Study of Ketamine Infusions Extended Stability (SKIES)
Received: 25-Feb-2024 / Manuscript No. jabt-24-128196 / Editor assigned: 27-Feb-2024 / PreQC No. jabt-24-128196 (PQ) / Reviewed: 12-Mar-2024 / QC No. jabt-24-128196 / Revised: 19-Mar-2024 / Manuscript No. jabt-24-128196 (R) / Accepted Date: 20-Mar-2024 / Published Date: 25-Mar-2024 QI No. / jabt-24-128196
Abstract
Background: Structurally similar to phencyclidine (PCP), ketamine hydrochloride is widely used for treatment in patients with resistant depression, as an anesthetic for surgical procedures, and more. The usage of ketamine infusions has increased in recent years. However, with a lack of extended stability information, it is difficult to determine if ketamine is safe to administer if diluted and stored for extended periods of time.
Objective: To evaluate the stability of ketamine hydrochloride infusion in 0.9% normal saline while in controlled ambient conditions.
Methods: A High-Pressure Liquid Chromatography (HPLC) analysis was performed after each time point to determine stability of Ketamine Hydrochloride 500mg diluted in 50ml of normal saline. An infusion was considered stable if the area under the curve was within the USP acceptable range of 95-105%.
Results: All samples remained physically unchanged over time. For all ketamine infusions, after each time point, the HPLC analysis was within 95-105%. Additionally, the analysis showed negative sterility samples and the pH remained stable throughout the study.
Conclusion: This study shows Ketamine HCL is stable in concentrations up to 10mg/ml in polyvinyl chloride (PVC) under ambient conditions for a maximum of 90 days.
Keywords
Ketamine; Ketamine HCL; Ketamine hydrochloride; Ketamine infusion; Extended stability; HPLC
Introduction
With the spike in hospital admissions due to various indications, the country has seen an increase in the use of ketamine therapy. While sometimes used in severe cases for sedation, ketamine is a popular choice for pain management. Classified as a non-barbiturate dissociative anesthetic, the use of ketamine includes indications ranging from depression and pain control to sedation. Typical dosing for ketamine infusions can range with a maximum infusion dosing of up to 15mg/kg/hr in indications such as refractory status epilepticus. With these ranges, ketamine usage can reach elevated levels in a short period of time, and preparing this therapy can be time consuming.
At the time of this study, little information was known for the stability of the drug. Recent searches bring up information on the stability of vials stored in different areas, one being an emergency service unit. Other studies have stability information of ketamine with morphine sulfate [1]. However, it is difficult to extrapolate storage information from vials to polyvinyl chloride (PVC) or in combination with other therapies [2].
With new updates to USP <797> taking effect November 2023, it calls for more robust information for using stability data. High Pressure Liquid Chromatography (HPLC) is becoming more of a standard analysis along with clear details on how the study was performed. Stability information consists of the drug concentration, the storage temperature and, the final container. The purpose of this study was to provide extended stability information of Ketamine Hydrochloride diluted into a 50ml PVC container and stored at room temperature (22℃ - 25℃). An overview of studies that tested Ketamine to compare the different beyond use dates (BUD) [3-5] (Table 1).
| Year | Details | Ketamine + X Drug | BUD | Reference |
|---|---|---|---|---|
| 2002 | 10mg/ml diluted with sterile water using Polypropylene syringes | N/A | 30 days @ RT | Gupta VD, 2016[3] |
| 2009 | 0.2 - 1mg/ml diluted with sodium chloride using PVC bags | Hydromorphone | 7 days @ RT | Ensom MHH, 2009[4] |
| 0.2mg/mL | ||||
| 2016 | 1-4mg /ml diluted with sodium chloride using Polyolefin bags | Butorphanol Tartrate | 15 days Ref or @ RT | ASHP Injectables[5] |
| 50, 100, or 150mcg/mL | Protect from light | |||
| 2016 | 1mg/ml diluted with sodium chloride using PVC bags | Morphine sulfate | 6 days @ RT | ASHP Injectables[5] |
| 1mg/ml | ||||
| 2016 | 25 mg/mL diluted with sodium chloride using PVC bags | Morphine sulfate | 6 days @ RT | ASHP Injectables[5] |
| 25mg/ml |
Table 1: An overview of studies that tested Ketamine to compare the different beyond use dates (BUD)
Methods
Preparation of Ketamine and Infusion Set-Up
All ketamine hydrochloride infusions were prepared in a certified ISO-5 primary engineering control area within an ISO-7 clean room. The 50ml saline bags were supplied by ICU Medical [lot: 5639986 exp: 06/30/2023] and the ketamine hydrochloride 500mg/5ml vials from Hikma [lot 21052331/21052332 exp: 11/30/2024] and Hospira [lot: 39015CD/39290CD exp: 01/03/2024]. The 50ml saline bags have a range of overfill from 52 to 62mls. All ketamine vials did not require reconstitution.
