Stewardship Implementation in Intensive Care Unit in a Tertiary Care Unit and the Role of Clinical Pharmacist
Received: 19-Aug-2022 / Manuscript No. JIDT-22-72430 / Editor assigned: 22-Aug-2022 / PreQC No. JIDT-22-72430(PQ) / Reviewed: 05-Sep-2022 / QC No. JIDT-22-72430 / Revised: 12-Sep-2022 / Manuscript No. JIDT-22-72430(R) / Published Date: 22-Sep-2022 DOI: 10.4172/2332-0877.23.S2.001
Abstract
We tried to analyse the stewardship interventions in a tertiary care hospital, optimize the antibiotic utility to combact the antibiotic resistance. Clinical pharmacists are trained and engaged in the stewardship management, they have an extensive role in optimizing the rationale use of antibiotics, combact the antibiotic resistance, implement interventions, prevent adverse drug events and improve the patient quality by decreasing the hospital costs and length of stay. As a result, rapid stewardship interventions by clinical pharmacist had significant outcomes.
Keywords: Clinical pharmacists; Antimicrobial stewardship; Intensive care unit; Multidrug resistance; Intervention; Antibiotics; ADR: Adverse Drug Reactions
Abbreviations
MDR: Multi Drug Resistance; ASO: Anti-Biotic Stewardship Programs; CRE: Carbapenem-Resistant Enterobacterales; CNS: Central Nervous System
Introduction
Antimicrobial resistance is the major concern worldwide, India is the hotspot for antimicrobial consumption with an increase in the number of infectious diseases [1]. Hence is crucial to restrict and rationalise the use of antimicrobials to prevent resistance, improve quality care and preserve the utility of last resort drugs available.
WHO defined the inappropriate usage of antimicrobial as; any prescribing antimicrobial agents without need or any improper dosage form, or short or long duration of treatment which usually leads to an increase in the risk of resistance [2].
Antibiotic resistance
The development of MDR gram-negative pathogens is multi factorial [3]. However, experience supports that improving the use of antibiotics in health care settings can play an important role in addressing antimicrobial resistance [4,5]. Although newly approved therapies for CRE infections have been associated with improved patient outcomes compared with older treatment options, their use requires close guidance from ASPs.
Antimicrobial stewardship pharmacists are uniquely positioned to help educate and provide guidance to optimize therapy [6]. It is essential to implement policies like antimicrobial stewardship not only to prevent the resistant strains but also to improve the clinical outcomes and preserve the antibiotic utility. Comprehensive education and resources on guidelines should be disseminated to clinicians to ensure compliance and optimal use in clinical practice [7].
Intensive care units
Various studies have illustrated that antibiotic utilization is maximum in the intensive care units with a wide range of antibiotics prescribed [8,9]. It is due to various factors like nosocomial hospital acquired illness, multiple previous hospitalizations, comorbidities and prescribing privileges of the physicians [10,11]. Although it is necessary to support the clinical judgment and condition of the patient, understanding drug utilization and prescribing factors helps us to achieve rational use, preserve antibiotic utility and also cost saving to the patient [12]. It is also prudent to analyse the local antibiogram, resistance patterns of the microorganisms periodically and modify the antibiotic prescribing patterns [13,14]. In our health care settings we tried to intensify the stewardship policies and recommendations to improve the antimicrobial prescribing patterns in accordance with the hospital's antibiotic policy [15-17].
Materials and Methods
Study design
It is a prospective study of 6 months carried out from September 2019 to February 2020. Both male and female patients of all age groups are taken into consideration. Pregnant and lactating women are not taken into consideration. A total of 531 patients are taken into consideration.
Study instrument
A standard clinical audit form is made which contains all the demographic details and clinical information and evidence for the selection of appropriate antimicrobial therapy.
