Research Article Open Access
Strain Reduction of Human Gingival Fibroblasts Induces the ATP Pathway
Nasir Gadban1, Evgeny Weinberg1, Adeeb Zoabi1, Malka Ashkenazi2, Avinoam Yaffe3 and Itzhak Binderman1*
1Department of Oral Biology, the Goldschleger School of Dental Medicine, Sackler Faculty of Medicine, Tel Aviv University, Israel
2Private practice, Petah-Tiqva, Israel
3Department of Prosthodontics, Hadassah Faculty of Dental Medicine, Hebrew University, Jerusalem
- Corresponding Author:
- Itzhak Binderman
Department of Oral Biology
School of Dental Medicine, Aviv University
Ramat Aviv 69978, Tel Aviv, Israel
Tel: +97-2505352344
E-mail: Binderman.itzhak@gmail.com
Received Date: November05, 2014; Accepted Date: December 16, 2014; Published Date: December 20, 2014
Citation: Nasir Gadban, Evgeny Weinberg, Adeeb Zoabi, Malka Ashkenazi, Avinoam Yaffe, et al. (2015) Strain Reduction of Human Gingival Fibroblasts Induces the ATP Pathway. J Interdiscipl Med Dent Sci 3:162. doi: 10.4172/2376-032X.1000162
Copyright: © 2015 Gadban N,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
Studies in rats demonstrated that surgical detachment of marginal gingiva from root surfaces induced alveolar bone resorption via activation of ATP receptor P2X4. Our aim was to study the effects of strain release of human gingival fibroblasts by detachment of collagen coat from culture dishes. Changes in cell shape, extracellular ATP, cell calcium and genes relevant to trigger alveolar bone resorption were measured. HGF cells from human marginal gingiva explants were seeded on collagen coated culture dishes. In the test group, the collagen coat was detached from culture dish 4-5 days after seeding. Quantification of extracellular ATP was measured by Bioluminescent Assay Kit and changes of intracellular calcium by FLU-AM fluorescence. Real-time PCR was used to examine expression of purinergic receptor P2X4 and P2X7, RANK-L and STC1. Cell length of HGFs grown on plastic surface and on collagen was similar. Reduction in cell length of 45% was measured after detachment of collagen coat. Extracellular ATP raised 10 folds 1 minute after detachment of collagen coat, then declined and returned to control levels 60 minutes later. Ionomycin increased extracellular ATP, while, pretreatment by EGTA, or BAPTA-AM, 30 minutes prior to Ionomycin, reduced significantly extracellular ATP. Reciprocally, addition of ATP increased influx of calcium into HGFs. At the molecular level elevated expression of STC-1, RANK-L and P2X7 was recorded by RT -PCR in detached HGFs, while expression of P2X4 was unchanged. Expression of STC-1, a modulator of cellular calcium and phosphate, P2X7 a calcium ionic channel purinergic receptor and RANK-L a powerful regulator of osteoclastic activity, all were up-regulated significantly.
Conclusion: We propose that drop of strain of HGF cells and change in their shape stimulated release of cellular ATP which is signaling through Pi/Ca modulators the molecular activation of osteoclasts.