A total of 121 samples with a total fill volume of 55 mls were compounded at the hospital in Lancaster, Pennsylvania and sent out to an analytical site in Oklahoma for testing. This was divided into two phases, the first phase consisted of 44 bags which were compounded on July 22nd, 2022 for system suitable testing which was initiated on July 28th, 2022. In the second phase, 77 bags were compounded on October 27th, 2022, and stability testing was initiated on November 3rd, 2022.
To prepare the infusion, 5mls of the ketamine were drawn up using a 10ml Luer-lock BD syringe [lot: 2033223 exp: 01/31/2027] and injected into the 50ml ICU Medical 0.9% normal saline bag. The final compounded items were then sent to the testing facility.
Testing consisted of particle counts, pH, assay, sterility, and endotoxin testing. Samples were evaluated at monthly intervals with time points consisting of 30, 60 and 90 days. Ketamine infusion bags were evaluated under ambient (25°C ± 2°C and 60% RH ± 5%) storage conditions. All testing was done under current good manufacturing practices (cGMP). The stability criteria were deemed acceptable for a range of 95.0-105.0%.
The pH was determined per USP <791> [6]. Endotoxin testing was performed per USP <85> with a specification of having no more than (NMT) 1.67 EU/mg [7]. Sterility testing was performed per USP<71> [8]. Particulate counts were performed per USP <788> [9]. Container closure was also tested to ensure it maintained its integrity throughout the duration of the study. An infusion was considered stable if it maintained 95.0-105.0% of label concentration, tested via an assay.
To comply with USP <71> standards, a minimum of 4 articles of the finished product were required to satisfy requirement at each specified time point. During T0, T60, and T90, a total of 17 infusion bags were tested at each time point from the 77 bags that were compounded during the second phase. At the thirty day interval, only 6 bags were used to test particulate matter, ketamine assay, and sterility. 4 out of the 6 bags were sterility tested and the two others were reserved for the 2 other variables. It is important to note that bags utilized for the ketamine assay were shared with the appearance and pH assays. Only 57 of the 77 bags were tested.
HPLC Instrumentation
The HPLC instrument, an Agilent system, consisted of a Kinetex 2.6-μm C18 (100x 4.6mm) silica core column (Phenomenex, Torrence, California), delivery pump, an automatic injection system equipped with a 200-μL injector, a sequence injector, and an ultraviolet detector (dual absorbance) set at 225nm. The column temperature was set to 30 degrees Celsius. The first mobile phase utilized consisted of 2.76g of sodium phosphate monobasic anhydrous added to 1000ml of HPLC grade water. Mobile phase B, the second mobile phase, consisted of methanol. The samples were eluted at room temperature via gradient and the flow rate was set at 1.0 mL/min to achieve timely chromatograms. A total of 25 samples were injected with each injection consisting of a volume of 10μl and a run time of 12 minutes.
Method Validation
For system suitability testing, 5 injections of ketamine 50mcg/ml were used. The percent relative standard deviation of peak areas was no more than 2.0% and resolution was no less than 1.5. Tailing was no more than 2.0, theoretical plates no less than 2,000 and all bracketing standard percent relative standard deviation (%RSD) was no more than 2.0% and was deemed acceptable.
For specificity of verification, a placebo of benzethonium chloride and water was used. This placebo was found in the highest amounts across multiple formulations of ketamine. The placebo and Ketamine were verified both at 50mcg/ml. There was no co-elution of the active pharmaceutical ingredient (API) or excipients in the chromatograms. Resolution was no less than 1.5 between the ketamine and neighboring peaks for the suitability injections. Peak to peak resolution was ≥ 1.5 between the ketamine and neighboring for the system suitability injections.
Three separate preparations of the API were studied for accuracy at 100% assay level. Each preparation was injected once for a total of 3 injections. All preparations were within 3% and deemed acceptable.
Physical Compatibility and Chemical Stability
The physical characteristics of the infusions were evaluated qualitatively at the time of preparation and at weekly intervals up to 91 days. Initial values were established for appearance, pH, particulate matter, endotoxin, sterility, container closure, fill volume, and ketamine content at time 0 (T0) and subsequently evaluated at each stability time point (T30, T60, T90). As batches were made, the same individual analyzed all bags for physical properties.
