Research Article Open Access
Study of Breath Acetone in a Rat Mode of 126 Rats with Type 1 Diabetes
Zhennan Wang1, Meixiu Sun1, Xiaomeng Zhao1, Chenyu Jiang1, Yingxin Li1 and Chuji Wang1,2*1Institute of Biomedical Engineering, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin 300192, China
2Department of Physics and Astronomy, Mississippi State University, MS 39759, USA
- *Corresponding Author:
- Chuji Wang
Department of Physics and Astronomy
Mississippi State University, MS 39759, USA
Tel: +16623259455
Fax: +16623258898
E-mail: cw175@msstate.edu
Received date: December 28, 2016; Accepted date: January 18, 2017; Published date: January 24, 2017
Citation: Wang Z, Sun M, Zhao X, Jiang C, Li Y, et al. (2017) Study of Breath Acetone in a Rat Mode of 126 Rats with Type 1 Diabetes. J Anal Bioanal Tech 8:344. doi: 10.4172/2155-9872.1000344
Copyright: © 2017 Wang Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
We investigate correlations of breath acetone with blood glucose (BG) and blood beta-hydroxybutyrate (BHB) in a rat model of 126 streptozotocin-induced Type 1 diabetic (T1D) and 32 healthy rats, and evaluate the role of breath acetone analysis as a non-invasive alternative in diabetes screening and management. We conducted breath acetone analysis using a T1D rat model under various conditions, including fasting and insulin-treated. Breath acetone concentrations were measured using a cavity ringdown breath acetone analyzer, LaserBreath-001. The results showed that the breath acetone, BG, and BHB in the T1D rat group were significantly different from those in the healthy group (P<0.05). Significant positive relationships between breath acetone and blood BHB existed in both T1D and healthy groups, as well as in a group of T1D rats under fasting condition and insulin treatment. When the BG concentrations were grouped as follows: 16.5-24.4, 24.5-27.7, and 27.8-41.7 mmol/L, a moderate negative correlation between breath acetone and BG was observed in the T1D group. Furthermore, the accuracy of diabetes screening using breath acetone was analyzed using the receiver operating characteristic (ROC) analysis method and results showed that the accuracy is low when the area under ROC curve (AUC) is <0.6425.
Keywords
Breath acetone; Type 1 diabetic (T1D) rat; Cavity ringdown spectroscopy; Blood glucose (BG); Blood beta-hydroxybutyrate (BHB)
Introduction
Breath acetone has long been known as a biomarker for diabetes. More than 50 independent studies of breath acetone in human subjects using various techniques and methods have been conducted during the past half-century. In these studies of clearly-defined Type 1 diabetes (T1D) subjects, elevated exhaled breath acetone concentration in T1D is an acknowledged fact [1]. At present, a commonly used clinical parameter for diabetes screening and management is the blood glucose (BG) concentration. It is still a challenge to use breath acetone analysis as a noninvasive alternative, because a quantitative relationship between the breath acetone and BG concentration remains unrevealed. From the 1950s, tremendous efforts have been made to address this issue in the course of conducting research involving human and animal subjects under various conditions.
Tassopoulos et al. found that breath acetone increased monotonically with elevated BG when 103 insulin-treated diabetic patients were grouped by their BG concentrations [2]. Likewise, a linear correlation between the mean group breath acetone concentration and the mean group BG concentration was observed in a study of 30 T1D subjects when they were grouped by different BG concentrations [3]. More recently, a weak positive relationship was found between BG and breath acetone in 113 children and adolescents with T1D [4]. It should be noted that the instable metabolic conditions of a subject could accordingly affect the breath acetone concentration. In a hypoglycaemic clamp study of 8 T1D patients, it was found that the breath acetone concentrations declined linearly with BG concentrations in each individual subject [5]. Whereas the experiment conducted on non-diabetic rats under glucose treatments showed a decreasing trend in breath acetone with increasing cumulative dose of glucose [6]. The above studies demonstrate the challenge in monitoring BG concentrations using a single breath acetone measurement, and indicate that the subject condition should be specifically controlled so that breath acetone analysis can contribute more useful information to BG prediction and monitoring.
It is desirable and necessary to investigate the correlation between breath acetone and BG under a condition that has minimal to no baseline effect resulting from individual physiological heterogeneity. The natural intra-individual biological and diurnal variability can easily cause wide variations in breath acetone concentration [7-10]. One of the ideal approaches to this end is the implementation of a rat model. Experimental rats do not have varying lifestyle choices and factors, such as medication, nutrition, or habits, and can be sampled via one breed living in the same housing and diet conditions [11]. Furthermore, a T1D rat model would be able to provide a relatively large number of sampling subjects in a short experimental period, as compared to usually small numbers of human subjects recruited in a single study.