Keywords
Strain release of human gingival fibroblasts; Extracellular ATP and purinergic receptor P2X7; Cellular calcium; Expression of STC-1 and RANK-L
Introduction
Teeth are anchored to marginal gingiva by bundles of Sharpey fibers that run from the cervical part of the root cementum, toward the basal membrane of papilla, toward the periosteum of the alveolar bone crest and toward adjacent teeth, creating a physical communication network between teeth and those tissues. These collagen fibers and other ECM components are lined by cellular network of adherent gingival fibroblasts that produce traction forces, viable for maintaining normal architecture of periodontium. Under physiological conditions, tensile forces are inherent in the cells, known as prestress or "tensegrity" [1]. Externally applied forces are transmitted by integrins or cadherins into and throughout the cell toward the nuclear membrane, by adhesive proteins that link to cytoskeleton structures [2]. Our previous studies [3-5] and others [6,7] have shown that surgical detachment of dento-gingival fibers from root surfaces is a major trigger for alveolar bone resorption, in a rat model because a sudden reduction of physiological strains of adherent fibroblasts ensued [8]. We found that a rapid release of ATP from the cells to the extracellular environment and up-regulated expression of ATP cell membrane receptor P2X4 is main trigger for alveolar bone resorption [9]. Normally, all cells contain high intracellular ATP concentration (1-5 mmol/l) and no extracellular ATP. At sites of tissue injury, in wounding or fracture ATP is released from the cells at high concentration, activating P2 receptors [10,11]. Once ATP is released it will immediately bind to specific purinergic receptors, activating a fast signaling process. Extracellular ATP triggers a variety of responses in several cell types, including contraction of smooth muscle cells, regulation of nitric oxide production from endothelium, stimulation of cytokine release from immune cells and modulation of several other metabolic pathways [10-12]. The receptors mediating these diverse processes have been described as purinoreceptors and are divided into two groups: P2Y and P2X receptors. P2Y receptors are a group of membrane-spanning and G protein-coupled receptors that are activated by various nucleotides, e.g. ATP, ADP and UTP. The P2X receptors are solely activated by extracellular ATP. P2X receptors are membrane ligand-gated channels that open in response to the binding of extracellular ATP, allowing a rapid increase in intracellular Ca+2 [13,14]. It is now evident that P2X and P2Y receptors are important local signaling molecules in bone, both osteoblasts and osteoclasts [15-17]. The P2X receptors have been implicated in the generation of osteoclasts [16]. In another study ATP was shown to elevate the receptor activator of nuclear factor-kB ligand (RANKL) mRNA and protein by UMR-106 cells [16]. In a recent study [9,18], inhibitors of purino receptors such as Coomassie Blue R and G or apyrase that degrades extracellular ATP reduced alveolar bone loss significantly when applied locally at sites of surgical detachment of marginal gingiva from root surfaces.
In the present study we investigated the effect of detachment of collagen coat from culture dishes resulting by abrupt reduction of traction forces of adherent human gingival fibroblasts (HGF). Our aim was to study the cellular and molecular changes after detachment of the collagen coat. Changes in cell shape, ATP release from cells, and cell calcium were measured. An attempt to elucidate the signaling molecular pathways that lead to activation of osteoclasts in response to strain relaxation of HGFs was also investigated.
Our hypothesis is that HGF are sensing a drop in physiological strain by immediate release of ATP to the extracellular environment, influx of calcium and changes in their cell shape. Then, activation of molecular changes that modulate osteoclastic activity and alveolar bone resorption follows.
Materials and Methods
Human Gingival Fibroblasts cultures on collagen coated dishes
Marginal gingiva tissue was dissected from patients during extraction of teeth as part of an orthodontic treatment plan in the Orthodontic clinic of the Dental School of Tel Aviv University. The procedures of the experiments were approved by Helsinki committee of the Tel Aviv University, and informed consent was obtained from all participants. Connective tissue fragments were cut into small pieces and placed in culture medium DMEM supplemented with 10% fetal calf serum (FCS) and incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air, to facilitate cell outgrowth. The outgrown cells were replated in new dishes. Cells from the second or third passage, demonstrating a typical fibroblastic morphology, were used.
It should be mentioned, that phenol red was not added as a pH monitor to the medium, since it is known to act as an antagonist to P2X receptors [19]. After two weeks, the cells were transferred from the flasks to experiment dishes, either collagen-coated or non-coated dishes. 30 mm culture dishes were coated with collagen gel using the collagen solution (Collagen R, type I isolated from rat trail collagen). After a 15- minute incubation the collagen gel was fixed to the surface of the dishes, and HGFs were seeded over the collagen. The HGF cells developed traction forces by attaching to collagen fibers.
After 48 hours the collagen coat was detached from the bottom of the dish resulting in deformation of collagen coat and strain release [20]. The detachment of the collagen coat was carried out by using a thin spatula in a circle movement beneath the collagen, hence detaching it from the bottom of the dish [21,22]. The control groups consisted of seeded cells in dishes with no collagen coat or cells seeded over a collagen coat.
Quantification of cell shape of HGF
Cultures were stained with Coomassie Blue, and the mean length of the cells was measured and calculated, using the ImageJ software. This provided information regarding the extent of changes in cell shape between strained cells on collagen coated dishes (group B) and on non-coated plastic surface (group A), versus cells that the collagen coat was detached (group C).
Quantitative measurement of extra cellular ATP
For this test the medium was removed from the culture dishes which were washed gently twice with clear DMEM medium (without phenol red). Then 0.75 ml of DMEM medium was added to the dishes. At this stage the collagen coat in one dish was detached as described above, and samples of 0.1 ml medium were obtained from dishes of each of the three experimental groups (A, B, C) and from a fourth dish which included medium only. Samples from all dishes were taken in three different time intervals; 1 minute, 10 minutes, and one hour after the collagen coat detachment.
