Stochastic Simulation of the Economic Impact of Cattle Brucellosis in Cote d Ivoire
Received: 28-Aug-2018 / Accepted Date: 05-Oct-2018 / Published Date: 10-Oct-2018 DOI: 10.4172/2332-2608.1000283
Keywords: Brucellosis; Cattle population; Loss of production; Matrix model; Côte d’Ivoire
Introduction
Brucellosis is predominantly a chronic zoonotic disease in livestock, wildlife and humans and is caused by Gram-negative bacteria Brucella spp. It is transmitted to people through the direct contact with infectious animal abortion/birth materials and contaminated products. Though the zoonosis has been eliminated in high-income countries, it induces every year 500,000 human cases worldwide [1]. Brucellosis is present in all sub-Saharan countries where it potentially induces major economic losses to livestock production through abortions, lower fertility and fecundity, reduced milk and meat productions, as well as mortality of weak newborns of infected females [2]. In contrast to cattle, the epidemiology and economic impacts of brucellosis are less evidenced in small ruminants in Africa [3]. Up to date, there is no B. melitensis isolate from Western Africa and our working hypothesis is that brucellosis is absent in small ruminants in Côte d’Ivoire.
Mangen [4] estimated a total additional income potential of USD 86–143 million per year by elimination of brucellosis in sub-Sahara. An older study on the economic significance of brucellosis in Central Africa has estimated up to 6% loss of the gross income per cattle [5]. In Western Africa, few older studies have assessed the cost of brucellosis at herd level. In Côte d’Ivoire, Camus [6] found an annual loss of FCFA 150 million (almost USD 260,000) in brucellosis infected herds due to a 10% decrease in the annual income of cattle breeders about 60% of milk produced in northern Côte d’Ivoire was infected by Brucella spp., and thus, would have needed to be boiled or pasteurized before human consumption.
A national brucellosis mass vaccination campaign was launched in 1978 by the Ivorian governmental agency Société de Développement des Productions Animales (SODEPRA). Breeding cows were primarily vaccinated, and in the following years heifers only. This resulted in a 40% reduction in the cattle abortion rates, and an important increase in milk yield in the country [7]. The last census of the Ivorian livestock population was conducted in 2001, when 1,336,000 cattle were estimated [8]. The collapse of SODEPRA in 1995 and the first armed conflict in 2002 led to the cessation of livestock diseases control activities, destruction of animal health services and production infrastructure, large numbers of stolen cattle herds that were culled or trekked abroad. Since then, the national supply of milk, meat and hides strongly depends on import [9]. This work simulates the Ivorian national cattle demography and the losses in meat, milk and hide productions attributable to cattle brucellosis over a 10-year period (2005-2015). The findings can be further used to assess the cost-effectiveness of brucellosis control in Côte d’Ivoire.
Materials and Methods
Ethical considerations
The study was approved by the Ministère de la Santé et de la Lutte contre le Sida (N°71/MSLS/CNER-dkn), and the Direction Générale de Recherche Scientifique et de l’Innovation Technologique du Ministère de l’Enseignement Supérieur et de la Recherche Scientifique in Côte d’Ivoire (N°089/MESRS/DGRSIT/KYS/tm). Further approval was obtained from the Ethics Commission of the Cantons of Basel-Stadt and Basel-Land (ref. 146/10).
Data collection
A seroprevalence of 4.6% (95% CI 2–10.6) derived from a previous cross-sectional study using a three-stage cluster sampling approach was employed, and 63 village herds in northern Côte d’Ivoire from 2012 to 2014 [10]. The national cattle herd composition and slaughter data were collected from the Regional and Departmental Veterinary Services and the Laboratoire de Recherches Veterinaires (LRK) in northern Côte d’Ivoire. Given the sparse data on cattle production parameters in the country, the literature on extensive livestock production systems in semi-arid sub-Saharan Africa to assemble parameters’ values was used but, where no published parameters for sub-Saharan Africa existed, also from elsewhere (e.g., Kyrgyzstan) [11].
