Strategies for Developing Genetically Engineered Plants for Viral Resistance-A Review
Received: 09-Jan-2023 / Manuscript No. ACST-23-86394 / PreQC No. ACST-23-86394 (PQ) / Reviewed: 25-Jan-2023 / QC No. ACST-23-86394 / Revised: 21-Apr-2023 / Manuscript No. ACST-23-86394 (R) / Published Date: 28-Apr-2023
Abstract
Plant viruses cause significant agricultural losses all around the globe. Cultural approaches and applications of biocide against arthropod, nematode and plasmodiophorid vectors had little efficacy in minimizing the effects of herbal viruses. The utmost efficient and cost effective method of reducing plant viral infections is to plant impervious farmers. Natural several causes of the opposition have been widely used in conventional breeding to generate virus resistant plants. Non-traditional approaches have also been employed effectively to give virus resistance by transferring virus derived genes into susceptible plants, containing viral coat protein, replicas, expression protein, nonsense interfering RNA, non-coding RNA, protease RNA viruses are not the only kind of genes since ribosome inactivating proteins, protease inhibitors, double stranded RNase and RNA modifying enzymes also fall under this category.
Additionally, scFvs and effectively employed in plant virus resistance engineering. There have just a few GE crops with viral authorized resistance for farming and none have been now accessible in underdeveloped nations. However, many commercially significant GEVR crops converted using viral genes are gaining popularity in underdeveloped countries. The main challenges issue with GEVR agricultural production and deregulation in developing countries generally include socioeconomic in nature and are connected to biosafety regimes and protections for intellectual property, the expense to create GE crops and resistance from members of non-governmental organizations. Proposals for resolving these issues satisfactorily, apparently leading to field testing and liberalization of GEVR plantation standards in emerging nations are provided.
Keywords: Genetic engineering, DNA, Plant viruses, RNA, Socioeconomic factors
Introduction
Genetically Modified (GM) plants that are grown as crops DNA has been genetically edited and used in agriculture. Physical techniques or Agrobacterium can be used to edit plant genomes to provide sequences housed in dual T-DNA vectors. The intent is to develop a novel characteristic, not something found in nature among plants [1]. This alteration is designed to make plants more resistant to climatic and environmental stressors, pests, diseases and herbicides [2].
Over these past several decades, a change in the push to forego choosing. In favor of adopting genetic technology (transgenic) improvement of agricultural yields. Simply said, plant modification takings numerous generations (i.e., a lengthy period) and is not always effective. Transgenic technology may be used to develop plants with desired characteristics and increased yields allow for additional harvesting and resilience to illnesses and pests. The premise is that desirable characteristics may be cultivated, as in traditional selection processes, enabling the rising population to have higher levels of nutrition and commodities to be further appealing [3].
The benefits of introducing GM crops have been extensively identified. GM biotechnology allows for the greater importance of nutrition over foods, the use of fewer chemicals and the manufacture of larger amounts of food. This is accomplished using highly specialized methods of modifying a crop’s genes deprived of changing its other characteristics. GM Plants could produce higher yields than traditional crops while also providing extra benefits. Items that are resistant to insecticides and weed killers are less expensive. Furthermore, using fewer pesticides on GM crops may reduce the quantity of gas emitted into the environment.