For the quantification of ATP Adenosine 5'-triphosphate (ATP) Bioluminescent Assay Kit [23,24] was used. In the ATP Bioluminescent Assay, ATP is consumed and light is emitted when firefly luciferase catalyzes the oxidation of D-luciferin.
Calcium influx assessment with histochemical means
To monitor changes in Ca+2 in response to different ATP concentrations, the cell cultures were loaded with 5 µM fluo-4 acetoxymethyl ester at 37C for 15 minutes. Then, fluorescence signals from the cells were observed at room temperature (25C) using the confocal microscope Zeiss LSM 510 META. An argon laser was used to excite fluo-4 acetoxymethyl ester at 488 nm. Intracellular calcium was quantified after obtaining the fluorescence images using the Carl Zeiss LSM software.
Gene expression analysis
Real Time PCR
Real-time PCR was used to examine expression of purinergic receptor P2X4 and P2X7, RANKL and STC1 in HGFs. RNA was isolated from HGFs cell cultures (groups A, B and C), using RNA extraction kit (Manual perfectPure RNA cell & Tissue; 5PRIME) following the manufacturer’s protocol. Since our model included collagen matrix, 10 μl Proteinase K per 400 μllysisbuffer (20 mg/ml, 5PRIME) were applied prior to the process of RNA purification, in order to obtain maximum RNA from the cells. Total RNA (1 μg) was converted to cDNA using I ScriptTMcDNA Synthesis Kit (BIO-RAD). For RT-QPCR, we used the Applied Bio Science 7300 real time machine. Each target gene was tested in triplicates in each group, and was normalized compared to the house keeping gene TBP also in triplicates.
Statistics
Statistical analyses were performed with SPSS software. Mean cell length was analyzed with one-way ANOVA test. The PCR data were analyzed using ANOVA with repeated measures within subject factors (treatments). The ATP data was analyzed using ANOVA with repeated measures within subject factors (treatments and times). Significance reporting criteria was P<0.05.
Results
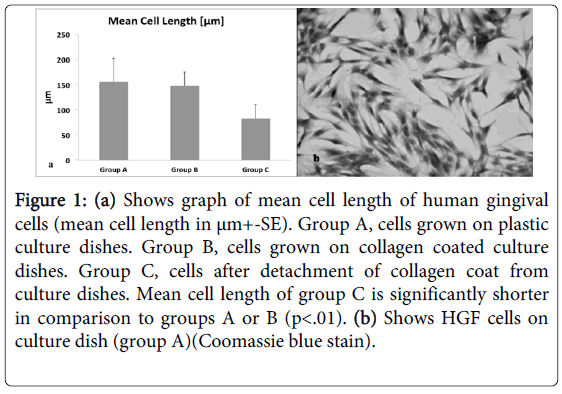
Normally, matrices like collagen are optimal substrate for cells to develop strong anchorage through strained cytoskeleton and transmembraneintegrins [25]. In fact, the cells developed a normal strained morphology in sub-confluent cultures in group B as well as in group A (Figure 1b) 4-5 days later, the collagen coat was detached from the plastic dish (group C), resulting in immediate deformation of collagen coat. Twenty minutes after detachment of the collagen coat the length of the cells was measured. HGFs grown on plastic surface (group A) and on strained collagen (group B) was similar, 155+/- 47 µm and 147 +/- 28 µm, respectively, while a significant reduction of 45% in cell length was measured in group C, 83+/- 27 µm where collagen was detached from the culture dishes (Figure 1a). These results were tested with one way ANOVA statistical test and showed significant reduction of long axis of cells in group C in comparison to groups A or B (p<0.01).
Figure 1: (a) Shows graph of mean cell length of human gingival cells (mean cell length in μm+-SE). Group A, cells grown on plastic culture dishes. Group B, cells grown on collagen coated culture dishes. Group C, cells after detachment of collagen coat from culture dishes. Mean cell length of group C is significantly shorter in comparison to groups A or B (p<.01). (b) Shows HGF cells on culture dish (group A)(Coomassie blue stain).
