Research Article Open Access
Study of Label-Free Detection and Surface Enhanced Raman Spectroscopy (SERS) of Fibrinogen Using Arrays of Dielectric Core-Metal Nanoparticle Shell
H Awad1, N Waly1, T Abdallah1, S Negm2 and H Talaat1*1Department of Physics, Faculty of Science, Ain Shams University, Abbassia, Cairo, Egypt
2Department of Physics and Mathematics, Faculty of Engineering (Shoubra), Benha University, Cairo, Egypt
- *Corresponding Author:
- Hassan Talaat
Department of Physics
Faculty of Science, Ain Shams University
Abbassia, Cairo, Egypt
Tel: +20-24854254
Fax: +20-24842560
E-mail: hassantalaat@hotmail.com
Received date: November 04, 2015; Accepted date: November 24, 2015; Published date: November 30, 2015
Citation: Awad H, Waly N, Abdallah T, Negm S, Talaat H (2015) Study of Label- Free Detection and Surface Enhanced Raman Spectroscopy (SERS) of Fibrinogen Using Arrays of Dielectric Core-Metal Nanoparticle Shell. J Anal Bioanal Tech S13:010. doi:10.4172/2155-9872.S13-010
Copyright: © 2015 Awad H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Label-free detection using arrays of SiO2 nanospheres (of diameters ~500 nm) core and gold nanoparticle seeding (~4 nm) shell, with additional gold electroplated films were used as biosensors for adsorption of human fibrinogen. Also, surface enhanced Raman spectroscopy (SERS) of fibrinogen was studied as a function of the thickness of the electroplated film. It is observed that the extinction spectra of these arrays show multiple extinction peaks resulting from the interference of beams reflected between the flat substrate and the surface of the dielectric spheres as reported in the literature. There is an increase in the peak intensity and a red shift with increasing plating time. The sensitivity of these arrays to the adsorption of fibrinogen was obtained from the further red shift in the extinction spectra. SERS of fibrinogen shows an increase in the Raman bands with the time of electroplating up to a surface roughness of mean value ~1.35 nm (measured by scanning tunneling microscopy (STM)). This was followed by a decrease in the intensity of peaks with more increasing electroplating. Similar SERS results were obtained of the inorganic dye cresyl violet (CV). These results are explained in terms of interplay of the surface field enhancement due to the growth in the nanoparticles size and the resulting hotspot effects and the reduction due to the increase in the metal shell thickness.
Keywords
Surface enhanced Raman spectroscopy; Fibrinogen; Dielectric core; Nanoparticle
Introduction
Recently, label free biosensors have become an effective tool for the minute analysis of biospecific interactions overcoming the known drawbacks of label based biosensors, like time consuming, and that the marker itself can affect the original functionality of the molecules being studied [1]. Great efforts have been made to establish a highly sensitive label-free detection scheme [2] through array structured biosensors formed from dielectric nanoparticle silica core-metal shell. These structures allow a combination of surface plasmon resonance (SPR) and reflectometric interference effects, to improve the sensitivity through the shift in the plasmon interference peaks.
On the other hand, the plasmonic nature of surface enhanced Raman scattering (SERS) of molecules adsorbed on specially prepared metal surfaces, results in enhancement of the molecules spectra up to 14 orders of magnitudes [3,4]. Such an enhancement allows the possibility for single molecule spectroscopy [5-8]. These large plasmonic enhancements are caused by the resonant surface plasmons associated strong electromagnetic (EM) fields in the substrate metallic nanostructrures. Localized surface plasmons are coherent oscillations of metal electrons in resonance with incident light at a certain wavenumber [9]. These resonances are functionally dependent on the shape (geometry) and size of the metal nanoparticles constituting the surface [10].
In this work, we used label-free biosensors composed of an array of SiO2 nanospheres coated with gold nanoparticles and an added different thickness electro-plated gold film. The sensitivity of these biosensors to the adsorption of fibrinogen was measured through the red shift in the resulting extinction interference peaks. SERS of fibrinogen adsorbed on these arrays is employed to compare with the sensitivity of the label-free technique. Furthermore, SERS for inorganic dye cresyl violet (CV) on similar substrates was carried out to ascertain the variation in intensity of Raman bands with the thickness of the electroplated gold film.
