hematol 13: 95-100.
Citation: Haggag R, Hussein O, El Moaty HA, Omran A, Ebian H (2016) Study of Plasma Endostatin Level in Patients with Acute Myeloid Leukemia. Adv Oncol Res Treat 1: 107.
Copyright: ©2016 Haggag R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 12314
- [From(publication date): 6-2016 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 11364
- PDF downloads: 950