Ivorian national cattle herd composition
In 2005, a total cattle population of 1,449,000 was estimated, and almost 80,000 cattle were slaughtered that year (MIRAH-DPP 2012). Both populations were used in the study as the baseline populations. Animals were grouped by sex and age: female and male calves (0-1.6 years), heifers and replacement bulls (≥ 1.6-3.6 years), adult bulls and breeding cows (>3.6 years) [12,13]. Tables 1 and 2 show the national cattle population structure and slaughter data respectively [8]. The national herds had a preponderance of breeding cows and heifers (almost 50% and 20%, respectively), while slaughter animals were predominantly cows and replacement males (approximately 30% each) and adult bulls (approximately 15%).
| National cattle herd structure | Population vector (Nt) | Mean proportions of sex and age classes in the population (Eigenvector) |
|---|---|---|
| Female calves | 167,452 | 0.1156 |
| Male calves | 167,452 | 0.1156 |
| Heifers | 278,460 | 0.1922 |
| Replacement males | 66,242 | 0.0457 |
| Cows | 693,933 | 0.4789 |
| Bulls | 75,457 | 0.0520 |
| Total | 1,449,000 | |
Table 1: Age and sex structure of the national cattle population (in equilibrium) used for the simulation from 2005-2015.
| Structure of slaughter cattle | Population vector (Nt) | Relative proportions of slaughter animals |
|---|---|---|
| Female calves slaughter | 7,185 | 0.09 |
| Male calves slaughter | 9,700 | 0.13 |
| Heifers slaughter | 3,897 | 0.05 |
| Replacement males slaughter | 20,970 | 0.27 |
| Cows slaughter | 24,478 | 0.32 |
| Bulls slaughter | 11,325 | 0.15 |
| Total | 77,555 | |
Table 2: Age and sex structure of the slaughter cattle population used for the simulation from 2005–2015.
Projection matrix model
A Leslie matrix which is a squared, discrete, sex and age-structured demographic model to project the population dynamics according to Vandermeer and Goldberg [14] was used, and the cost of brucellosis infection in cattle estimated. The economic evaluation included income losses: decreased milk, meat and hide production in infected herds. Possible socio-economic impacts of the disease on human health as well as other potential indirect costs were not included in this model.
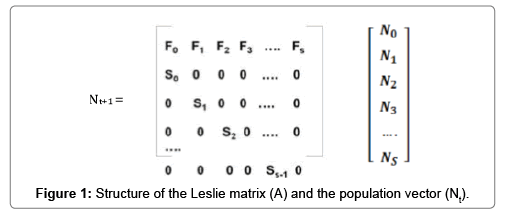
The projection model was available as an Excel spread sheet which showed all calculations and used a series of charts to illustrate outputs of modifying various model parameters. It was also linked to the add-in program Ersatz (version 1.3.3, EpiGear International, 2015, Pty Ltd, QLD, Australia) which included variance of the parameters values to shift from deterministic to stochastic Monte Carlo method for analyses of uncertainty. The modeling was based on the hypothesis of exponential growth in the population and the absence of inward and outward migration of cattle. The demographic process and the cattle population vector (Nt+1) for each following time step was simulated by multiplying the Leslie Matrix (A) by the age and sex-structured stable population vector (Nt) for the previous time step (Figure 1) [14].