Literature Review
Transgenic plants that are herbicide tolerant
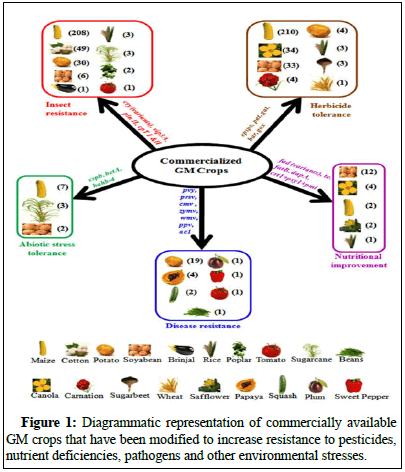
When agricultural plants and weeds compete for resources, including soil, water, sunshine and space, the former suffer severe losses in yield. There has been an increase in the usage of pesticides and other active management is necessary because weeds reduce agricultural yields. But because most weeds are herbaceous, protecting the crop plant while selectively eliminating weeds is not always possible. Consequently, one solution that has the potential to enable the versatile usage of potent non-selective and broad spectrum herbicides is of development of herbicide tolerance characteristics in the core crop. Both selective and non-selective mechanisms of action are used by the many weed killers on the market. Glyphosate and glufosinate are the frequently used non-selective herbicides. The overwhelmingly most HT transgenic plants have been designed to be resistant to the herbicides glyphosate and glufosinate. Glyphosate works by blocking an enzyme called 5-Enolpyruvyl Shikimate 3-Phosphate Synthase (EPSPS), which is essential for aromatic amino acid biosynthesis through the shikimate pathway. Glyphosate is safe for usage around people, birds, insects and furthermore, animals since the shikimate pathway do not exist in the animal kingdom. Chemically produced genes or glyphosate resistance genes expressed heterologous resistant versions of EPSPS Arthrobacter globiformis were similar to the epsps grg23 gene of that organism. Tumefaciens strain CP4 or a mutant variant of maize EPSPS [4]. Soybean with herbicide resistant crops was originally created using the cp4epsps gene and it was commercialized in 1996. Most glyphosate resistant crops sold commercially include this gene. In addition, several commercially available transgenic crops have the gene for Glyphosate Oxidoreductase (GOX) and Glyphosate Acetyltransferase (GAT), produced by Bacillus licheniformis or either from Ochrobactrum anthropi. In cooperation with these enzymes, break down glyphosate into safer compounds. Glufosinate or phosphinothricin another non-selective herbicide, serves plays a competitive inhibitory role against the glutamine synthetase enzyme [5]. The production of glutamine from glutamate and ammonia is catalyzed by this enzyme. This enzyme is blocked by glufosinate, which causes ammonia to build up and slow down photosystem I and II activity [6]. Crops resistant to glufosinate were created using two separate genes from the bacterium Streptomyces spp., pat and bar. These genes both code for the enzyme that acetylates the herbicide, known as Phosphinothricin Acetyl Transferase (PAT). Recently, transgenic crops that have been engineered to be resistant to a wide variety of herbicides, including 2,4-D, dicamba, isoxaflutole, mesotrione, oxymel and sulfonylurea, have entered the market. Benefits to farmers from the broad use of HT transgenic crops include higher yields thanks to easier and more effective weed control and lower overall weed management expenses [7]. The economic advantage of glyphosate resistant soybean is projected to be 62% owing to reduced weed control expenses and 38% due to higher yield [8]. Additionally, weed management’s environmental effect has been mitigated thanks to HT crops. Herbicides like glyphosate and glufosinate, which break down rapidly after application are two examples of the types of pesticides that have replaced others that are less eco-friendly. In addition, lower greenhouse gas emissions resulted from decreased tractor use as a consequence of the shift from plow based to reduced, minimal or no agricultural tillage systems enabled by HT technology [9].
Insect resistant transgenic plants
Crop losses are substantial due to insect pests and illnesses. There are more than 67,000 different kinds of insects that may damage economically important crops. They feed on plant sap and parts including leaves, stems and roots, causing damage to crops. Insects can transmit a number of plant diseases to crops as they eat [10]. In order to manage and control insect pests, farmers sometimes have to spend a lot of money on chemically produced pesticides. This kind of crop protection is costly for farmers and detrimental to the environment. Emerging methods, such as genetically modifying crops to boost pest resistance, provide a potential solution to these problems with conventional insecticide usage. So far, ten transgenic crops with insect resistance have been approved in order to produce commercially. Many of these commercialized plants have been genetically modified to add insecticidal genes (most often versions of the Cry gene and in a few cases, the VIP gene) to battle dangerous insects. That feed on agricultural produce [11]. About 23.3 million acres are used for cultivating transgenic crops that are resistant to insects. At this time, 304 events have been approved for widespread commercial production. There are 208 approved maize production events that include several IR genes due to the prevalence of insect pests. Tomatoes (1), cucumbers (1), peppers (1), eggplants (1), cabbage (1), cotton (49 occurrences), potatoes (30 events), soybeans (6), rice (3), sugarcane (3), poplar (2), brinjal (1) and tomatoes (1) all contain many IR genes (Figure 1).