Release of ATP from HGF cells into culture medium
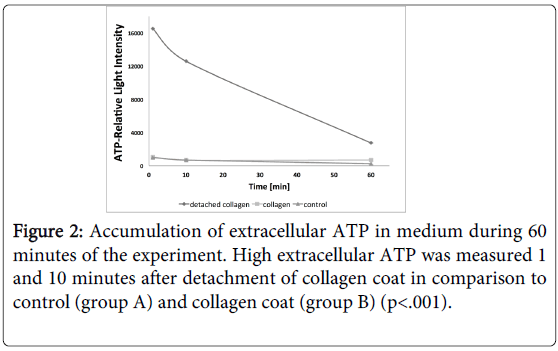
The release of ATP from HGFs into the culture medium in response to detachment of collagen coat was measured during 60 minutes. Figure 2 shows accumulation of ATP in medium that is released from HGF cells. 1 minute after detachment of collagen coat (group C) ATP raised more than 10 folds in comparison to strained cells grown on collagen (group B) or on plastic surface of culture dishes (group A). After 10 minutes the amount of extracellular ATP declined and returned to control levels 50 minutes later (Figure 2). The extracellular ATP level in group A and group B was low during 60 minutes of the experiment. A significant difference of ATP levels at 1 and 10 minutes between group C and groups A and B was measured (p<0.001), while the ATP level returned to control at 60 minutes. It is possible to predict that the presence of enzymes which degrade ATP in the extracellular environment may account for its decay after 50 minutes.
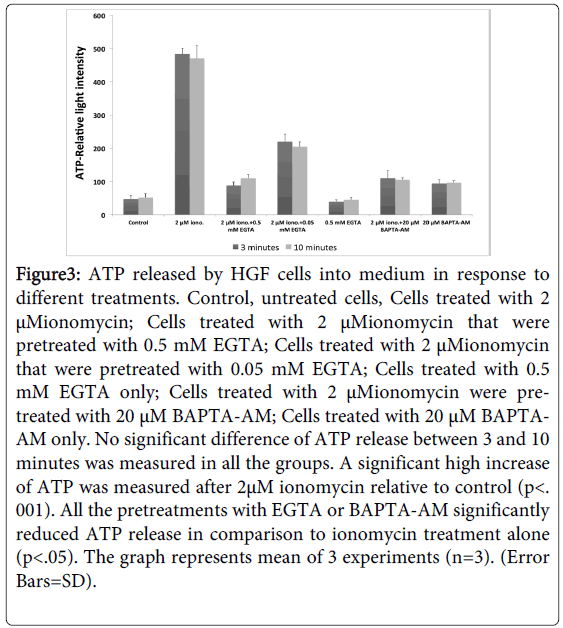
We further hypothesized that the release of ATP from HGF cells is regulated by changes and fluxes in cellular calcium. In fact, addition of 2 µM of Ionomycin, an agent that increases Ca+2 influx into the HGF cells, increased significantly the extracellular ATP (p<0.01), (Figure 3). In contrast, addition of 0.5 mM of EGTA, an extracellular chelator of calcium or addition of 20uM of BAPTA-AM which reduces intracellular calcium, 30 minutes prior to addition of Ionomycin, reduced significantly extracellular ATP (p<0.05) (Figure 3). These data support the notion that cellular influx of Ca+2 stimulates secretion of ATP from HGF cells. No significant difference of ATP release between 3 and 10 minutes was measured in all the groups.
Figure 3: ATP released by HGF cells into medium in response to different treatments. Control, untreated cells, Cells treated with 2 μMionomycin; Cells treated with 2 μMionomycin that were pretreated with 0.5 mM EGTA; Cells treated with 2 μMionomycin that were pretreated with 0.05 mM EGTA; Cells treated with 0.5 mM EGTA only; Cells treated with 2 μMionomycin were pretreated with 20 μM BAPTA-AM; Cells treated with 20 μM BAPTAAM only. No significant difference of ATP release between 3 and 10 minutes was measured in all the groups. A significant high increase of ATP was measured after 2μM ionomycin relative to control (p<. 001). All the pretreatments with EGTA or BAPTA-AM significantly reduced ATP release in comparison to ionomycin treatment alone (p<.05). The graph represents mean of 3 experiments (n=3). (Error Bars=SD).