The first two rows in the Leslie matrix indicate the probability that a cow of any given age will produce a female/male calf (i.e., sex and age-specific fecundity Fs). Note that local Ivorian cattle breeds do not reach sexual maturity before the age of 3.5 years [12]. The rows (below the first two) present the likelihood that an animal of a given age will survive for another year (i.e., age-specific survivorship Ss). The matrix also included the persistence of heifers (i.e., 1-1/years as heifer or 1-1/2) in replacement herds and cows (1-1/years as breeding cow or 1-1/4) and bulls (i.e., 1-1/4) in breeding herds. Table 3 shows the parameters and their values used in the Leslie matrix for the simulation of the population growth. To consider uncertainty and variability in the projection matrix, each demographic and production parameters were specified with a PERT (or Beta PERT) distribution according to expert’s minimum, maximum and mode (or most likely central value) (Table 3). Cattle brucellosis is rarely fatal (i.e., about 1% mortality rate in cows) [13]; thus, it was assumed that it induces 15% decrease in the baseline calving rate in seropositive herds according to Bernués [15]. The fertility in brucellosis-infected herds can be written as follow:
Fi =basline fertility × (1-(0.15 × seroprevalence))
| Productivity parameters (Units) | Mean value | Min | Max | Distribution (Source) |
|---|---|---|---|---|
| Fertility rate for female calves (Calve × Year-1) | 0.220 | 0.219 | 0.221 | Pert+ (n.r.0) |
| Fertility rate for male calves (Calve × Year-1) | 0.220 | 0.219 | 0.221 | Pert+ (n.r.0) |
| Survival female calves (Calve × Year-1) | 0.876 | 0.875 | 0.877 | Pert+ (n.r.0) |
| Survival male calves (Calve × Year-1) | 0.841 | 0.840 | 0.842 | Pert+ (n.r.0) |
| Survival heifers (Heifer × Year-1) | 0.929 | 0.928 | 0.930 | Pert+ (n.r.0) |
| Survival bulls (Bull × Year-1) | 0.300 | 0.299 | 0.301 | Pert+ (n.r.0) |
| 1–1/years in replacement herds as heifer | 0.500 | 0.499 | 0.501 | Pert+ (n.r.0) |
| 1–1/years in breeding herds as cow | 0.683 | 0.682 | 0.684 | Pert+ (n.r.0) |
| 1–1/years in breeding herds as bull | 0.750 | 0.749 | 0.751 | Pert+ (n.r.0) |
| Slaughter rate for female calves (Calve × Year-1) | 0.085 | 0.084 | 0.086 | Pert+ (n.r.0) |
| Slaughter rate for male calves (Calve × Year-1) | 0.100 | 0.099 | 0.101 | Pert+ (n.r.0) |
| Slaughter rate for heifers (Heifer × Year-1) | 0.040 | 0.039 | 0.041 | Pert+ (n.r.0) |
| Slaughter rate for replacement males (Young male × Year-1) | 0.400 | 0.399 | 0.401 | Pert+ (n.r.0) |
| Slaughter rate for breeding cows (Cow × Year-1) | 0.300 | 0.299 | 0.301 | Pert+ (n.r.0) |
| Slaughter rate for breeding bulls (Bull × Year-1) | 0.200 | 0.199 | 0.201 | Pert+ (n.r.0) |
+ =Assigned by authors following consultations with veterinary clinicians and other experts n.r.0=No reference
Table 3: Cattle productivity and slaughter parameters used, and their distribution for the projection of the Ivorian cattle population.
A multivariate sensitivity analysis by Monte Carlo simulations to calculate Spearman’s rank correlation coefficients (RCC) for model parameters and cattle productivity with and without the disease was performed. The simulations were run for 10,000 iterations to identify the most sensitive parameters and to show how changes in their values could affect the population structure and productivity. To make reliable inferences on model outputs, Monte Carlo errors were assessed via checks for potential lack of convergence in parameter trace plots.