Abiotic stress tolerant transgenic plants
Drought, heat, cold, feeding, salt and other abiotic stresses have a detrimental influence on wheat plant growth and development, resulting in a loss of grain crop [12]. As a result of these abiotic pressures, anticipated to grow as climatic conditions change. Plants modify their metabolism in a variety of ways to cope with abiotic stresses, including activating signaling regulating proteins and cascades, raising/altering anti-oxidant defense systems to maintain cellular homeostasis, synthesizing then accumulating compatible solutes (polyamines, sugars, retains, proline and so on) that aid in osmotic adjustment and so on [13]. Abiotic stress causes plants to respond by changing how the stressors assist in mitigating harmful plants benefit from this because the environment is kept at a level that is almost ideal for plant development. Abiotic stressors affect the molecular expression of many genes.
Options for creating genetically engineered virus and their vector resistance
Technological advances in gene splicing, the discovery of promoters that guarantee the constitutive expression of genes, as well as the refinement of procedures that allow the transformation of a wide variety of agricultural plants have made it possible to introduce and express various nucleotide sequences, both viral and not in seedlings to impart virus resistance. It’s been close to 25 years since the initial report on the creation of TMV resistant tobacco plants was published [14]. Several empirical investigations have successfully created plants with permanent viral resistance [15]. In this brief overview, we discuss the antiviral genes that have been used effectively in plant genetic engineering to confer virus resistance. There is also a brief introduction to the genes that are used to modify plant host genes, as well as ways for blocking the spread of viruses via insect vectors.
Antiviral genes with viral ancestry
Protein mediated resistance: Protein genes are covered. The Coat Protein (CP) genes were used for creating virus resistance in crops, either entirely or partly. The amount of safeguard offered genes for CP caused a wide range of effects, from complete immunity to a postponement of symptoms. In certain cases, there would be widespread opposition [16]. CP genes are employed in the creation of all presently commercially marketed virus resistant crops. The molecular processes behind CP mediated resistance are unclear and seem to vary amongst viruses [17]. Persistence is ascribed to the viral protein RNA, a mix of the two.
Proteins are involved in the replication. In poor countries, several single stranded DNA viruses are commercially significant. These viruses’ reproduction needs contact between Rep and the host. Cellular polymerases are essential for DNA replication. Rep plays an essential role in viral replication [18]. In section 3, we see several examples of successful GEVR plant growth in response to the Gemini virus’s African Cassava Mosaic Virus (ACMV) and TYLCV using the Rep technique.
The RNA polymerase genes. Tobacco plants that harbored a component virus specific RNA polymerase (RdRp or replicase) demonstrated strain specific resistance to TMV [19]. To impart resistance, TMV 54 kDa protein production was needed, which matches the carboxyl terminus of the 183 kDa RdRp. Similarly, to induce resistance to another Tobamovirus, Pepper mild mottle virus, full length 54 kDa protein expression or 30% of it was needed. This resistance was then connected to RNA silencing by further studies. Replicase genes derived from Potex, Potty, Alfamo and Cucomoviruses were used to effectively develop resistance [20]. It is apparent that transplanting native or reduced variants of replicase genes may result in a high degree of resistance to several plant viruses.
Genes that control movement. Tobacco plants GE with a TMV mutant Movement Protein (MP) inhibited not just TMV movement but also the movement of various other plant viruses, comprising several by means of a DNA genome. Surprisingly, the production of native MP promoted virus infection by a movement deficient virus strain.
A 13 kDa protein that is an integral component of the protein complex encoding the 3' block of genes regulates the movement of Potex-, Carla-, Hordei- and Furoviruses.
The production of mutated variants of 13 kDa having protein in one’s system increases one’s defenses against the parallel viruses and those that very rely on the triple gene blocks protein complex for motion.
Genes for proteases. The protease gene family Picornaviruses are characterized by having a polyprotein that encodes their RNA genome and is digested by a protease that is produced by the virus. Poty viruses employ a protease called NIa. Significant resistance to virus infection was seen in plants that expressed the protease domain of viruses NIa, most likely as a result of blocking viral replication requires the conversion of polyprotein precursors into active multiplication. Although protease activity is necessary, it is not sufficient to induce protease mediated resistance in potatoes to PVY unless the protease enzyme’s active location is intact.
RNA mediated resistance: Plants’ RNA silencing may mediate viral resistance. The generation of exchangeable or non-translatable RNA, as well as antisense RNA matching the CP cistron of two potyviruses, offered great resistance to mechanical inoculation and aphid infection in tobacco plants. This kind of resistance might potentially be used to defend plants against RNA and DNA viral contagion by forming tiny hairpin RNAs. (shRNAs). This method through which virus specific small interfering RNAs (siRNAs) are created.