In a previous study [12], we reported on the correlation of breath acetone to BG and blood beta-hydroxybutyrate (BHB) in a rat model of 20 T1D and 18 healthy rats. Significant positive relationships between breath acetone and blood BHB were found in both the T1D and the control groups, the relationship between breath acetone and BG in the T1D group was significantly negative, which was weakly positive in 5 insulin-treated T1D rats. Nevertheless, only non-fasting breath samples were measured in the previous study, and the number of insulin-treated rats was limited. In the present work, we extended breath acetone analysis using a T1D rat model under various conditions including fasting, non-fasting and insulin-treated and using a relatively large number of subjects. Moreover, the receiver operating characteristic (ROC) analysis method was used to evaluate the prediction accuracy of diabetes using breath acetone.
Materials and Methods
LaserBreath-001
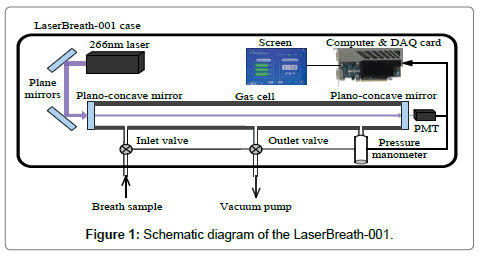
The LaserBreath-001 is a standalone, automated, portable instrument based on the CRDS technique, its schematic diagram is shown in Figure 1. The detailed information on the instrument design, validation, and measuring principle can be seen elsewhere [13,14]. Briefly, a 266 nm laser source (Changchun New Industries Optoelectronics, MPL-F-266 nm, China), two plane mirrors (Thorlabs, PF05-03-F01, U.S.), a gas cell (ringdown cavity, CRD Optics Inc., U.S.), two high reflectivity plano-concave mirrors (Los Gatos Research, R=99.99%, radius of curvature=1 m), a micro pressure manometer (MKS, 870B, U.S.), and a miniature photomultiplier tube (PMT, Hamamatsu, H10721, Japan) were the major components of the instrument. The gas cell had three ports, one was connected with a pressure manometer to monitor the inside cell pressure, two electromagnetic valves were connected to the other two ports as the sample inlet valve and outlet valve, respectively. The ringdown decay waveform was captured by the PMT and then input to a data acquisition card and a computer for determination and display of breath acetone concentration (ppm). Meanwhile, the instrument operation, including the atmospheric background measurement, breath gas measurement, nitrogen purge, and parameter setting could be completed by simply interacting with a touch screen in the instrument. All parts were housed in a metal case with dimensions of L638 mm × W237 mm × H143 mm and a weight of 19.4 kg. A single measurement takes ~1 second. With an average over 30 measurement events, a detection limit of 0.05 ppm (1-sigma limit) can be reached, it takes ~1 minute including the time for the sample introduction from a breath gas collection bag, gas cell flush, data processing and display. The LaserBreath-001 has a VGA and two USB ports on its rear panel for the embedded software update, onsite and remote troubleshooting.
Acetone concentration measuring method
In this work, a background subtraction method was used to determine the acetone concentration in breath samples, which was described in detail in our previous works [3,15]. In brief, the absorbance of the laboratory atmospheric air Aatm is used as the background baseline, and subtracted from the breath gas absorbance Abreath to calculate the absolute breath acetone concentration (the upper limit) n, which can be obtain by
ΔA = Abreath − Aatm = σ(ν)nd (1)
where ΔA is the absorbance difference between the laboratory air and the breath gas, σ(ν) is the absorption cross-section of the transition line at frequency ν, d is the gas cell length, which is 0.42 m in the LaserBreath-001.
The background subtraction method for the measurement of acetone concentration at 266 nm is based on an assumption that the absorbance difference in Equation (1) is attributed to the absorption of acetone alone. The absorption interference of acetone at 266 nm from other atmospheric molecules and high abundance breath volatile organic compounds (VOCs) in breath gas of human subjects was discussed in our previous work [15] to evaluate the reliability of this assumption.
A rat has the similar gas exchange process to human being based on the inhalation of air. In order to evaluate the reliability of the background subtraction method in rats, the top ten non-methane VOCs of a breath gas sample from a healthy rat under anesthesia were measured using gas chromatography-mass spectrometry (GC-MS, Agilent Technologies, U.S.A., 7890A/5975C, the State Key Laboratory on Odor Pollution Control, Tianjin Academy of Environmental Science). Table 1 summarizes the measured or estimated absorbance of these compounds. Compared to the ten most abundant VOCs in the human breath [13], the high abundance of ethanol and chloroform mainly might result from the alcohol disinfection and anesthesia. The absorbances of acetone and m-xylene at 266 nm were about 3.1 × 10-5 and 2.5 × 10-5 with the absorption cross-sections of 4.5 × 10-20 and 0.5 × 10-18 cm2/mole, respectively, at atmospheric pressure and room temperature. Using the relative molar absorption coefficient of toluene and styrene at 266 nm, 102.1 and 103.17 L/(mol·cm), the absorbances were estimated to be 9.3 × 10-8 and 4.8 × 10-7, respectively, which are approximately two orders of magnitude smaller than that of acetone. Likewise, the absorbance of 2-methyl-2-methacrylate was determined to be 3.6 × 10-10. The rest of the VOCs, such as ethanol, chloroform, tetrahydrofuran, dichloromethane and pentane, have negligible absorbance at 266 nm, which is represented by zero in Table 1.