Economic evaluations of livestock production
It was assumed that the public cost of cattle brucellosis was negligible (since the study did not further consider human health impact); hence, the economic evaluations from the private livestock holder’s perspective were conducted according to Tschopp et al. [16]. Given limited research data on cattle product values in Côte d’Ivoire specifically and sub-Saharan Africa in general, some values were assigned after consultations with veterinary clinicians and other experts familiar with the cost distributions of these. The annual milk, meat and hide production were calculated in the scenarios without and with brucellosis, adjusting the baseline fertility rate (F=0.220) to the seroprevalence-dependent reduction in fertility (Fi =0.218). The losses associated with the annual milk production were estimated by multiplying the population of lactating cows with the average yearly milk yield and price per liter (Table 4), as according to Roth et al. [17] and Godet [18]. The annual slaughter value was computed by summing up the products of the sex and age-structured slaughter cattle population with respective average carcass weight and meat price per Kg (Table 4). The annual hide production value was obtained by multiplying the total number of hides (i.e., total slaughtered cattle) by the average hide weight and price per Kg. The net present values (NPV) of livestock production were a function of milk, meat and hide production with and without the disease, and was calculated in Microsoft Excel 2010 using the following equation:
| Variables name (units) | Average | Min | Max | Distribution (Reference) |
|---|---|---|---|---|
| Carcass weight (kg), female calves | 60 | 50 | 70 | Normal+ (n.r.0) |
| Carcass weight (kg), male calves | 70 | 60 | 80 | Normal+ (n.r.0) |
| Carcass weight (kg), heifers | 100 | 80 | 190 | Normal+ (BNETD 2012) |
| Carcass weight (kg), young males | 120 | 110 | 200 | Normal+ (BNETD 2012) |
| Carcass weight (kg), cows | 280 | 150 | 360 | Normal+ (Mangen et al.) |
| Carcass weight (kg), bulls | 325 | 200 | 400 | Normal+ (Mangen et al.) |
| Meat price (FCFA per kg) | 2,300 | 1,400 | 3,000 | Normal+ (BNETD 2012) |
| Milk yield (liter/cow/year) | 600 | 400 | 680 | Normal+ (Domenech et al.) |
| Milk price (FCFA per liter) | 350 | 250 | 400 | Normal+ (MIRAH-DPP 2012) |
| Hide weight (kg/animal) | 20 | 10 | 25 | Normal+ (n.r.0) |
| Hide price (FCFA per kg) | 800 | 650 | 1,000 | Normal+ (n.r.0) |
| Adult cattle price (FCFA) | 210,000 | 125,000 | 600,000 | Normal+ (BNETD 2012) |
| Replacement cattle price (FCFA) | 125,000 | 90,000 | 170,000 | Normal+ (BNETD 2012) |
| Calve price (FCFA) | 80,000 | 60,000 | 100,000 | Normal+ (n.r.0) |
| Reduced milk yield in brucellosis infected herds (%) | 15 | 10 | 25 | (Bernués et al.) |
| Reduced fertility rate in brucellosis infected herds (%) | 15 | (Bernués et al., Domenech et al. and Camus) |
+ =Assigned or adjusted after consultations with veterinary clinicians and other livestock experts
n.r.0= No reference
Table 4: Cattle production parameters and product prices (in FCFA) used for brucellosis economic impacts estimation in 2015.
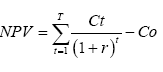
Where Ct = cattle production values during the period t;
Co =initial cattle production value;
r=discount rate,
and t=time period in years.
A discount rate of 5% was used to consider the time value of the local currency FCFA and the possible risk or uncertainty of future cash flows, as well as an exchange rate of USD 1.00=FCFA 512.950 in 2005; and USD 1.00=FCFA 579.682 in 2015 (http://www.oanda.com/currency/converter). The total production losses were estimated by subtracting the NPV of cattle productivity with brucellosis from the NPV of cattle productivity without brucellosis. A summary statistic of mean values with 95% confidence intervals was tabulated for NPV and the loss of production. The annual asset value of the live animals was estimated by summing up the products of age-structured live cattle populations and the average market prices in each scenario. The cost breakdown calculations were done to assess the individual contribution of milk, meat and hide production that represented the total domestic production value.