Study process
List of restricted antibiotics is formulated based on the hospital antibiogram and antibiotic usage. Antibiotics included in this list can be released by the pharmacy for only forty hours after which a justification form for the restricted antibiotic is mandatory. The justification form is filled and signed by the prescribing doctor before the initiation of the antibiotics. It provides evidence for restricting the antibiotics and deters clinicians from prescribing restricted antimicrobials and results in de-escalation of antibiotics. Culture reports are evaluated (usually the culture results are available within 24 hours-48 hours after specimen collection) and the condition of the patient is discussed with the primary consultant. Clinical interventions are documented and discussed with the consultants and culture reports are examined to reassess the initial therapy prescribed and the selection of antibiotics is evaluated along with the patient's clinical condition and other clinical investigations. It is ensured that guidelines compliance treatment (rational therapy) is maintained, the duration of the drug and dosage is optimized accordingly.
Results
Patient demographics
The total of 531 patients in Intensive care units in a period of 6 months are considered (Table 1).
| S.No | Demographics | Total number of patients | Percentage |
|---|---|---|---|
| I | Age | ||
| 0-20 years | 45 | 8.47% | |
| 20-40 years | 92 | 17.32% | |
| 40-60 years | 177 | 33.33% | |
| 60-80 years | 198 | 37.28% | |
| 80-100 years | 19 | 3.57% | |
| II | Gender | ||
| Male | 342 | 64.40% | |
| Female | 189 | 35.50% | |
Table 1: Patient demographics in percentage (%).
Culture sensitivity pattern
Blood and other specimens are collected and sent for microbiology assay and culture sensitivity patterns. The results are reported within forty eight hours of the specimen collection. A total of 489 samples were sent among which respiratory samples were highest with 50.93%. Among 489 samples 255 samples have reported microbial growth which included pathogens, colonisers or contaminents (Table 2).
| S.No | Lab cultures | Total number of patients | Percentage |
|---|---|---|---|
| I |
Total cultures |
||
| Culture sent | 489 | 92.09% | |
| Culture positive | 255 | 52.14% | |
| No growth | 234 | 47.85% | |
| II |
Culture samples |
||
| Respiratory samples | 164 | 50.93% | |
| Blood samples | 84 | 26.08% | |
| Urine samples | 43 | 13.35% | |
| Fluid samples | 24 | 7.45% | |
| CSF cultures | 5 | 1.55% | |
| Clostridium difficle | 2 | 0.62% | |
Table 2: Culture sensitivity patterns in percentage (%).
Factors for prescribing antibiotics
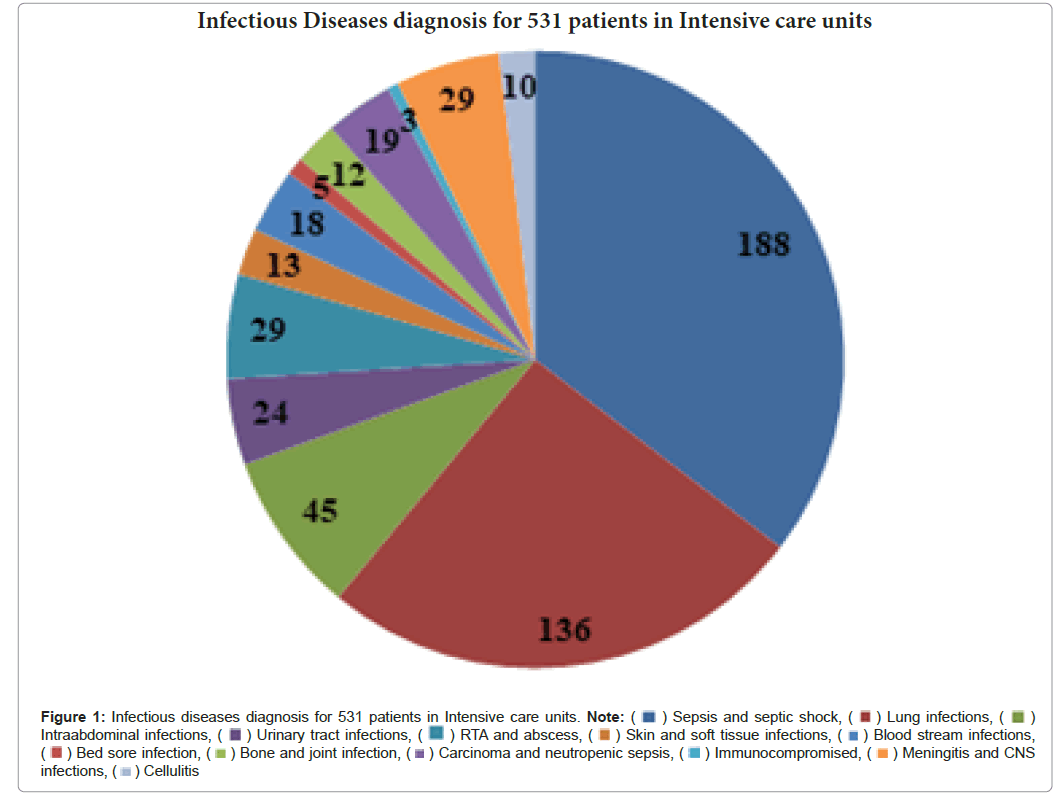