Non-viral origin antiviral genes
Plant derived R genes and micro RNA: Plant virus Resistance (R) genes have typically been discovered via a collaborative effort between plant pathologists, germplasm scientists and breeders. When possible, plants with introduced R DNA have been synthesized and cultured. The method may have timescales of 5-10 years or more are typical when the desired genotype is not yet commercially accessible or distributed. Several plant viral resistance genes have been cloned and proven plant virus resistance as transgenes. Tobacco and tomato both benefit from the N gene, which was initially cloned from the Samsun NN tobacco strain. Single dominant resistance genes with molecular structures comparable to those of plant resistance genes against bacteria, fungi and nematodes make up the vast majority of virus specific R genes cloned too far. Plants contain recessive resistance genes, in addition to R genes, that diminish susceptibility to viral infection ribosome inactivating proteins.
Ribosome Inactivating Proteins (RIPs) are enzymes that deglycosylate one particular base eukaryotic 28S rRNA. They are made of two polypeptide chains A and B. Domain A catalyzes, whereas domain B binds galactose. Only chain A is present in the overwhelming majority of plant RIPs. A RIP known as Pokeweed Antiviral Protein (PAP) has been proven to prevent the spread of the infection. Transgenic N. tabacum plants producing PAP showed broad spectrum resistance to various viruses when challenged by mechanical inoculation and aphid transmission; CMV, PVY and PVX are examples (PVX). This method has also been found to be effective against a plant DNA virus. PAP may degrade bacterial 23S rRNA and 28S rRNA. Initially, it was assumed that PAP mediated viral resistance was induced by the inactivation of the 28S rRNA.
However, lacking depuration function, a PAP transgenic plant with the deletion mutant of the 28S rRNA's C-terminal region showed viral resistance. In GE plants, the resistance mechanism underlying PAP or RIP type 1 production is unclear at this time.
Protease inhibitors: The genomic RNAs of several plant viruses, including the comoviridae, potyviridae and closteroviridae, code for proteases that catalyze the disassembly of polyproteins into their constituent parts. Viral proteases are likely to be inhibited by protease inhibitors. Resistance to TEV and PVY, but not TMV, was seen in rice cystatin conveyed in tobacco plants (a cysteine proteinase inhibitor). In theory, the transgene’s product might provide resistance to viruses that employ proteases to produce and replicate their genomes. The use of GEVR to successfully cultivate food crops are not documented anywhere.
Eukaryotic antiviral genes
Expression of dsRNAse and RNA modifying enzymes: RNA viruses and viroids in plants employ dsRNA as an intermediary in their replication. Pac 1 is a yeast gene that genes for a ribonuclease that can digest dsRNA isolated this gene from the yeast Schizosaccharomyces pombe expressed in N. tabacum cv. Xanthi-NC plants. When confronted with the Tomato mosaic virus, these plants demonstrated a reduction in lesion numbers and delay in the onset of symptoms. Further research on potato plants expressing Pac 1 indicated that viroid infection of potato spindle tubers was prevented in these plants and the tubers were also viroid free. In animals, when it comes to fighting viruses, the interferon system delivers. Interferons have a crucial role in fighting viral infections. Drive the production of novel proteins that change virus replication. 2u-5u oligo Adenylate synthetase (2-5Ase) is a critical enzyme that, when activated by dsRNA, polymerizes ATP to form 2u-5u oligo adenylates, which activate RNase L, an endoribonuclease that destroys both cellular and viral RNAs. Plants of the potato species that had been genetically modified to express a rat cDNA encoding the 2-5Ase were resistant to PVX infection in the wild. The GE potato lines with the PVX CP transgene were shown to be the most virus resistant, although there were other lines that were also resistant. The appearance of a human 2-5Ase similarly protected tobacco plants against CMV and TMV. Generations of tobacco plants containing both human RNase L and 2-5Ase transgenes showed increased resistance to CMV and PVY infection compared to parent GE lines containing either RNAse L or 2-5Ase transgenes alone.
Host genes that are involved in viral reproduction are silenced: Several host genes are important in viral replication. TMV multiplication in two strains was completely abolished by mutations in the A. thaliana genes TOM1 and TOM3. In N. tabacum cv. Samsun, resistance to several Tobamoviruses was achieved by silencing homologs of these genes by RNA interference. The lack of two host gene products did not seem to affect the development of silenced plants. Surprisingly, this resistance was only found in Tobago viruses and not in a CMV strain, indicating that viral RdRps choose distinct host proteins. Silencing TOM1 and TOM3 homologs in N. benthamiana also inhibited Tobamovirus replication. This method may potentially be used for any plant virus as long as the host genes that need to be silenced are identified and then described and found many host characteristics that impact viral infection, allowing opportunities to utilize this approach.