Results
Demographic dynamic of Ivorian national cattle herds from 2005 to 2015
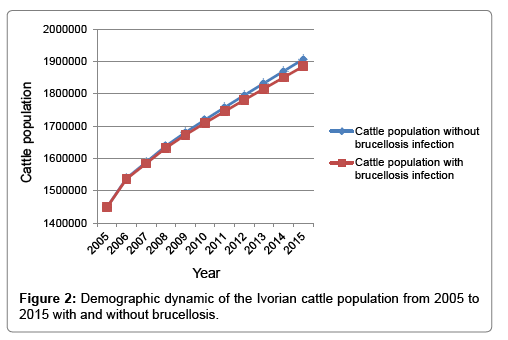
The simulation projected the cattle population to be 1,885,123 (95% CI: 1,876,483–1,893,484) in the scenario with brucellosis and 1,906,961 (95% CI: 1,894,404–1,919,484) without brucellosis (Figure 2). An overall intrinsic population growth rate (i.e., Eigenvalue) of 1.8% and an annual offtake rate of 17.4% for domestic meat production were found. The sensitivity analysis of the projection model showed that the persistence of breeding cows (RCC=0.78) and heifers (RCC=0.45) in the herds, fertility rate (RCC=0.71) and survival of female calves (RCC=0.27) as well as the survival of heifers (RCC=0.26) influenced most the population growth.
Cattle productivity and cost of brucellosis from 2005 to 2015
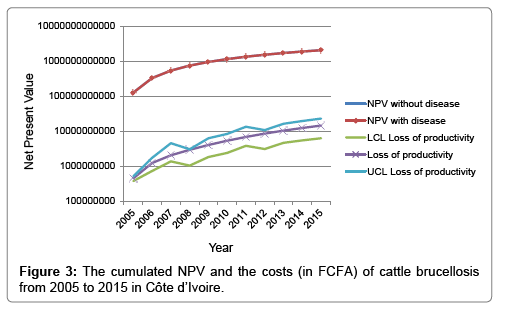
The total cattle gross production value without brucellosis was FCFA297, 482 × 106 (i.e. about USD 500,000 × 103) and was composed of 72% meat, 26% milk and 2% hide production values in 2015. The net present value (NPV) of these products in the baseline year was FCFA 127,669 × 106 (i.e., about USD 224,748 × 103). During the 10 years period, the cumulated NPV was estimated at FCFA 2,062,415 × 106 (95% CI: 1,536,407–2,217,122) in the scenario with brucellosis, and FCFA 2,076,871 × 106 (95% CI: 1,685,643–2,437,478) without brucellosis. The cumulated net present losses caused by brucellosis were FCFA 14,455 × 106 (95% CI: 6,278–22,906) which is the equivalent of USD 23,662 × 103 (95% CI: 10,276–37,496).
Figure 3 shows the growth of the NPV and the cost of brucellosis over the 10-year period of simulation. In the sensitivity analysis, the overall costs of brucellosis were most influenced by the fertility rate for female calves (RCC=0.41), persistence (RCC=0.43) and the slaughter rate of breeding cows (RCC=0.32), persistence (RCC=0.19) and survival of heifers (RCC=0.17), fertility rate for male calves (RCC=0.16), and milk prices (RCC=0.16) as well as meat prices (RCC=0.16). The overall live cattle asset value was as high as FCFA 359,307 × 106 (95% CI: 234,786-545,744) with the disease scenario, and FCFA 364,606 × 106 (95% CI: 238,663-554,850) without the disease scenario in 2015. Thus, the simulated difference of the asset value between cattle flock in both scenarios was about FCFA 3,826 × 106 (95% CI: 1-7,623) or USD 6,340 × 103 (1,657-12,629) in 2015. The overall asset value was most influenced by the market prices of replacement cattle (RCC=0.9).
Discussion
To the best of our knowledge, this is the first stochastic simulation of cattle demographics and costs estimates of the cattle production with and without brucellosis in Côte d’Ivoire, and indeed for a West African country. The findings suggest that the cattle population and the costs of brucellosis gradually increase absolutely and relatively in the next years.