| Name | Molecular formula | Concentration (ppm) | Absorption wavelength (nm) in UV[Ref] | Absorbance at 266 nm |
|---|---|---|---|---|
| Ethanol | C2H6O | 4.349 | <207[15] | 0 |
| Chloroform | CHCl3 | 1.308 | 105-147[16] | 0 |
| Tetrahydrofuran | C4H8O | 0.692 | 160-200[17] | 0 |
| Acetone | (CH3)2CO | 0.654 | 225-320[15] | 3.1�?10-5 |
| Toluene | C7H8 | 0.395 | 230-275[17] | 9.3�?10-8 |
| 2-Methyl-2-methacrylate | C5H8O2 | 0.193 | 180-265[17] | 3.6�?10-10 |
| Styrene | C8H8 | 0.172 | 220-270[17,18] | 4.8�?10-7 |
| Dichloromethane | CH2Cl2 | 0.185 | 112-129[18] | 0 |
| M-Xylene | C8H10 | 0.045 | 243-277[19] | 2.5�?10-5 |
| Pentane | C5H12 | 0.060 | <160[15] | 0 |
Table 1: Top 10 non-methane VOCs of the breath gas sample from a healthy rat after anesthesia.
As mentioned above, in the exhaled breath of a healthy rat, m-xylene is the only VOC that could contribute a comparable amount of absorbance with that of acetone at 266 nm. It should be noted that the exhaled acetone concentration in the diabetic rats is higher than that in the healthy ones [12]. From this perspective, the background subtraction method is still valid to determine the upper limit of breath acetone concentration in rat.
T1D rat model and breath gas sampling
The study was carried out with the approval of the Ethical Committee of Animal Research at the Institute of Biomedical Engineering, Chinese Academy of Medical Sciences (IBME). The Sprague-Dawley rats (male, initial weigh range 200-300 g, 10 weeks old) were obtained from Beijing HFK Bioscience (Beijing, China), and housed in the Laboratory Animal Center of IBME under controlled conditions (temperature 18-25°C, humidity 40-70%). The rats had free access to tap water and commercially available pellet food throughout the experiment period.
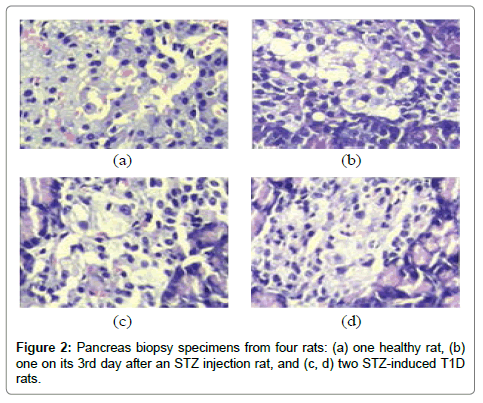
The rats were randomly assigned to healthy or T1D groups, the healthy group was regarded as a control group, while the T1D group was produced into a T1D rat model by a single intravenous injection of streptozotocin (STZ, Sigma-Aldrich, USA), which was dissolved in citric acid-sodium citrate buffer (pH 4.4) at the concentration of 20 mg/mL, with a dose of 45 mg/kg body weight. Rat BG concentration was measured in the blood sample collected from the tail vein using a blood glucose and ketone monitoring system kit (Optium Xceed, Abbott, USA) at one week after an STZ injection. Rats with nonfasting BG (NFBG) >16.7 mmol/L were considered diabetic [16-20]. Furthermore, four rats, including one healthy rat, one rat on its 3rd day after an STZ injection (3DSI) and two STZ-induced T1D rats, were randomly selected to perform pancreas biopsies (hematoxylin-eosin stain, Tianjin Yishengyuan Biotech Co. Ltd, China).
As can be seen in Figure 2, the pancreas biopsy specimens show a continuous change from the healthy to the T1D condition. The pancreatic islets from the healthy rat had clear islet boundaries and a relatively large quantity of healthy β-cells (Figure 2 (a)), however, the pancreatic islets from the two T1D rats appeared collapse within the atrophic pancreases, meanwhile, reduced quantities of normally shaped β-cells and local inflammation were observed. This pathophysiological feature indicates that the T1D rat model was developed successfully.