The pH was evaluated using a Fisher Scientific Accumet AB150 meter. Particulate matter was determined using a HIAC 9703 Particle Counter. Endotoxin levels were tested using Biotek ELx808. Additionally, sterility testing was determined by membrane filtration and container closure was determined using a vacuum decay method. Finally, obtaining ketamine levels was performed via assay using a stability indicating HPLC method.
Results
The results of the study showed a robust outcome when analyzing the entire study. The data from all time points indicates ketamine is stable when diluted to approximately 10mg/ml in PVC containers when stored at room temperature. Little variation was seen between time points. Each time point was express as a precentage compare to the standard 10mg/ml concentration.
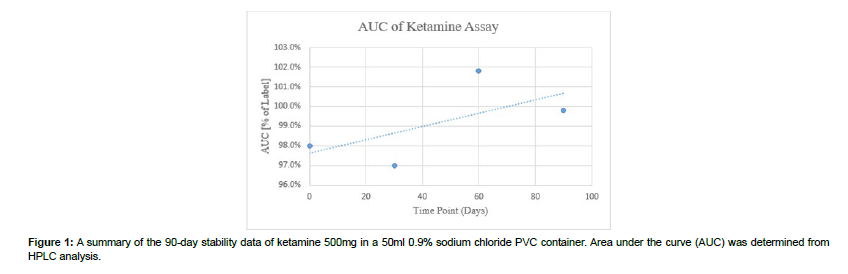
Figure 1 depicts the percentage of ketamine compared to our time points in days. The 90-day mark records a reading of 99.8%. These results demonstrate the type of behavior ketamine experiences when stored at room temperature. No excipient peaks or degradation were noted on the HPLC analysis. This also indicates that the therapy does not produce any unknown compounds which can lead to deviations in treatment. Depicted in the graph is a trend line which shows that throughout the study period and at each time point, at least 90% of the ketamine was maintained in the infusion. Concurrently, sterility testing was also performed parallel to the stability study and concluded no growth.
There were no notable changes in appearance, pH, fill volume, or container closure. Throughout the study, all 121 ketamine infusion bags conformed to the requirement of staying a clear, colorless solution that is free of visible particles. The pH was determined per USP <791> [6] and shows the different variables studied and the concluding results at each time point.
All tested samples depicted pH ranges of 4.0 to 4.4. This falls within the pH range (3.5-5.5) of ketamine which is found to be slightly acidic [2, 10] throughout the study, testing the container closure was vital as we needed to ensure an aseptic barrier was maintained. Testing was conducted via vacuum decay method and no anomalies were reported throughout the duration of the study.
USP<85> sets forth the requirement and limit for endotoxin testing [7] For our study, the infusion samples, when tested, were required to have no more than 1.67 EU/mg of endotoxin. As depicted in (Table 2), throughout the 90 days, the ketamine infusions samples tested below the detectable limits. To ensure no viable microorganisms are present, sterility testing was performed in accordance with USP <71> [8]. Sterility testing for the samples showed no growth. Furthermore, particulate counts were measured per USP <788> requirements [9]. Testing reports no anomalies throughout the duration of the study.
Other studies looked at the usage of ketamine in combination with other narcotics. This was referenced earlier in (Table 2). After analyzing our data, we set out to illustrate the difference in stability data of ketamine studies. It is important to note that prior to this study, the only viable data point was ketamine with no combination, and it was using a concentration of 0.6mg/ml [11]. All ketamine studies used PVC containers. From this graph, we can extrapolate how ketamine infusions may have the longest stability and the raw data from this study supports this idea.
| Stability Data For Ketamine in Ambient Condition Controlled Ambient Condition (25 |
|||||
|---|---|---|---|---|---|
| Attribute | Specification | Initial (T0) | T30 | T60 | T90 |
| Appearance | Clear, colorless solution, free of visible particulates | Conforms | Conforms | Conforms | Conforms |
| pH | Report Value | 4.4 | 4.3 | 4 | 4 |
| Container Closure | Pass/Fail | Pass | NT | Pass | Pass |
| Particulate Matter | 30 0 | 37 11 | 22 0 | 110 22 | |
| Sterility | Sterile | Sterile | Sterile | Sterile | Sterile |
| Endotoxin | NMT 1.67EU/mg | NT | |||
| Ketamine Assay | % of Label (90.0-110.0%) | 98.00% | 97.00% | 101.80% | 99.80% |
| Fill Volume | Report Value | 65mL | 60mL | 60mL | 60mL |
Table 2: Date from the ketamine study up to the 90-day time point
Discussion
After, obtaining the results we set out to compare our data against other studies that both looked at the usage of ketamine in PVC or syringes alone as well as studies that tested stability of ketamine in conjunction with other agents.