The effect of detachment of collagen coat or addition of ATP on intracellular Ca+2 in HGF cells
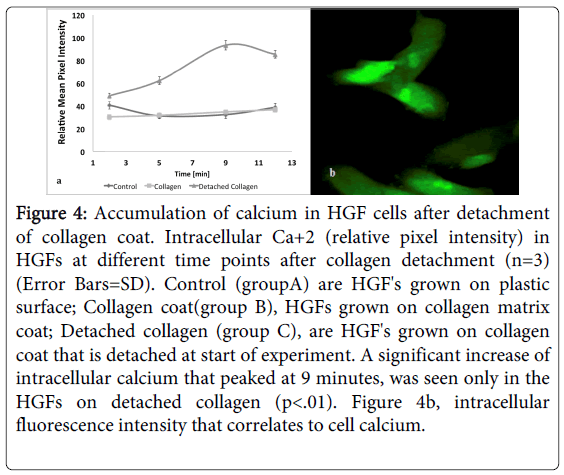
The changes in intracellular calcium were monitored and images visualized using Zeiss LSM 510 META confocal live cell microscopy by a fluorescent FLU-4AM specific Ca+2 probe that loaded HGF cells. The HGF cells which were seeded over collagen coat (group B) showed a low expression of intracellular Ca+2, similar to that seen in the cells that were seeded on plastic surface (group A)(Figure 4a). The abrupt reduction of cellular strain after detachment of collagen coat (group C) sharply increased influx of Ca+2 inside the HGF cells (Figure 4a and 4b). A significant increase of intracellular calcium that peaked at 9 minutes, was seen only in the HGFs after detachment of collagen coat (p<0.01).
Figure 4: Accumulation of calcium in HGF cells after detachment of collagen coat. Intracellular Ca+2 (relative pixel intensity) in HGFs at different time points after collagen detachment (n=3) (Error Bars=SD). Control (groupA) are HGF's grown on plastic surface; Collagen coat(group B), HGFs grown on collagen matrix coat; Detached collagen (group C), are HGF's grown on collagen coat that is detached at start of experiment. A significant increase of intracellular calcium that peaked at 9 minutes, was seen only in the HGFs on detached collagen (p<.01). Figure 4b, intracellular fluorescence intensity that correlates to cell calcium.
In another experiment, where 0.3 mM of ATP was added to HGF’s cultures an immediate increase of intracellular Ca+2 was measured (p=0.05). When extracellular calcium was chelated by EGTA, prior to ATP treatment, no significant increase in intracellular Ca+2 was observed.
Gene expression of P2X4, P2X7, RANK-L and STC-1 in HGF cells after detachment of collagen coat
Differential display of genes was performed 30 minutes after detachment of collagen coat, by microarray analysis. Changes in expression of only 19 genes were recorded between groups (A) and (B), while detachment of collagen coat from the dish surface (group C) induced changes of much larger number of genes (189 genes).
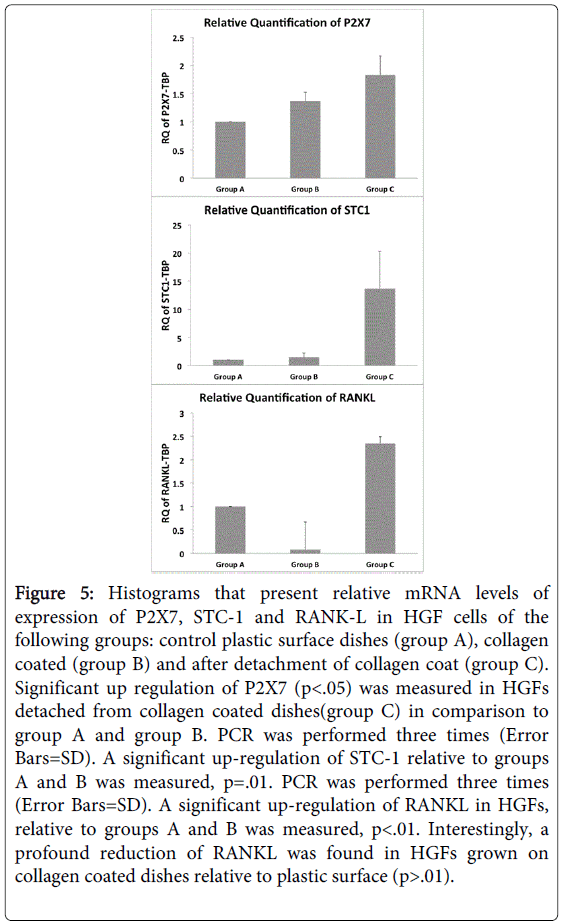
It was striking to find high and significant up-regulation of stanniocalcin-1 (STC-1) in cells of group C, in comparison to groups A and B (p=0.01). STC-1 mRNA was found in a wide variety of mammalian tissues having an autocrine or paracrine role modulating phosphate and calcium fluxes between extracellular and intracellular compartments [26]. Quantitative measurement of STC-1 by qPCR confirmed the elevated expression in HGF cells after detachment of collagen coat (Figure 5).