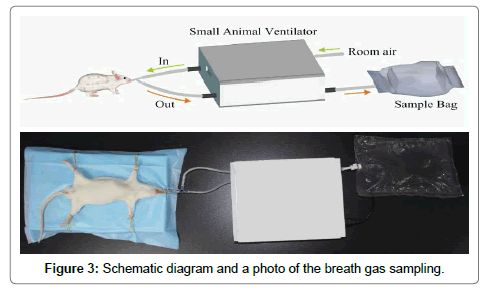
The schematic diagram and photo in Figure 3 shows the procedure of collecting breath gas from a rat using an oral intubation method and a small animal ventilator (ALC-V8S, Shanghai Alcott Biotech Co. Ltd, China). First of all, a rat was anesthetized by an intraperitoneal injection of chloral hydrate aqueous solution (10%) with a dose of 0.3 mL/100 g by weight. Then, a flexible plastic tube was placed into the trachea through oral cavity and connected to a 3-way stopcock, the other two ports of the stopcock were connected to the gas channel (“In”) and return channel (“Out”) of the ventilator, respectively. A fluorinated ethylene propylene (FEP) breath sample bag (~1 L) was connected to the expired air channel of the ventilator for exhaled breath gas collection. The ventilator was operating at a volume controlled mode with a tidal volume of 6.0 cm3, a respiration rate of 70 breath/ min, and inspiration/expiration ratio of 1:1. Right after a breath sample was collected from a rat, its BG and BHB concentrations in the tail vein were measured. Meanwhile, the acetone concentration in the collected breath sample was tested using the LaserBreath-001. All the rats were sacrificed after the experiment.
Results and Discussion
Instrument stability and reproducibility
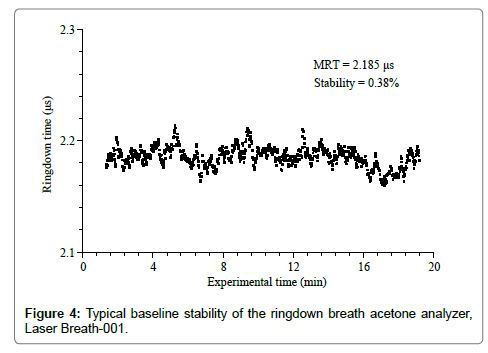
The stability and reproducibility of the ringdown breath acetone analyzer were evaluated prior to breath measurements. A typical baseline scan of the LaserBreath-001 is shown in Figure 4. Each data point was generated by averaging over 128 ringdown events. The ringdown time baseline stability is defined as the relative standard deviation of the obtained ringdown time. As can be seen in Figure 4, the mean ringdown time (MRT) was 2.185 μs, and the baseline stability was 0.38%. Given the LaserBreath-001 gas cell length of 0.42 m, the mirror reflectivity was determined to be 99.94%. Additionally, using the acetone absorption cross-section at the atmospheric pressure and room temperature, σ266=4.5 × 10-20 cm2/mole, the theoretical 3-sigma detection limit of the LaserBreath-001 for acetone was determined to be 0.13 ppm [15].
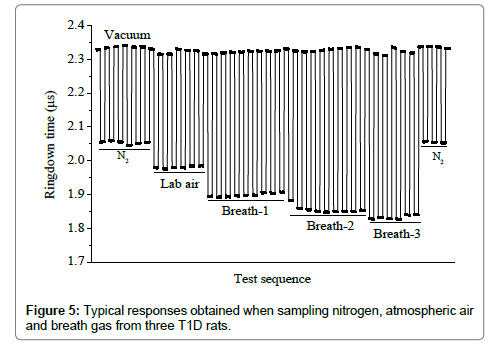
Figure 5 shows a typical response of the LaserBreath-001 to the laboratory air and breath samples from three T1D rats. The ringdown time difference was obvious when the gas cell was filled with the laboratory air or a breath sample. Among the several successive measurements of each sample, it showed a same ringdown time. After each sample measurement, the gas cell was evacuated and the corresponding ringdown times returned to the same baseline concentration. The nitrogen ringdown times at both the beginning and the end of the test were the same too. The sharp rising and falling edges illustrated the rapid responses (~1 s) of the LaserBreath-001. This test demonstrated the good reproducibility and fast response of the LaserBreath-001.
Comparison of breath acetone with blood glucose
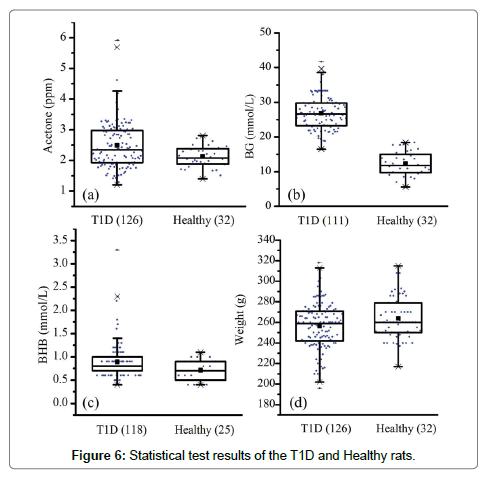
In total, the breath samples from 126 T1D rats and 32 healthy rats were collected. However, due to poor health and anesthesia, some rats died after the breath sample collection, as a result, the tail vein BG and BHB tests were not conducted in these subjects. Figure 6 gives the statistical information and test results. The data analysis was performed using the Software of Statistical Product and Service Solutions (SPSS 13.0). Statistical significance was set at P<0.05.