Culture report was taken into consideration and infectious diseases diagnosis is made. Among the patients admitted in ICUS, the majority of the patients had been diagnosed with sepsis and septic shock (34.5%) with underlying comorbidities. The other conditions were lung infections 25.61% Intra Abdominal infections 8.47%, Meningitis and CNS infections 5.46%, RTA 5.46%, Urinary tract infections 4.51%, Immunocompromised with carcinoma and neutropenic sepsis 4.13%, Bloodstream infections 3.89%, Skin and soft tissue infections 2.44%, Bone and joint infections 2.25%, Bedsore infections 0.94% , cellulitis 1.88% (Figure 1).
Figure 1: Infectious diseases diagnosis for 531 patients in Intensive care units. Note: ( ) Sepsis and septic shock, (
) Sepsis and septic shock, ( ) Lung infections, (
) Lung infections, ( ) Intraabdominal infections, (
) Intraabdominal infections, ( ) Urinary tract infections, (
) Urinary tract infections, ( ) RTA and abscess, (
) RTA and abscess, ( ) Skin and soft tissue infections, (
) Skin and soft tissue infections, ( ) Blood stream infections, (
) Blood stream infections, ( ) Bed sore infection, (
) Bed sore infection, ( ) Bone and joint infection, (
) Bone and joint infection, ( ) Carcinoma and neutropenic sepsis, (
) Carcinoma and neutropenic sepsis, ( ) Immunocompromised, (
) Immunocompromised, ( ) Meningitis and CNS infections, (
) Meningitis and CNS infections, ( ) Cellulitis
) Cellulitis
Prescribing patterns of restricted antibiotics
We analysed a category of 18 antibiotics marked as restricted. A total of 1186 restricted antibiotics were prescribed for the above conditions. The average consumption of restricted antibiotics for a patient was 2. It is similar to the study done by Williams RM, et al. where in a mean ( ± SD) of 2.09 ( ± 1.27) antibiotics/prescription has been reported [15].
Study done by Hanssens Y, et al. reported an average of almost three antibiotics/prescription in MICU [18]. A study conducted in Southern India reported the mean of 1.60 ( ± 0.77) antibiotics/prescription which is lower than current study [19,20]. Meropenem (38.44%) consumption is highest among the restricted antibiotic usage in our study with empirical pssrescription of 84.64(%). Although carbapenem usage against nosocomial gram negative bacteria can be considered as an effective antibiotic therapy in life threatening conditions [21], the rampant emergence of carbapenem resistant enterobacteriaceae is worrisome [22]. It may limit the antibiotic therapy and furthermore worsen the patient's condition. Hence it is advisable to restrict the empiric usage of carbapenems to prevent the emergence of widespread carbapenemase producing enterobacteriaceae [23,24]. In our study we identify that meropenem was given empirically in 49 cases (56.97%) in 86 MDR cases which might be a major probability of development of multi drug resistant organisms [25,26]. The other restricted antibiotics prescribed are in Figure 2.