Experiment
Samples
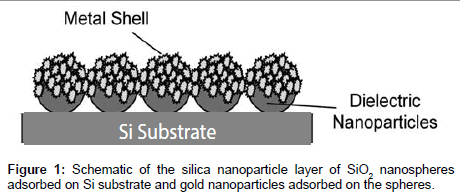
Dielectric amine terminated SiO2 nanoparticles were deposited on the substrate by self-assembly floating technique [11]. Seed nanoparticles were prepared by reduction of the gold chloride hydrate, (H(AuCl4). H2O) with a strong reducing agent (sodium borohydride, NaBH4) and stabilized by citrate. To coat the amine terminated silica particles with metal seed nanoparticles, the surfaces were first incubated in a 2:1 (v:v) solution of polyethyleneimine (PEI)/phosphate-buffered saline (PBS) for 20 min to charge the surface positively. The surfaces were rinsed with Millipore water, dried in air atmosphere and afterwards incubated in seed solutions for 12 h. Then the solution was exchanged with fresh seed solution, in which the substrate was kept for another 12 h. To remove non-bound nanoparticles, the surfaces were gently rinsed with Millipore water and dried in air atmosphere (Figure 1).
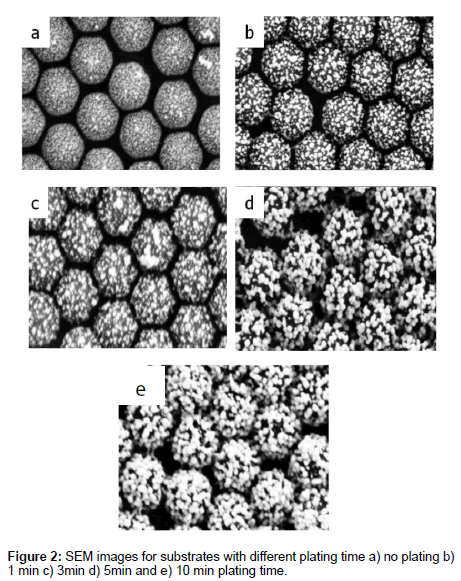
In electrolysis gold plating [12], the plating solution consists of 0.1 wt% AuCl4- and 0.04M NH2OH mixed in a ratio of 7:3. The solution was applied to the surface for different periods of time, 60 sec, 180 sec, 300 sec and 600 sec to grow the nanoparticle seeds up in size and form a contiguous metal shell around the dielectric. Then the surfaces were rinsed with Millipore water, dried in air atmosphere and either stored in N2 atmosphere or used immediately. Figure 2 shows the SEM images for dielectric spheres covered with gold nanoparticles. Coverage starts on the isolated gold nanoparticles and by increasing the time of electrolysis plating, these spherical gold particles increase in size but are still isolated. On increasing the plating time further, these particles grow in diameter and connect forming surface that may be described as continuous one. It is observed that as the plating time increases the surface roughness decreases, and becomes smoother due to the growth of surface confined Au nanoparticles.
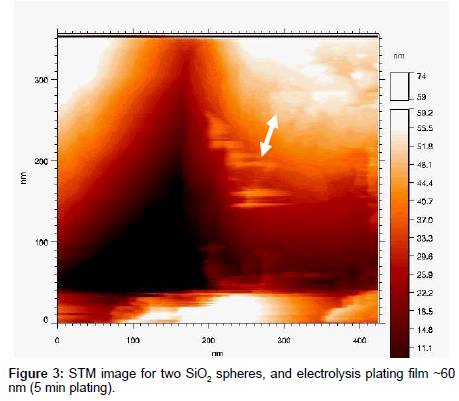
To measure the roughness of these electro-plated films, scanning tunneling microscope (STM) images of these samples were carried out. The STM is an OMICRON system, operating in ultra-high-vacuum below 4 × 10-9 Torr, at room temperature, and at the constant current mode. Figure 3 shows the STM image of two SiO2 spheres of diameter ~500 nm in contact, and the gold layer thickness of ~60 nm (5 min. plating).