In general, the differences in breath acetone, BG, BHB and body weight between the healthy and T1D groups were all found to be significant at the 0.05 level. On the one hand, the mean breath acetone, BG and BHB concentrations were relatively higher in the T1D group, while the mean body weight was higher in the healthy group. The BG difference, 26.8 ± 5.0 mmol/L versus 12.3 ± 3.5 mmol/L, was especially obvious between these two groups. The obtained results are consistent with our previous work [12], and confirm the common diabetes-related symptoms, such as weight loss, increased BG and ketone bodies. On the other hand, the data of each parameter were more dispersed in the T1D group, which can be seen from the standard deviation and range values, although the housing condition, food supply, sampling procedure were all the same for both the healthy and the T1D rats. This phenomenon was also observed in the human subjects [21,22]. Nevertheless, the mean breath acetone concentration in the healthy rats was higher than that in healthy people [1].
It has been proved that the administration of chloral hydrate anesthesia leads to a glucose intolerance in rat, both the BG and insulin concentrations elevate significantly in rat plasma [23,24]. In this study, elevated BG concentrations were observed in both the health and the T1D groups after the anesthesia with chloral hydrate, as shown in Table 2. The BG concentrations in 4 healthy and 4 diabetic rats were measured before and at 10 min after the chloral hydrate injection. As expected, the BG concentrations were higher in the diabetic rats because of the impaired glucose tolerance, whereas the BG increase rates in the healthy rats were more significant than that in the diabetic rats, i.e., 40.1-132.4% and 0.7-16.1%, respectively. These data suggest that chloral hydrate induces fewer effects on the BG homeostasis in the T1D rats as compared to that in the healthy rats. As a consequence of diminished glucose uptake and utilization, ketone bodies production in the liver is provoked to provide energy, thus, elevated breath acetone concentration can be observed. This might be one of the reasons that such high breath acetone concentrations were observed in the healthy rats under chloral hydrate anesthesia.
| Subject | Blood glucose (mmol/L) | Increase rate (%) | |
|---|---|---|---|
| Before anesthesia | 10 min after anesthesia | ||
| H1 | 8.0 | 13.4 | 40.1 |
| H2 | 7.6 | 12.1 | 59.2 |
| H3 | 8.7 | 15.3 | 75.9 |
| H4 | 7.7 | 17.9 | 132.4 |
| D1 | 24.9 | 27.6 | 10.8 |
| D2 | 24.2 | 27.7 | 14.5 |
| D3 | 23.6 | 27.4 | 16.1 |
| D4 | 26.7 | 26.9 | 0.7 |
Note: H represents healthy rat and D represents STZ-induced T1D rat
Table 2: The increase rate of the blood glucose concentration following the anesthesia in the rats.
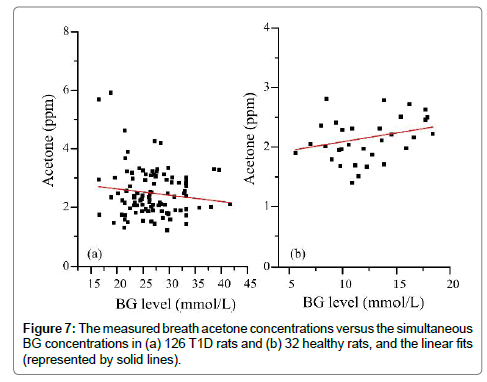
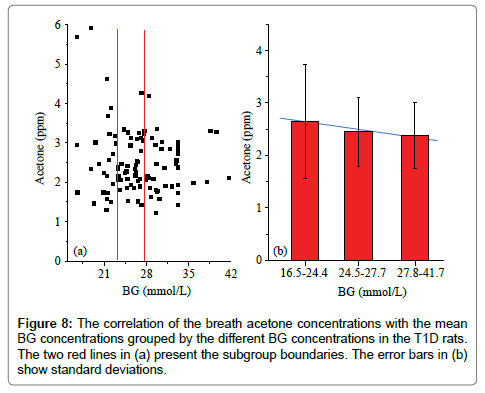
Figure 7 shows the measured breath acetone concentrations and simultaneous BG concentrations in the 126 T1D rats and 32 healthy rats. There appeared to be a downward trend in the breath acetone concentrations with increasing BG concentrations in the T1D rats, but an upward trend in the healthy rats. This observed trend in the T1D group was contrary to that of previously published studies in T1D human subjects, in which the subjects with high BG concentrations tended to have higher breath acetone concentrations [2,3,5]. It should be noted that the diabetic condition of the T1D rats was uncontrolled completely, whereas that of the human T1D subjects was under control with medical treatments, more or less. However, the linear trend was hardly significant in neither the diabetic rat group (Pearson’s r=-0.13) nor the control rat group (Pearson’s r=0.29). For the T1D group, when the BG concentrations were grouped into three subgroups: 16.5- 24.4 (border concentration), 24.5-27.7 (high BG concentration), and 27.8-41.7 (very high BG concentration) mmol/L, a moderate negative correlation between the mean breath acetone concentrations and mean BG concentrations was observed (Pearson’s r=0.678, P<0.05), as can be seen in Figure 8(b), which agrees with the results in our previous work [12]. The STZ-induced rats were deemed to be diabetic when their NFBG concentration was higher than 16.7-22.2 mmol/L [18] or the fasting BG concentration was higher than 11.1 mmol/L [25,26]. The increase rate in the BG concentration induced by the anesthesia ranged from 0.7 to 16.1% and the mean value was about 10% in the four T1D rats, as shown in Table 2. Therefore, the BG value below the dividing line of 24.4 mmol/L was considered as the border concentration. The range of the BG concentration from 27.8 to 41.7 mmol/L was set at a very high BG concentration owing to the noticeable changes seen in the liver and kidney functions of the rats with the BG concentration in this range. The effect of anesthesia on the BG concentration was considered when the BG concentrations were grouped (Figure 8).