Given the lack of official and reliable data on cattle population for the period of simulation, the precision of the estimates about the population growth could not be assessed. Sex and age-stratified stochastic projection would have generated more precise estimates, as it captures essential features of the population growth [14]. The annual growth rate was reported to be 4% from 1974 to 1995 in the country, but then gradually decreased to 0.14% in 2001 at the beginning of the civil unrest [9]. Restoring peace and re-opening Côte d'Ivoire to neighboring countries for cattle import provided a new national cattle population with a growth rate about 2.4% in 2005 [9]. Therefore, our estimate of 1.8% annual growth rate over a 10-year period seems realistic, despite that this fixed rate does not reflect alternations of normal and drought years with generally higher mortality rates of cattle in Africa. The 17.4% annual meat offtake rate found was within the range reported in the literature – albeit, still far below the maximum level of offtake at which a livestock population can be maintained. The persistence and survival of cows and heifers, and the calving rate for female calves were the most influencing parameters in our Leslie matrix, as they maintained the demographic growth [19]. Consequently, the model was sensitive to any change in their values. However, since input data sources were sparse and not always specific to the Ivorian context, there may be aberrations in parameter values which, in turn, might impact the predictions [20].
Though the meat products contributed 70% of the overall gross productions values, the sensitivity analysis showed that variations in meat prices had limited influence on the model outcomes when compared to the basic reproduction parameters of fertility, persistence and survival. The confidence intervals of NPV and live cattle asset values with and without the disease overlapped; therefore, the cost estimates were statistically not significantly different. This does not mean that there was not a real cost of brucellosis, but probably shows the rather high variability of demographic and production parameters, as well as market prices. In analogy with endemic cattle tuberculosis at relatively low levels (< 1%) [16], this study does not preclude the need to control cattle brucellosis in endemic areas in Côte d’Ivoire.
Some of the limitations of this study were that the Leslie matrix considered the Ivorian cattle population to be closed to inward and outward cattle mobility, which is not the case, particularly since the post-electoral armed conflict in 2010 [10]. The model was neither adjusted to co-morbidities and density-dependent factors that can also limit the population growth and productivity, nor to normal and drought years. The demographic projection assumed that animals within the same sex and age classes had the same chance of survival and reproduction (i.e., individual homogeneity). These assumptions have likely influenced the model outputs.
It would be interesting to reassess the population dynamics and the losses of productions due to brucellosis by including the abovementioned limitations in the stochastic model, as well as an intervention scenario with different achieved vaccination coverages. This will give valuable insight into the more complex cost-effectiveness assessment of brucellosis control in the country. More importantly, a future assessment must include human health costs, and health burden due to uncontrolled brucellosis to assess the societal benefits of brucellosis control in Côte d’Ivoire specifically, and in Western African countries more generally.
Conclusion
The presented study is the first stochastic demographic model for the Ivorian cattle population and can serve as a backbone for a more detailed cost-effectiveness assessment of brucellosis control at herd and national levels that also includes human health costs. The findings provide stakeholders and decision-makers with evidencebased information about the costs of cattle brucellosis in terms of meat, milk and hide productions, as well as live cattle asset values. The matrix can be used in future with more accurate and context specific input data for better cost estimations of other zoonotic diseases, and for comparable cattle populations in other West African countries. In the future, regional exchange in Western Africa with its high cross-border mobility of cattle should be assessed in total. This should foster regional collaboration in appropriate control programs of brucellosis, of other important zoonoses and transboundary livestock diseases.
Acknowledgements
The authors thank the Ivorian authorities, the Regional and Departmental Veterinary Services and the Laboratory for their collaboration in data collection. This study was funded by the Swiss National Centre of Competence in Research North-South, the City of Basel and the Freiwillige Akademische Gesellschaft.
References
- Seleem MN, Boyle SN, Sriranganathan N (2010) Brucellosis: A re-emerging zoonosis. Vet Microbiology 140: 392-398.
- McDermott JJ, Deng KA, Jayatileka TN, El Jack MA (1987) A cross-sectional cattle disease study in Kongor rural council, southern Sudan: Prevalence estimates and age, sex and breed associations for brucellosis and contagious bovine pleuropneumonia. Prev Vet Med 5: 111-123.
- Ducrotoy MJ, Bertu WJ, Matope G, Cadmus S, Conde-Ãlvarez R, et al. (2015) Brucellosis in sub-Saharan Africa: Current challenges for management, diagnosis and control. Acta Tropica pp: 1-15.