Fibrinogen was dissolved in PBS by sonication for 30 min and adsorbed on biosensor surfaces by incubating the surface in a 1 mg/ml fibrinogen solution for 180 min in the fridge. The surface was continuously washed in a container with Millipore water to remove non-adsorbed fibrinogen effectively from the solution and dried in air atmosphere. SERS spectra were carried out on a Renishaw Micro spectrometer series 5000 (Kaiser optical systems, Inc, Ann Arbor, MI) using the 50X objective. The excitation wavelength is 785 nm from a diode laser and exposure time was 25 times per second.
In the case of CV adsorption on the biosensor surfaces, solution with concentration 10-4M of the dye cresyl violet perchlorate of analytical grade purity in pure ethanol was prepared and deposited on the surface of the substrates. A droplet (about 2 μl) of the sample solution was deposited on the substrate surfaces as well as on the surface of a Si wafer which was used as a reference sample. SERS was performed using HORIBA JOBN YVON Ramanor HG2S spectrometer equipped with a micro-Raman attachment, and Ar ion laser (Spectra Physics) for excitation 514.5 nm. The SERS spectra for the adsorbed CV were recorded after evaporation of the solvent. The laser beam was focused on SERS substrate by means of microscope objective (Olympus plan 50X). The scattered light was collected by the same objective in the back scattering configuration. The laser power was kept constant for all samples including the reference.
Results and Discussion
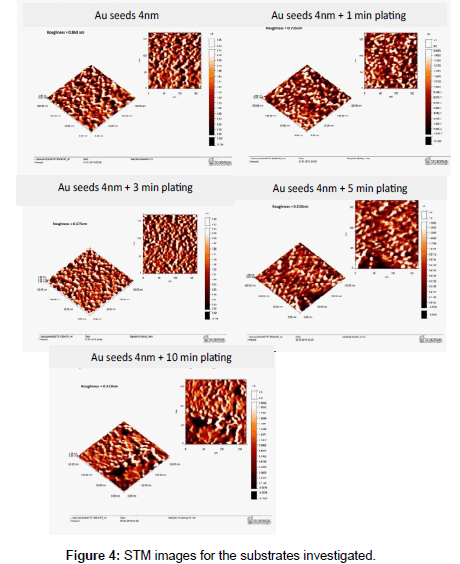
The Surface roughness of the SiO2 core-gold nanoparticle shell before and after plating was determined by the STM images that were taken for all samples with increasing electroplating time. The results are shown in Figure 4. The analysis of roughness was carried out across similar selected designated areas of each sample.
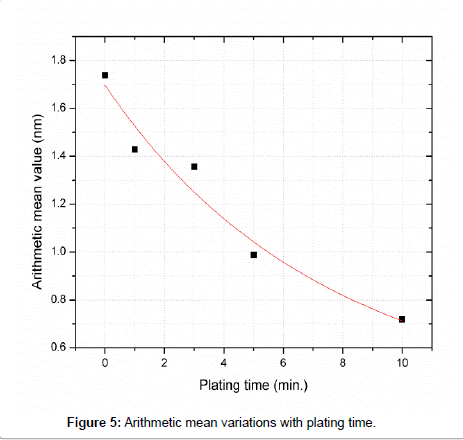
The arithmetic mean values for five samples of different time of plating (0, 1, 3, 5 and 10 min.) were obtained and shown in Figure 5. It is clear from the figure that as the plating time increase from 0 to 10 minutes, the arithmetic mean decrease from 1.738 nm to 0.718 nm and the surface becomes smoother due to the growth of surface confined Au nanoparticles.