Statistic evaluation of diabetic screening using breath acetone
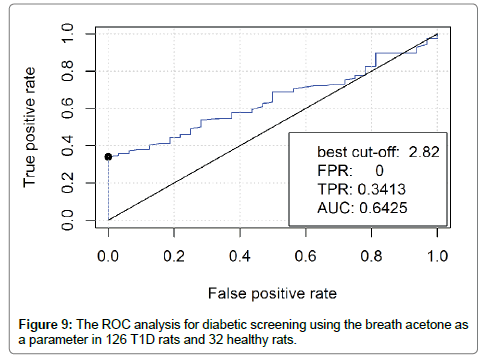
The method of the Receiver Operating Characteristic (ROC) curve [27] was used for sensitivity and specificity analysis of diabetic screening using breath acetone. It is a plot of the true positive rate (TPR) versus the false positive rate (FPR) for the different possible cutpoints of a diagnostic test. The true-positive rate is also known as sensitivity, the false-positive rate is known as (1-specificity). The breath acetone data from the 126 T1D rats and the 32 healthy rats were included in the ROC analysis as an evaluation of using breath acetone for potential diabetic screening. As shown in Figure 9, when the best cut-off was determined to be 2.82, which was higher than the mean breath acetone concentration of 2.5 ppm in the T1D group and any of the obtained breath acetone concentrations in the healthy group, the TPR that represents the accuracy of T1D screening was 0.3413, FPR that represents a misdiagnosis rate for healthy rats was 0.00. In this case, the area under the ROC curve (AUC) was determined to be 0.6425, which indicates a low accuracy of the diagnosis test. As far as the obtained breath acetone data were concerned, the T1D group had a higher mean value and a more dispersed distribution as compared to the healthy group. However, the ROC analysis suggests that the dispersed and overlapping concentration distribution of breath acetone makes it inadequate to be used solely for diabetic screening.
Comparison of breath acetone with blood BHB
The blood BHB concentration in the T1D group was significantly different from that in the healthy rat group (P<0.05), as can be seen in Table 2. The blood BHB ranged from 0.4 to 3.3 mmol/L in the 118 T1D rats and from 0.4 to 1.1 mmol/L in the 25 healthy rats. The mean value was determined to be 0.9 ± 0.4 mmol/L in the T1D group, which was higher than that of 0.7 ± 0.2 mmol/L in the healthy group. The effect of anesthesia process on the blood BHB concentration was also studied, as shown in Table 3. The blood BHB concentrations in 7 healthy rats were tested both before and at 10 min after the chloral hydrate injection. The blood BHB increase rate induced by the anesthesia changed in a range of 14.3-133.0% with a mean value of 52.6 ± 38.9%.
| Samples | Blood BHB (mmol/L) | Increase rate (%) | |
|---|---|---|---|
| Before anesthesia | After anesthesia | ||
| H1 | 1.1 | 1.6 | 45.0 |
| H2 | 0.8 | 1.1 | 37.5 |
| H3 | 0.7 | 0.8 | 14.3 |
| H4 | 0.6 | 1.4 | 133.0 |
| H5 | 0.7 | 1.0 | 42.8 |
| H6 | 0.7 | 0.9 | 28.6 |
| H7 | 0.6 | 1.0 | 66.7 |
Note: H represents healthy rat
Table 3: The effect of the anesthesia process on the blood BHB in seven selected healthy rats.
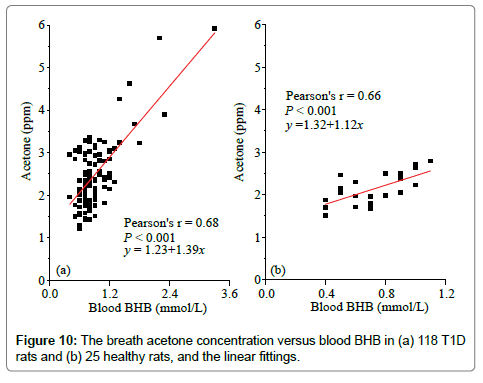
Diabetic ketoacidosis is an acute and one of the most severe complications in the untreated or inadequately treated T1D subjects, the accumulation of ketone bodies in the blood is usually detected by blood or urine BHB concentration measurement. Figure 10 shows the linear relationships between breath acetone concentration and blood BHB concentration in the T1D and healthy rats, respectively. The strong positive correlations (Pearson’s r>0.6) in both groups demonstrates the effectiveness of predicting ketone concentrations using the noninvasive breath acetone measurement as an alternative. The similar increasing trend in breath acetone as blood BHB was also reported in the studies with both human [4,28,29] and rat subjects [6,30] under different conditions. However, it should be noted that the slope of the linear regression in the T1D group was larger than that of the healthy group, 1.39 versus 1.12, as shown in Figure 10. This means that a T1D rat trends to have a higher breath acetone concentration than a healthy rat even when they are at the same blood BHB concentration. But, it is hardly to observe this phenomenon due to the large dispersion of breath acetone concentrations in the T1D rats.