Arthropod vector resistance GE or plant virus transmission: Another strategy for reducing plant virus transmission is to develop insect vector resistance. GE rice lines expressing a lecithin gene dramatically decreased the survival, fertility and Nilaparvatha lugens (brown plant hopper) development. N. lugens is a significant pest of rice, as well as a carrier of viruses responsible for the grassy stunt, ragged stunt and tungro.
Despite substantial studies into RNAi-mediated viral silencing, this technology has yet to be fully used to manage plant virus vectors owing to plants’ incapacity to induce silencing in insects. The investigation of the silencing effects of dsRNA in a gossypol containing artificial diet in A. thaliana was an important advance in this field. Plant derived siRNA could not trigger silencing in insects, but shRNA might in insects served a diet laced with shRNA targeting insect genes. ShRNA mediated silencing has yet to be exploited in agricultural crops to create viral resistance via vector control.
Symbionin, a GroEL homolog, is another protein of relevance. This protein is generated by insect bacterial endosymbionts, including plant viral vectors like aphids and whiteflies. Symbionin has the ability to attach to the virions of chronically transmitted, circulative viruses. These viruses depend on this protein for safe transit via the use of insect hemolymph and later, saliva gland release. A GroEL gene derived from whiteflies (Bemisia tabaci) provided resistance to TYLCV and CMV when expressed in transgenic N. benthamiana. Because the virus does not spread consistently, the resistance of plants harboring the GroEL gene to CMV was unexpected. A B. tabaci GroEL homolog has been demonstrated usage for binding icosahedral and geminate RNA viruses. The full potential of GroEL expressing GEVR plants has not yet been unknown.
Examples of virus resistant genetically engineered crops produced with success in developing countries
Many yields have been genetically modified to be viral resistant. However, no GEVR cultivars have been released for general cultivation in developing nations by governmental organizations or the business sector to date. Table 1 lists GEVR crops that have been evaluated in both the laboratory and the field and may be released in poor countries in the future.
| Crop plant | Virus |
|---|---|
| Cassava | African cassava mosaic virus (Begomovirus) |
| Cereals | Barley yellow dwarf virus (Luteovirus) |
| Citrus | Citrus tristeza virus (Closterovirus) |
| Maize | Maize streak virus (Mastrevirus) |
| Cucumber | Cucumber mosaic virus (Cucumovirus) |
Table 1: GEVR crops are evaluated in both the laboratory and the field.
Discussion
Assessment of socioeconomic factors that prevent the adoption and culture of genetically engineered virus resistant crop plants in developing countries
The identification of a wide variety of viral and non-viral genes has greatly expanded the possibility of introducing long lasting transgenic virus resistance into attractive plant genotypes. More than 90% of Hawaii’s historically papaya growing field was currently used for growing GE PRSV resistant papayas after their regulation was lifted in 1998. Papayas grown with GEVR technology have been consumed in Hawaii and the continental United States for the last decade. No adverse effects on human health from consuming these papayas have been reported. Results from a battery of studies on papayas grown in GEVR greenhouses showed no differences in allergen city between the GE and non-transgenic varieties. The GEVR summer squashes that have been tested have also shown resistance against CMV, WMV and ZYMV, revealing no allergenic characteristics.
Potential environmental safety hazards associated with the growth of GEVR plants, as well as key social also ethical considerations that are probable to restrict their acceptance in underdeveloped countries. The environmental concerns of recombination, transgenic transfer via pollen and the influence on non-target species were thoroughly examined. These were recently evaluated and debated. For example, no recombinant viruses were discovered on plum trees engineered to carry a virus’s CP gene (PPV) cultivated during a 10 years period in Spain and Romania. Furthermore, no discernible change in the quantity and kind of aphids visiting GE and non-GE plum trees was seen in an experimental orchard in Spain. Furthermore, testing PPV resistant plums with three unrelated viruses did not result in the collapse of the engineered PPV resistance. Taken together, the findings reveal those hazards harmful to ecosystems and people alike, a major worry two decades ago, is no longer seen as barriers to the cultivation of GEVR crops. Other difficulties, like those discussed below, can be seen as substantial impediments to testing and derestricting GEVR crops in underdeveloped nations.