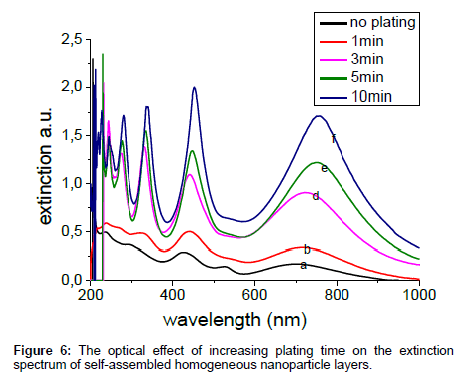
The UV-Vis spectroscopy for the samples is referenced to a substrate without dielectric spheres. As shown in Figure 6, there are several prominent SPR peaks, due to interference of the beams multiply reflected between the substrate and the surface of the sphere. The coherence conditions are satisfied as the diameter of spheres is about 500 nm. By increasing the time of plating there is an increase in the intensity of the peaks, as well as slightly red shift with increasing growth time of the plating.
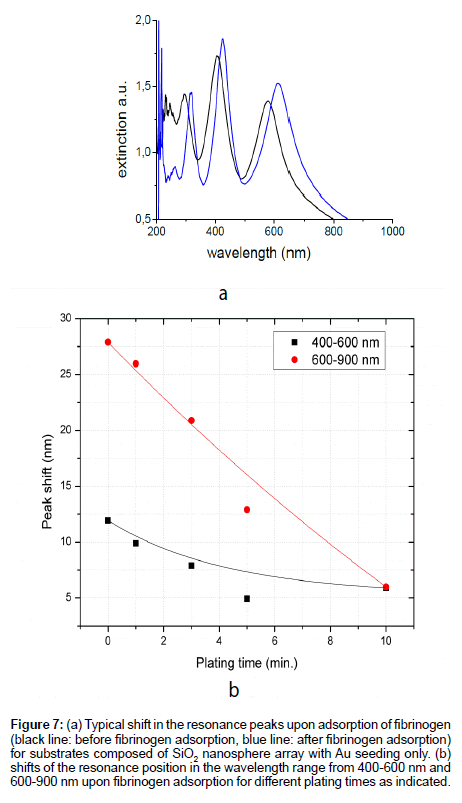
Sensitivity was determined by measuring the wavelength shift of the resonance peaks in response to adsorption of fibrinogen onto the surface. Figure 7a shows a typical shift of the resonance peaks upon adsorption of fibrinogen on the substrate composed of SiO2 layer with Au seeding only. It is found that the sensitivity of the peaks in the range from 400 to 900 nm decrease nearly exponentially with increasing electroplating time. Figure 7b show the peak shift for substrates with increasing plating time in the wavelength range from 400-600 nm and 600-900 nm respectively as indicated.
Figure 7: (a) Typical shift in the resonance peaks upon adsorption of fibrinogen (black line: before fibrinogen adsorption, blue line: after fibrinogen adsorption) for substrates composed of SiO2 nanosphere array with Au seeding only. (b) shifts of the resonance position in the wavelength range from 400-600 nm and 600-900 nm upon fibrinogen adsorption for different plating times as indicated.
These results are consistent with Jain et al. [13] where he observed that the change in the extinction wavelength peak position of dielectric core-metal shell structures in solution decreases near-exponentially with an increase in the shell thickness-to-core radius ratio, due to the decrease in the near-field. On comparing Figures 7b and 5 it is observed that the surface roughness is an important parameter that affects the sensitivity of surface plasmon resonance SPR biosensors [2]. As the surface roughness decreases, the sensitivity of SPR biosensors was found to decrease for both localized surface plasmons and propagating surface plasmons based biosensors [14-17].