Figure 5: Histograms that present relative mRNA levels of expression of P2X7, STC-1 and RANK-L in HGF cells of the following groups: control plastic surface dishes (group A), collagen coated (group B) and after detachment of collagen coat (group C). Significant up regulation of P2X7 (p<.05) was measured in HGFs detached from collagen coated dishes(group C) in comparison to group A and group B. PCR was performed three times (Error Bars=SD). A significant up-regulation of STC-1 relative to groups A and B was measured, p=.01. PCR was performed three times (Error Bars=SD). A significant up-regulation of RANKL in HGFs, relative to groups A and B was measured, p<.01. Interestingly, a profound reduction of RANKL was found in HGFs grown on collagen coated dishes relative to plastic surface (p>.01).
In our previous study we have shown that detachment of marginal gingiva from molar tooth roots of rats induced specifically the P2X4 receptor [9]. In the present study, P2X7 (Figure 5) but not P2X4 (data not shown) was significantly expressed in human gingival fibroblasts in group C after collagen detachment in comparison to group A or group B (p<0.05) (Figure 5). Another important finding was that RANK-L, which induces differentiation and activity of osteoclasts is highly expressed in HGF cells after detachment of collagen coat in comparison to groups A and B (p<0.01) (Figure 5). It should be noted that HGF’s grown on collagen exhibited a significant lower RANKL gene expression than HGF’s grown on plastic surface (Figure 5).
Discussion
In our previous studies, we have shown that surgical detachment of collagen fibers from the root surface resulted in a fast release of ATP to the extracellular environment and increase of ATP receptor P2X4 expression, in a rat model [9]. Three weeks later, a significant bone loss on the periodontal aspect of alveolar bone that extends toward bone crest was recorded [4,18]. In the present study, human gingival fibroblasts (HGF) that were seeded on collagen coated culture dishes normally developed traction forces between the HGF cells and collagen [27-29]. Detachment of the collagen coat from culture dish surface produced an immediate sharp rise of extracellular ATP, an increased influx of calcium and a significant change in cell shape. Also, Ionomycin drug that induces a rapid Ca+2 influxes into the HGF cells, increased significantly the release of ATP from cells grown on plastic culture dishes. Vice versa, addition of ATP to medium of cultured HGFs increased significantly calcium influx into the cells. This reciprocal relation between levels of extracellular ATP and intracellular calcium could be possibly explained by the up regulation of STC-1in response to detachment of HGF cells. Also, Boudreault and Grygorczyk [30] found that ATP release was tightly synchronized with cytosolic calcium elevations and therefore proposed that intracellular Ca2+ elevation is a critical step in ATP release.
STC-1 mRNA was found in a wide variety of mammalian tissues having an autocrine or paracrine role modulating phosphate and calcium fluxes between extracellular and intracellular compartments [26]. Similar to our observations were described by Block et al. [31] that employed an injury model in which epithelial monolayers were disrupted. They found that STC1 mediated calcium activation downstream of ATP in lung epithelial cells. Moreover, the increased expression of STC-1 in the present experiment might be an initial response to changes in cell shape activating calcium influx and release of ATP from the HGF cells, as proposed by Block et al. [31].
The fast release of ATP from the cells due to reduction of cell strain might be the consequence of the striking changes in HGF cell shape [32]. It seems that a decrease in the basal tensile prestress of HGFs, activation of ion channels by ATP and increase in intracellular calcium evokes a chain of molecular pathways including the MAPK pathway [11] and changes in actin microfilaments and microtubules in osteoblasts and osteoclasts [33]. Our RT-PCR results have shown elevation in gene expression of the purinoreceptors P2X7 but not P2X4 and a significant high gene expression of RANKL due to stress relaxation of HGF cells, after detachment of collagen coat. It is noted that P2X4 is up-regulated in rat gingival fibroblasts after strain relaxation [9], while P2X7 is increased after detachment of human gingival fibroblasts. As bone remodeling is a localized process, the local release of ATP from HGF cells may play a critical role in local alveolar crest bone remodeling [20,33,34]. Further, our findings suggest that local increase of extracellular ATP initiates a sequenced chain of reactions leading to increased expression of RANK-L that activate osteoclasts specifically on the PDL aspect of alveolar bone [35]. P2X receptors have been implicated in the generation of osteoclasts via up-regulation of osteoblast-expressed receptor, an activator of nuclear factor-B ligand (RANK-L), and an important link in the formation and activation of osteoclasts by several group of investigators [16,36]. In fact, significant up regulated expression of the purinoreceptor P2X7 and RANKL (receptor activator of nuclear factor-kappaB ligand), 20 minutes after detachment of HGFs is in good agreement with our in vivo findings in the rat model [9,18]. A most interesting discovery in HGFs was the high up-regulation of RANKL gene expression after detachment of collagen coat while HGF is depressed when cells grown on strained collagen coat in comparison to cells grown on plastic culture dish surface. It will be of interest to explore the possibility that collagen substrate may depress RANK-L expression.