Intellectual property rights
In the 1960’s, public sector research organizations in rising regions of Asia, Africa and Latin America had access to the fruits of developed nations’ research into the development of high yielding cultivars (hybrids and composites). In the present day, multinational firms dominate agricultural research on Genetically Engineered (GE) crops. These firms own patents on the great majority of presently utilized technology, including CP mediated plant viral resistance. Discusses the importance of patents in GE agricultural development. Developing nations lack the financial capacity to cover the expenditures connected with the development and deregulation of GE crops. Thus, a collaboration between international corporations and developing country public sector research agencies is critical for the creation of relevant GE crops for poor countries. On humanitarian grounds, it is still possible to get access to technology that helps marginal farmers without having to pay royalties, although innovations created locally in poor nations are more likely to be favored over those created by international businesses.
Biosafety regulations
Present biosafety regulation regimes remained primarily created to cope with the emergence of new technologies and to address the initial demands of industrial countries. They mainly aimed to inhibit dangerous items from accessing the market. To detect dangers to public safety and environmental safety, many criteria have been utilized. Regulations should be written in a clear and uniform way, taking into account the interests of product creators, distributors, farmers and consumers. Policies in the United States vary from individuals in the European Union. Several nations in Asia, Africa and Latin America have biosafety rules in place. They have developed differently in various nations. The creation of this legislation was impacted by history, public opinion, economic and trade incentives and geographical factors. South Africa is among the few nations that recognize the crucial need to establish a reasonable regulatory framework as well as a national biotechnology strategy. The recent steps made by the Malawi government in East Africa to adopt a national biotechnology strategy might be mentioned because it shows how serious a developing nation may be about bringing GE crops to market. Cost of generation of GE crops and enforcement of biosafety regulations.
The expense of GE crop cultivation and the application of biosafety rules are two of the most significant barriers to field testing and deregulating GE crops in underdeveloped nations. For example, India spends approximately $500 million per year on agricultural research but barely $50 million on biotechnology research. In comparison, Monsanto spends about $490 million on biotechnological research. Developing nations contribute less than 9% of global biotechnology research investment. Many seed firms in underdeveloped nations want to buy rights to cultivate and use GE crops instead of putting resources into developing GEVR crops. Since the expertise to transfer the resistance to locally suited crop cultivars via conventional breeding is readily accessible in governmental institutions and the business sector in many developing countries, this method is generally used. For a developing country, China’s People’s Republic stands out because it has taken the lead in adopting Genetically Engineered (GE) crops and invests extensively in biotechnologies. Although rural farmers in developing nations may benefit greatly from the introduction of new technology for crops like sorghum, pearl millet and groundnut/peanut, multinational corporations are reluctant to shoulder the financial burden. Introducing Genetically Engineered (GE) crops with the same characteristic in India is more expensive for multinational corporations than in the People's Republic of China, according to a study comparing the costs of enforcing biosafety standards in the two countries. Deregularizing BT cotton in India is expected to cost almost US $ 1 million for a single transformation event, but in China, the same cost is just US $ 90,000.
Campaigns by non-governmental activists
Several international groups actively work against the creation of GEVR crops. Concerns about the long term effects of genetically engineered crops on humans also eco-friendly health is at the heart of their major argument.
Conclusion
Despite the success of field experiments and the scientific consensus in favor of releasing GE PRSV resistant papaya in Thailand, the crop was not deregulated due to pressure from environmental groups like greenpeace. It’s no secret that several Asian nations have played a pivotal role in the fight against genetic engineering. Activists have been known to vandalize experimental plots as part of their ongoing efforts against BT cotton, BT brinjal and other crops. The trips of activists were also widely publicized in the mainstream media. In January 2009, Mr. Jeffrey Smith, executive director of the institute for responsible technology, spoke publicly in Hyderabad, India, on the health dangers associated with eating foods resulting from Genetically Engineered (GE) crops. A widely distributed daily newspaper covered his speech. Also, a ban on field testing of GE plants might be an obstacle to maximizing the part of GE crops in expanding India’s food supply.
References
- Catacora-Vargas G, Binimelis R, Myhr AI, Wynne B (2018) Socio-economic research on genetically modified crops: A study of the literature. Agric Human Values 35: 489-513.