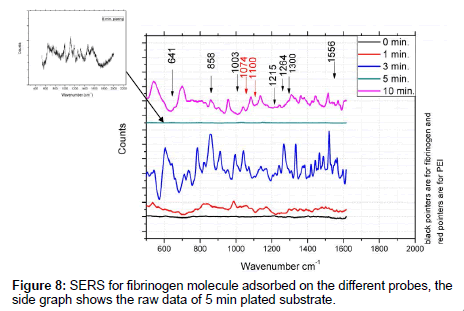
The SERS for fibrinogen molecules adsorbed on the five substrates are shown in Figure 8. The 780 nm excitation source is used since it is close to the interferometric resonance peak of the structure (Figure 6). After appropriate analysis of the spectra like back ground subtracting, and selecting the best spectra for each sample from three spectra recorded at three different spots but of similar appearance on the microscope. This procedure was carried out due to lack of reproducibility. Actually the spectra at different spots differ partially due to irregularities of the array substrate (as seen through the microscope but not shown here). Furthermore, molecular positioning on the corrugated surface may cause broadening and band shifts. The SERS spectra for PEI were also present giving a Raman bands at 1074 cm-1, and 1100 cm-1. In the Raman spectra of fibrinogen, the aromatic side chains of tyrosine (Tyr) at 641 cm-1, 858 cm-1, 1215 cm-1, the chains of phenylalanine (Phe) and tryptophan (Trp) at 1003 cm-1, 1300 cm-1, and 1556 cm-1, give strong Raman bands. Also the amide III band at 1264 cm-1 [18] was selected for comparison. It is clear from the spectra that the 3 minute plating gives the highest Raman intensity.
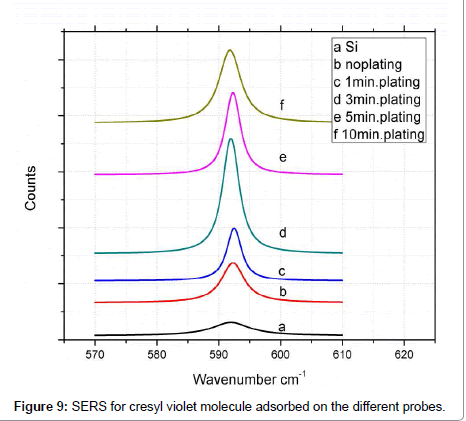
To check the above results, SERS was carried out for the CV adsorbed on the substrates investigated and are shown in Figure 9. The intensive band in Raman spectrum of the CV that appears at about 592 cm-1 due to the in plane vibrational mode, was used to monitor the enhancement of the Raman intensity of the substrates. In this case however, the incident wavelength (514 nm) is close to the surface Plasmon resonance of the Au spheres. From the obtained spectra it is easily observed that the Raman intensity increases up to the 3 minutes plating then decreases with increasing thickness, which is in agreement with the observation in the case of fibrinogen, that the 3 minute plating, of surface roughness 1.356 nm, gives the highest intensity.
However, on comparing these SERS results with the absorption or extinction peaks for the corresponding samples that was presented above there is a lack of consistency. The increase in the plating time results in the growth of the nanoparticle size and their approaching each other reducing the interparticle distance in the nanoshell arrays and creating periodic density of hot spots that produce an intense nearfield enhancement. This results in the enhancement of Raman spectra. However at the same time the increase in the thickness of the film tends to decrease the local field coupling between the two interfaces. Such interplay between these two effects, results in an optimum thickness of roughness ~1.35 nm corresponding to the 3 min. plating. Furthermore, a specific study of the effect of interparticle distance on the hot spot field enhancement has shown a minimum distance to give a large enhancement in the surface fields that decreases with further decreasing of the interparticle distance [19].
Conclusion
We have studied arrays of a dielectric core-metal nanoparticle shell with additional electroplated metal films as biosensors. The extinction spectra for these arrays show several peaks due to the interference of beams reflected between the flat substrate and the surface of the dielectric spheres. There is an increase in the peaks intensity as well as a red shift with increasing the electro plated film thickness. The STM images show a decrease of the surface roughness with the increase of the thickness of the plating. There is a further shift in the peak wavelength on the adsorption of fibrinogen. However, the sensitivity of the arrays to fibrinogen adsorption decreases with the increase of the gold nanoparticle shell thickness due to the decrease in coupling between the inner and outer surface of the metal film. SERS spectra for adsorbed organic fibrinogen show an increase in the intensity in the Raman band up to roughness of arithmetic mean value of 1.356 nm, and then a decrease, the same results were obtained using CV. These variations are discussed in terms of interplay between the field enhancement due to the approach of the shell nanoparticles resulting in hot spots, and the field decrease with increasing the shell thickness.