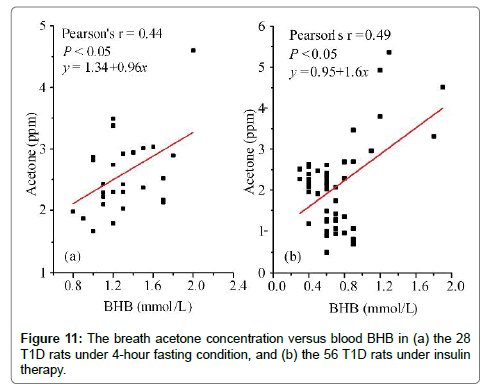
For a person who has type 1 diabetes mellitus, it is imperative to monitor the fasting blood glucose and to inject insulin for the daily diabetes management. Therefore, the relationship between breath acetone and blood BHB was also studied in the rats under the fasting condition and under the insulin therapy, respectively. In total, 28 T1D rats under 4-hour fasting condition and 56 T1D rats under the insulin therapy were randomly selected for this study. The insulin therapy was carried out with a daily dose of 8 unit/kg body weight for 5 days before the breath acetone and blood BHB tests. The moderate positive correlations were found in both groups, as can be seen in Figure 11. Likewise, the breath acetone concentration had different increasing rate as the variance of the blood BHB concentration in the rats under these two different conditions. In the T1D rat group under the insulin therapy, the blood BHB values mainly distributed in a range of 0.4-1.2 mmol/L, as its distribution in the healthy rats (Figure 10b).
Conclusions
In this paper, in total 126 T1D and 32 healthy rats were used to investigate the correlations between breath acetone and BG, and blood BHB, respectively. To date, this is the largest one on the T1D subject ever done in a single study with both human and animal subjects. The breath samples were collected from the rats under various conditions and tested using a ringdown breath acetone analyzer, LaserBreath-001.
The obtained data of each parameter, such as breath acetone, BG, blood BHB, and body weight, were more dispersed in the T1D rats. For example, the ranges of breath acetone were 1.2-5.9 ppm in T1D group and 1.4-2.8 ppm in the healthy group, respectively. Also, both the BG concentration and the blood BHB concentration were found to increase post-anesthesia using chloral hydrate, and the BG increase rates in the healthy rats were more significant than that in the T1D rats, i.e., 40.1- 132.4% and 0.7-16.1%, respectively. In the uncontrolled T1D rats, one with an elevated BG concentration trended to have a relatively low breath acetone concentration, whereas there appeared to be an upward trend in the healthy rats. The ROC analysis suggests that the dispersed and overlapping breath acetone concentration distribution makes it inadequate to be used solely for diabetic screening. A moderate/strong positive correlation was found in both the healthy rats and the T1D rats under various conditions, such as completely uncontrolled, under fasting, and under insulin therapy. This study using a relatively large number of subjects yet with minimum baseline effects supports that the breath acetone is a reliable indicator of blood BHB in both T1D and healthy rats.
Acknowledgements
This work was supported by the National Science Foundation of China (Grant No. 81471701).
References
- Wang Z, Wang C (2013) Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J Breath Res 7: 37109.
- Tassopoulos CN, Barnett D, Fraser TR (1969) Breath-acetone and blood-sugar measurements in diabetes. Lancet 293: 1282-1286.
- Wang C, Mbi A, Shepherd M (2010) A study on breath acetone in diabetic patients using a cavity ringdown breath analyzer: exploring correlations of breath acetone with blood glucose and glycohemoglobin A1C. IEEE Sens J 10: 54-63.
- Blaikie TPJ, Edge JA, Hancock G, Lunn D, Megson C, et al. (2014) Comparison of breath gases, including acetone, with blood glucose and blood ketones in children and adolescents with type 1 diabetes. J Breath Res 8: 46010.
- Turner C, Walton C, Hoashi S, Evans M (2009) Breath acetone concentration decreases with blood glucose concentration in type I diabetes mellitus patients during hypoglycaemic clamps. J Breath Res 3: 46004.
- Fink T, Albrecht FW, Maurer F, Kleber A, Hüppe T, et al. (2015) Exhalation pattern changes during fasting and low dose glucose treatment in rats. Anal BioanalChem 407: 3763-3773.