In summary, the present study supports our previous observations that gingival fibroblasts of the marginal gingiva are very sensitive to changes of strains in their environment and therefore are key in regulating the remodeling of the periodontal tissues. We propose that extracellular ATP may act as a regulator and/or transducer of mechanical signaling by regulating a cell’s set point for prestress [37]. Taken together, the results show that abrupt strain relaxation of human gingival fibroblasts stimulated a chain of cellular reaction, all being related to signaling of Pi/Ca modulators that affect the propagation toward osteoclastogenesis.
Acknowledgments
We acknowledge the support of local funds of School of Dental Medicine to IB to perform the present study. NG and IB contributed equally to the present study. EW and MA provided the human marginal gingiva explants and cells. AZ and AY helped in data analysis and discussion.
Conflict of Interest
The authors declare no potential conflict of interest with respect to the authorship and publication of this article.
References
- Ingber DE (2003) Mechanobiology and diseases of mechanotransduction. Ann Med 35: 564-577.
- Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260: 1124-1127.
- Yaffe A, Fine N, Alt I, Binderman I (1995) The effect of bisphosphonate on alveolar bone resorption following mucoperiosteal flap surgery in the mandible of rats. J Periodontol 66: 999-1003.
- Binderman I, Adut M, Zohar R, Bahar H, Faibish D, et al. (2001) Alveolar bone resorption following coronal versus apical approach in a mucoperiosteal flap surgery procedure in the rat mandible. J Periodontol 72: 1348-1353.
- Yaffe A, Herman A, Bahar H, Binderman I (2003) Combined local application of tetracycline and bisphosphonate reduces alveolar bone resorption in rats. J Periodontol 74: 1038-1042.
- Grevstad HJ (1993) Doxycycline prevents root resorption and alveolar bone loss in rats after periodontal surgery. Scand J Dent Res 101: 287-291.
- Kaynak D, Meffert R, Bostanci H, Gunhan O, Ozkaya OG (2000) A histopathological investigation on effects of the bisphosphonate alendronate on resorptive phase following mucoperiosteal flap surgery in the mandible of rats. J Periodontol 71: 790-796.
- Binderman I, Bahar H, Yaffe A (2002) Strain relaxation of fibroblasts in the marginal periodontium is the common trigger for alveolar bone resorption: a novel hypothesis. J Periodontol 73: 1210-1215.
- Binderman I, Bahar H, Jacob-Hirsch J, Zeligson S, Amariglio N, et al. (2007) P2X4 is up-regulated in gingival fibroblasts after periodontal surgery. J Dent Res 86: 181-185.
- Cook SP, McCleskey EW (2002) Cell damage excites nociceptors through release of cytosolic ATP. Pain 95: 41-47.
- Loomis WH, Namiki S, Ostrom RS, Insel PA, Junger WG (2003) Hypertonic stress increases T cell interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J BiolChem 278: 4590-4596.
- Burnstock G (2002) Potential therapeutic targets in the rapidly expanding field of purinergicsignalling. Clin Med 2: 45-53.
- Yu H, Ferrier J (1994) Mechanisms of ATP-induced Ca2+ signaling in osteoclasts. Cell Signal 6: 905-914.
- Jørgensen NR, Henriksen Z, Sørensen OH, Eriksen EF, Civitelli R, et al. (2002) Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J BiolChem 277: 7574-7580.
- Dixon JS, Sims SM (2000) P2 purinergic receptors on osteoblasts and osteoclasts: potential targets for drug development. Drug Dev Res. 49: 187-200.