- Han H, Yu Q, Owen MJ, Cawthray GR, Powles SB (2016) Widespread occurrence of both metabolic and target site herbicide resistance mechanisms in Lolium rigidum populations. Pest Manag Sci 72: 255-263.
[Crossref] [Google Scholar] [PubMed]
- Barragan-Ocana A, Reyes-Ruiz G, Olmos-Pena S, Gomez-Viquez H (2019) Transgenic crops: Trends and dynamics in the world and in Latin America. Transgenic Res 28: 391-399.
[Crossref] [Google Scholar] [PubMed]
- Lea PJ, Joy KW, Ramos JL, Guerrero MG (1984) The action of 2-amino-4 (methylphosphinyl)-butanoic acid (phosphinothricin) and its 2-oxo-derivative on the metabolism of cyanobacteria and higher plants. Phytochemist 23: 1-6.
- Wild A, Sauer H, Ruhle W (1987) The effect of phosphinothricin (glufosinate) on photosynthesis I inhibition of photosynthesis and accumulation of ammonia. Z Naturforsch C 42: 263-269.
- Brookes G, Barfoot P (2018) Farm income and production impacts of using GM crop technology 1996-2016. GM Crops Food 9: 59-89.
[Crossref] [Google Scholar] [PubMed]
- Green JM (2012) The benefits of herbicide resistant crops. Pest Manag Sci 68: 1323-1331.
[Crossref] [Google Scholar] [PubMed]
- Brookes G, Barfoot P (2015) Environmental impacts of Genetically Modified (GM) crop use 1996-2013: Impacts on pesticide use and carbon emissions. GM Crops Food 6: 103-133.
[Crossref] [Google Scholar] [PubMed]
- Mahmood-ur-Rahman KH, Khan MA, Bakhsh A, Rao AQ (2021) An insight of Cotton leaf curl virus: A devastating plant pathogenic Begomovirus. Pure Appl Biol 1: 52-58.
- Keresa S, Baric M, Grdisa M, Igrc Barcic J, Marchetti S (2008) Transgenic plants expressing insect resistance genes. Sjemenarstvo. 25: 139-153.
- Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203: 32-43.
[Crossref] [Google Scholar] [PubMed]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, et al. (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134: 1683-1696.
[Crossref] [Google Scholar] [PubMed]
- Abel PP, Nelson RS, de B, Hoffmann N, Rogers SG, et al. (1986) Delay of disease development in transgenic plants that express the Tobacco mosaic virus coat protein gene. Science 232: 738-743.
[Crossref] [Google Scholar] [PubMed]
- Baulcombe DC (1996) Mechanisms of pathogen derived resistance to viruses in transgenic plants. Plant Cell 8: 1833-1844.
[Crossref] [Google Scholar] [PubMed]
- Beachy RN, Loesch-Fries S, Tumer NE (1990) Coat protein mediated resistance against virus infection. Annu Rev Phytopathol 28: 451-472.
- Dasgupta I, Malathi VG, Mukherjee SK (2003) Genetic engineering for virus resistance. Curr Sci 84: 341-354.
- Goldbach R, Bucher E, Prins M (2003) Resistance mechanisms to plant viruses: An overview. Virus Res 92: 207-212.
[Crossref] [Google Scholar] [PubMed]
- Prins M, Laimer M, Noris E, Schubert J, Wassenegger M, et al. (2008) Strategies for antiviral resistance in transgenic plants. Mol Plant Pathol 9: 73-83.
[Crossref] [Google Scholar] [PubMed]
- Wilson TM. Strategies to protect crop plants against viruses: Pathogen derived resistance blossoms. Proc Natl Acad Sci USA 90: 3134-3141.
[Crossref] [Google Scholar] [PubMed]
- Elmer JS, Brand L, Sunter G, Gardiner WE, Bisaro DM, et al. (1988) Genetic analysis of the Tomato golden mosaic virus II. The product of the AL1 coding sequence is required for replication. Nucleic Acids Res 16: 7043-7060.
[Crossref] [Google Scholar] [PubMed]
Citation: Ngomle S (2023) Strategies for Developing Genetically Engineered Plants for Viral Resistance-A Review. Adv Crop Sci Tech 11: 594.
Copyright: © 2023 Ngomle S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2427
- [From(publication date): 0-2023 - Nov 16, 2025]
- Breakdown by view type
- HTML page views: 2086
- PDF downloads: 341