Acknowledgements
The help with sample preparation of R. Dahint group at Heidelberg University, Germany, and the help with Raman spectroscopy, of S. R. Panikkanvalappil (M. El-Sayed group) at Georgia Institute of Technology, USA, are greatly appreciated. The authors value the generous support of STDF ID 3746.
References
- Cunningham B, Lin B, Qiu J, Li P, Pepper J, et al. (2002) A plastic colorimetric resonant optical biosensor for multiparallel detection of label-free biochemical interactions. Sens Actuators B: Chem 85: 219-226.
- Guvenc HO (2013) Label-free Detection of Biospecific Interactions in Peptide Arrays Using Core-shell Nanoparticle Films. Universität Heidelberg, Heidelberg.
- Lee SJ, Guan Z, Xu H, Moskovits M (2007) Surface enhanced Raman spectroscopy and nanogeometry: the plasmonic origin of SERS. J Phys Chem C 111: 17985-17988.
- Special Issue on SERS (2005) J Raman Spectrosc 36: 465.
- Kneip K, Kneip H, Manoharan R, Itzkam I, Dasari RR, et al. (1998) Surface-enhanced Raman scattering (SERS)-a new tool for single molecule detectionand identification. Bioimaging 6: 104-110.
- Johansson P, Xu H, Kall M (2005) Surface-enhanced Raman Scattering and fluorescence near metal nanoparticles. Phys Rev B 72: 035427.
- Xu H, Wang XH, Persson MP, Xu HQ, Käll M, et al. (2004) Unified treatment of fluorescence and raman scattering processes near metal surfaces. Phys Rev Lett 93: 243002.
- Xu H, Bjerneld EJ, Käll M, Borjesson L (1999) Spectroscopy of Single Homoglobin Molecules by Surface Enhanced Raman Scattering. Phys Rev Lett 83: 4357-4360.
- Willets KA, Van Duyne RP (2007) Localized surface plasmon resonance spectroscopy and sensing. Annu Rev Phys Chem 58: 267-297.
- Lee KS, El-Sayed MA (2006) Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. J Phys Chem B 110: 19220-19225.
- Rybczynski J, Ebels U, Giersig M (2003) Large-scale, 2D arrays of magnetic nanoparticles. Colloids and Surfaces a-Physicochemical and Engineering Aspects 219: 1-6.
- Brown KR, Natan MJ (1998) Hydroxylamine seeding of colloidal Au nanoparticles in solution and on surfaces. Langmuir 14: 726-728.
- Jain PK, Ei-Sayed MA (2007) Surface plasmon resonance sensitivity of metal nanostructures: Physical basis and universal scaling in metal nanoshells. Journal of Physical Chemistry C 111: 17451-17454.
- Chen SJ, Chien FC, Lin GY, Lee KC (2004) Enhancement of the resolution of surface plasmon resonance biosensors by control of the size and distribution of nanoparticles. Optics Letters 29: 1390-1392.
- Byun KM, Yoon SJ, Kim D (2008) Effect of surface roughness on the extinction-based localized surface plasmon resonance biosensors. Applied Optics 47: 5886-5892.
- Chen X, Pan M, Jiang K (2010) Sensitivity enhancement of SPR biosensor by improving surface quality of glass slides. Microelectronic Engineering 87: 790-792.
- Kanso M, Cuenot S, Louarn G (2007) Roughness effect on the SPR measurements for an optical fibre configuration: experimental and numerical approaches. Journal of Optics a-Pure and Applied Optics 9: 586-592.
- Marx J, Hudry-Clergeon G, Capet-Antonini F, Bernard L (1979) Laser Raman spectroscopy study of bovine fibrinogen and fibrin. Biochim Biophys Acta 578: 107-115.
- Abdallah T, El-Brolosy TA, Mohammed MB, Easawi K, Negm S, et al. (2012) Effect of shape and interstice on surface enhanced Raman scattering (SERS) of molecules adsorbed on gold nanoparticles in the near-dipole and quadrupole regions. J Raman Spectroscopy 43: 1924-1930.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11382
- [From(publication date):
specialissue-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10482
- PDF downloads : 900