- Buckley KA, Hipskind RA, Gartland A, Bowler WB, Gallagher JA (2002) Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-kappa B ligand. Bone 31: 582-590.
- Gartland A, Buckley KA, Bowler WB, Gallagher JA (2003) Blockade of the pore-forming P2X7 receptor inhibits formation of multinucleated human osteoclasts in vitro. Calcif Tissue Int 73: 361-369.
- Yaffe A, Bahar H, Binderman I (2006) Topical application of drugs influencing cytoskeleton and cell contractility affects alveolar bone loss in rats. J Periodontol 77: 826-831.
- King BF, Liu M, Townsend-Nicholson A, Pfister J, Padilla F, et al. (2005) Antagonism of ATP responses at P2X receptor subtypes by the pH indicator dye, Phenol red. Br J Pharmacol 145: 313-322.
- Qi J, Chi L, Faber J, Koller B, Banes AJ (2007) ATP reduces gel compaction in osteoblast-populated collagen gels. J ApplPhysiol (1985) 102: 1152-1160.
- Richards J, Guzman R, Konrad M, Yang J, Nandi S (1982) Growth of mouse mammary gland end buds cultured in a collagen gel matrix. Exp Cell Res 141: 433-443.
- He Y, Grinnell F (1994) Stress relaxation of fibroblasts activates a cyclic AMP signaling pathway. J Cell Biol 126: 457-464.
- Ford SR, Hall MS, Leach FR (1992) Enhancement of firefly luciferase activity by cytidine nucleotides. Anal Biochem 204: 283-291.
- Chittock RS, Lidzey DG, Berovic N, Wharton CW, Jackson JB, Beynon TD (1993) The quantum yield of Luciferase is dependent on ATP and enzyme concentrations. Mol. Cryst. Liq. Cryst. 236: 59-64.
- Lee TL, Lin YC, Mochitate K, Grinnell F (1993) Stress-relaxation of fibroblasts in collagen matrices triggers ectocytosis of plasma membrane vesicles containing actin, annexins II and VI, and beta 1 integrin receptors. J Cell Sci 105 : 167-177.
- Yoshiko Y, Aubin JE, Maeda N (2002) Stanniocalcin 1 (STC1) protein and mRNA are developmentally regulated during embryonic mouse osteogenesis: the potential of stc1 as an autocrine/paracrine factor for osteoblast development and bone formation. J HistochemCytochem 50: 483-492.
- Rhee S, Grinnell F (2007) Fibroblast mechanics in 3D collagen matrices. Adv Drug Deliv Rev 59: 1299-1305.
- Greiner AM, Chen H, Spatz JP, Kemkemer R (2013) Cyclic tensile strain controls cell shape and directs actin stress fiber formation and focal adhesion alignment in spreading cells. PLoS One 8: e77328.
- Kim MC, Neal DM, Kamm RD, Asada HH (2013) Dynamic modeling of cell migration and spreading behaviors on fibronectin coated planar substrates and micropatterned geometries. PLoSComputBiol 9: e1002926.
- Boudreault F, Grygorczyk R (2004) Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol 561: 499-513.
- Block GJ, DiMattia GD, Prockop DJ (2010) Stanniocalcin-1 regulates extracellular ATP-induced calcium waves in human epithelial cancer cells by stimulating ATP release from bystander cells. PLoS One 5: e10237.
- Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, et al. (2008) Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4: 155-170.
- Hazama R, Qu X, Yokoyama K, Tanaka C, Kinoshita E, He J, Takahashi S, Tohyama K, Yamamura H, and Tohyama Y (2009) ATP induced osteoclast function: the formation of sealing-zone like structure and the secretion of lytic granules via microtubule-deacetylation under the control of Syk. Genes Cells 14: 871-884.
- Orriss IR, Key ML, Hajjawi MO, Arnett TR (2013) Extracellular ATP released by osteoblasts is a key local inhibitor of bone mineralisation. PLoS One 8: e69057.
- Nobuto T, Imai H, Suwa F, Kono T, Suga H, et al. (2003) Microvascular response in the periodontal ligament following mucoperiosteal flap surgery. J Periodontol 74: 521-528.
- Hoebertz A, Townsend-Nicholson A, Glass R, Burnstock G, Arnett TR (2000) Expression of P2 receptors in bone and cultured bone cells. Bone 27: 503-510.
- Ingber DE (1997) Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59: 575-599.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 14114
- [From(publication date):
February-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 9551
- PDF downloads : 4563