- Turner C, Špan�?l P, Smith D (2006) A longitudinal study of ammonia, acetone and propanol in the exhaled breath of 30 subjects using selected ion flow tube mass spectrometry, SIFT-MS. PhysiolMeas 27: 321-337.
- Špan�?l P, Dryahina K, Smith D, Spanel P (2007) Acetone, ammonia and hydrogen cyanide in exhaled breath of several volunteers aged 4-83 years. J Breath Res 1: 11001.
- Schwarz K, Pizzini A, Arendacká B, Zerlauth K, Filipiak W, et al. (2009) Breath acetone-aspects of normal physiology related to age and gender as determined in a PTR-MS study. J Breath Res 3: 27003.
- Špan�?l P, Dryahina K, Rejskova A, Chippendale TWE, Smith D (2011) Breath acetone concentration, biological variability and the influence of diet. PhysiolMeas 32: N23-N31.
- Wolf A., Baumbach JI, Kleber A, Maurer F, Maddula S, et al. (2014) Multi-capillary column-ion mobility spectrometer (MCC-IMS) breath analysis in ventilated rats: a model with the feasibility of long-term measurements. J Breath Res 8: 16006.
- Sun M, Zhao X, Yin H, Wang Z, Jiang C, et al. (2015) Study of breath acetone and its correlations with blood glucose and blood beta-hydroxybutyrate using an animal model with lab-developed type 1 diabetic rats. RSC Adv 5: 71002-71010.
- Gong Z, Sun M, Jiang C, Wang Z, Kang M, et al. (2014) A ringdown breath acetone analyzer: performance and validation using gas chromatography-mass spectrometry. J Anal Bioanal Tech S7: 1-8.
- Sun M, Jiang C, Gong Z, Zhao X, Chen Z, et al. (2015) A fully integrated standalone portable cavity ringdown breath acetone analyzer. Rev Sci Instrum 86: 95003.
- Wang C, Surampudi AB (2008) An acetone breath analyzer using cavity ringdown spectroscopy: an initial test with human subjects under various situations. Meas Sci Technol 19: 105604.
- Singh PJ, Shastri A, D��?Souza R, Jagatap BN (2013) Rydberg states of chloroform studied by VUV photoabsorption spectroscopy. J Quant SpectroscRadiatTransf 129: 204-213.
- http://webbook.nist.gov/chemistry/
- Zobel CR, Duncan ABF (1955) Vacuum ultraviolet absorption spectra of some halogen derivatives of methane. Correlation of the spectra. J Am ChemSoc 77: 2611-2615.
- Fally S, Carleer M, Vandaele AC (2009) UV Fourier transform absorption cross sections of benzene, toluene, meta-, ortho-, and para-xylene. J Quant SpectroscRadiatTransf 110: 766-782.
- Neuhaus S, Seifert L, Vautz W, Nolte J, Bufe A, et al. (2011) Comparison of metabolites in exhaled breath and bronchoalveolar lavage fluid samples in a mouse model of asthma. J ApplPhysiol 111: 1088-1095.
- Rooth G, �?stenson S (1966) Acetone in alveolar air, and the control of diabetes. Lancet 288: 1102-1105.
- Jiang C, Sun M, Wang Z, Chen Z, Zhao X, et al. (2016) A portable real-time ringdown breath acetone analyzer: Toward potential diabetic screening and management. Sensors 16: 1199.
- Arola L, Palou A, Remesar X, Herrera E, Alemany M (1981) Effect of ether, sodium pentobarbital and chloral hydrate anesthesia on rat plasma metabolite concentrations. Rev EspFisiol 37: 379-386.
- Hindlycke M, Jansson L (1992) Glucose tolerance and pancreatic islet blood flow in rats after intraperitoneal administration of different anesthetic drugs. Upsala J Med Sci 97: 27-35.
- Pushparaj P, Tan CH, Tan BK (2000) Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J Ethnopharmacol 72: 69-76.
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, et al. (1998) Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 4: 1025-1031.
- Lasko TA, Bhagwat JG, Zou KH, Ohno-Machado L (2005) The use of receiver operating characteristic curves in biomedical informatics. J Biomed Inform 38: 404-415.
- Prabhakar A, Quach A, Wang D, Zhang H, Terrera M, et al. (2014) Breath acetone as biomarker for lipid axidation and early ketone detection. Glob J Obesity, Diabetes MetabSyndr 1: 012-019.
- Tanda N, Hinokio Y, Washio J, Takahashi N, Koseki T (2014) Analysis of ketone bodies in exhaled breath and blood of ten healthy Japanese at OGTT using a portable gas chromatograph. J Breath Res 8: 46008.
- Likhodii SS, Musa K, Cunnane SC (2002) Breath acetone as a measure of systemic ketosis assessed in a rat model of the ketogenic diet. ClinChem 48: 115-120.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 3343
- [From(publication date):
February-2017 - Jun 30, 2024] - Breakdown by view type
- HTML page views : 2666
- PDF downloads : 677