Ketamine therapy has grown in popularity in recent years; however, there is limited data on extended stability. With the increased usage of ketamine, the lack of data supporting safe administration of ketamine if diluted and stored for extended stability poses problems to both hospitals (due to shortages) as well as patients. Until the time of this study, ketamine infusions were prepared “on an as needed” basis by pharmacy staff of Lancaster General Health. Infusions were given an arbitrary beyond use date of 24 hours. To the authors’ knowledge and at the time this study was conducted, there are no published extended stability studies for ketamine hydrochloride infusions compounded in 50ml PVC bags.
Limitations
A limitation to this study was accounting for the prefilled saline bags used during compounding. The 50ml prefilled 0.9% sodium chloride was used and 5 mls of ketamine 100mg/ml was injected into each container. These saline bags contain approximation overfill amounts ranging from 52 to 62ml. This, in theory, could adjust the concentration from 10mg/ml to a range from 8.7-7.5 mg/ml. During the sample analysis, the bags are weighed, and a fill volume is determined. This helps limit the stated variation between samples. In an ideal setting, to obtain the precise concentration, the sodium chloride should be measured out along with the ketamine. It is important to consider this strategy as it also increases the risk of contamination. This is because one more manipulation is introduced to the compound. All institutions should determine what procedure is best practice.
Additionally, it is imperative to note that with the new USP <797> changes of beyond use dates (BUD), facilities should consult the monograph to understand the limits with category 2 products [12]. Ultimately, final BUDs will be based on each individual institutions’ interpretation of the USP monograph and if the institution is providing internal testing to justify category 3 products.
Conclusions
To reduce the frequency of waste and improve cost-savings, a stability test is needed to justify its use. After conducting this study, ketamine hydrochloride was shown to be stable in PVC containers when stored at ambient conditions for up to 90 days. The sterility test also performed showed no growth, thus proving the internal aseptic technique. While each intuitions test result may vary depending on practices
After serial qualitative assessments of physical appearance, pH, ketamine hydrochloride potency assay, particulate matter, endotoxin testing, sterility testing, fill volume, and container closure assessments, ketamine hydrochloride 500mg infusions stored in 50-ml polyvinyl chloride bags were stable for up to 90 days at room temperature.
Acknowledgements
A special thanks to Jessica Munson and Vanessa Stewart who provided the analytical testing at ARL Bio Pharma and to the ARL Bio Pharma Team.
References
- Groetzinger L M, Rivosecchi R M, Bain W, Bahr M, McVerry et al. (2018) Ketamine infusion for adjunct sedation in mechanically ventilated adults. The Journal of Human Pharmacology and Drug Therapy, 38: 181-188.
- Rosenbaum SB, Gupta V, Patel P, et al. Ketamine.
- Gupta VD (2002) Stability of Ketamine Hydrochloride Injection After Reconstitution in Water for Injection and Storage in 1-mL Tuberculin Polypropylene Syringes for Pediatric Use. Int J Pharm Compd 6: 316-317.
- Ensom MH, Decarie D, Leung K, Montgomery C (2009) Stability of Hydromorphone-Ketamine Solutions in Glass Bottles, Plastic Syringes, and IV Bags for Pediatric Use. Can J Hosp Pharm. 62: 112-118.
- https://epic-med.com/resources/ASHP-Extended-Stability-2017.pdf
- ⟨791⟩ pH.
- ⟨85⟩ Bacterial Endotoxins Test.
- ⟨71⟩ Microbiology Tests/ Sterility Test.
- ⟨788⟩ Particulate Matter in Injections.
- Ketamine [monograph].
- Donnelly RF (2009) Physical compatibility and chemical stability of ketamine-morphine mixtures in polypropylene syringes. Can J Hosp Pharm. 62: 28-33.
- USP compounding compendium. Rockville (MD): US Pharmacopeia Convention (2016) USP General Chapter <797> pharmaceutical compounding—sterile preparations. 39–84.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Lee DT, El-Sayed AM, Breznak RJ (2024) Study of Ketamine InfusionsExtended Stability (SKIES). J Anal Bioanal Tech 15: 608.
Copyright: © 2024 Lee DT, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 622
- [From(publication date): 0-2024 - Apr 11, 2025]
- Breakdown by view type
- HTML page views: 444
- PDF downloads: 178