- Mangen MJ, Otte J, Pfeiffer D, Chilonda P (2003) A methodological framework for crude estimation of production losses attributable to diseases, using bovine brucellosis in sub-Saharan Africa as an example.
- Domenech J, Coulomb J, Lucet L (1982) Bovine brucellosis in Central Africa: An evaluation of its economic incidence and a cost-benefit calculation of eradication campaigns. Revue D’élevage et de Médecine Vétérinaire Des Pays Tropicaux 35: 113-124.
- Camus E (1984) Brucella vaccination of cows in northern Ivory Coast. Developments in Biological Standardization 56: 755-757.
- Camus E (1995) Evaluation of trypanosomiasis and brucellosis control in cattle herds of Ivory Coast. Agric Human Values 12: 90-94.
- MIRAH-DPP (2012) Annuaire des statistiques des ressources animales et halieutiques. Direction de la planification et des programmations, Ministère des ressources animales et halieutiques, Abidjan, Côte d’Ivoire, p: 26.
- BNETD (2012) Rapport Du BNETD sur le marché du bétail et de la viande en Côte d’Ivoire: Analyse des circuits d’approvisionnement, évolution des coûts et des prix du bétail et de la viande.
- Kanouté YB, Gragnon BG, Schindler C, Bonfoh B, Schelling E (2017) Epidemiology of brucellosis, Q fever and Rift Valley fever at the human and livestock interface in northern Côte d’Ivoire. Acta Tropica 165: 66-75.
- Kasymbekov J, Schelling E, Roth F, Zinsstag J (2014) Unpublished societal cost of brucellosis to Kyrgyzstan.
- Sokouri DP, Yapi-Gnaore CV, N’guetta ASP, Loukou NE, Kouao BJ, et al. (2010) Performances de reproduction des races bovines locales de Côte d’Ivoire. J Applied Biosciences 36: 2353-2359.
- Mangen MJ, Otte J, Chilonda P, Pfeiffer J (2002) Bovine brucellosis in sub-Saharan Africa: Estimation of seroprevalence and impact on meat and milk offtake potential. FAO Livestock policy discussion paper 8: 53.
- Vandermeer JH, Goldberg DE (2003) Population Ecology: First Principles Princeton University Press, Princeton, New Jersey.
- Bernués A, Manrique E, Maza MT (1997) Economic evaluation of bovine brucellosis and tuberculosis eradication programs in a mountain area of Spain. Prev Vet Med 30: 137-149.
- Tschopp R, Hattendorf J, Roth F, Choudhury AAK, Shaw A, et al. (2013) Cost estimate of bovine tuberculosis to Ethiopia. Curr Top Microbiol Immunol 365: 249-268.
- Roth F, Zinsstag J, Orkhon D, Chimed-Ochir G, Hutton G, et al. (2003) Human health benefits from livestock vaccination for brucellosis: Case study. Bulletin of the WHO 81: 867-876.
- Godet G, Landais E, Poivey JP, Agabriel J, Mawudo W (1981) La traite et la production laitière dans les troupeaux villageois sédentaires au nord de la Côte d’Ivoire. Rev Elev Med Vet Pays Trop 34: 63-71.
- Schärrer S, Presi P, Hattendorf J, Chitnis N, Reist M, et al. (2014) Demographic model of the Swiss cattle population for the years 2009-2011 stratified by gender, age and production type. PLoS ONE 9: e109329.
- Upton M (1989) Livestock productivity assessment and herd growth models. Agricultural Systems 29: 149-164.
Citation: Kanouté YB, Gragnon BG, Bonfoh B, Schelling E, Zinsstag J (2018) Stochastic Simulation of the Economic Impact of Cattle Brucellosis in Côte d’Ivoire. J Fisheries Livest Prod 6: 283. DOI: 10.4172/2332-2608.1000283
Copyright: © 2018 Kanouté YB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3200
- [From(publication date): 0-2018 - Mar 29, 2025]
- Breakdown by view type
- HTML page views: 2413
- PDF downloads: 787