Multidrug resistant organisms
Out of 255 samples that reported microbial growth 33.72% had multidrug resistant organisms. Among them respiratory samples had 70.87% of multi drug resistant organisms. Klebsiella pneumoniae is the most common gram negative organism with multi drug resistance in our hospital settings with 44.66%.
Although multiple factors favours the development of multi drug resistant organisms [27,28]. The antibiotic resistance should be managed by the rationale antibiotic usage, other factors responsible for MDR’s are older age, chronic pathologies, trauma, surgery and persistent infections which may cause acquired immune suppression that involves both innate and adaptive immunity [29,30] (Table 3).
| Intervention characteristics | Total percentages among interventions | Accepted percentages |
|---|---|---|
| De-escalation | 35.97% | 94.10% |
| Escalation | 11.10% | 71.40% |
| Dose adjustment | 7.40% | 100% |
| Prolonged duration | 2.60% | 20% |
| Wrong indication | 20.60% | 51.10% |
| Dual coverage | 15.30% | 44.80% |
| Drug interaction | 2.10% | 100% |
| ADR | 4.70% | 100% |
Table 3: MDR samples and organisms in percentage (%).
Interventions
In a period of 6 months of 531 studied cases drug interventions were noticed in 116 cases (21.84%). The total number of drug interventions in 116 cases were 189 (61.37%). The average drug intervention observed per case is +/- 1.6. Stewardship interventions were accepted in 88 cases (75.86%) and not accepted in 28 cases (24.13%) (Tables 4 and 5).
| Intervention characteristics | Total percentages among interventions | Accepted percentages |
|---|---|---|
| De-escalation | 35.97% | 94.10% |
| Escalation | 11.10% | 71.40% |
| Dose adjustment | 7.40% | 100% |
| Prolonged duration | 2.60% | 20% |
| Wrong indication | 20.60% | 51.10% |
| Dual coverage | 15.30% | 44.80% |
| Drug interaction | 2.10% | 100% |
| ADR | 4.70% | 100% |
Table 4: Intervention characteristics among interventions in percentage (%).
| Outcome parameters | Accepted cases | Not accepted cases |
|---|---|---|
| Improved | 60.22% | 21.42% |
| Readmitted (within 30 days of admissions) | 0.45% | 17.85% |
| LAMA | 13.63% | 32.14% |
| Death | 21.59% | 28.57% |
Table 5: Study of outcome measures of stewardship intervention.
Discussion
It is evident from our study that in spite of widespread awareness of antibiotic resistance, physicians continue to prolong the duration of therapy for greater than 14 days in the absence of infection parameter [31], dual coverage of antibiotics is widely practised which is not advisable [32], Use of multiple drugs active against anaerobes is not necessary and puts the patients at risk for additional drug toxicities [33]. No data or guidelines support the use of two anti anaerobic drugs in clinical practice [34]. Dual coverage de-escalation were accepted in only 15.3% commonly observed dual coverage include: Carbapenems and metronidazole (dual anaerobic) clindamycin and teicoplanin (dual MRSA coverage), linezolid and clindamycin (dual GPC, dual anaerobic coverage). However rapid de escalation was observed in 94.1% and escalation of organism specific antibiotics was observed in 71.4%.
Conclusion
In conclusion, the implementation of antimicrobial stewardship by clinical pharmacist significantly reduced antimicrobial use and improved the patient outcomes. Hence there is a requirement of antimicrobial stewardship program in Intensive care units to reduce the emerging antibiotic resistance and for a better patient outcome. Life threatening adverse drug reactions and drug-drug interactions are carefully observed and notified. ADR intervention acceptance rate is 100%, drug-drug interactions were analysed and necessary substitution of the other category of drug or time gap between the administration of the drugs is maintained.
References
- Walia K, Madhumathi J, Veeraraghavan B, Chakrabarti A, Kapil A, et al. (2019) Establishing antimicrobial resistance surveillance & research network in India: Journey so far. Indian J Med Res 149:164-179.
[Crossref] [Google Scholar] [PubMed]
- Antimicrobial stewardship programmes in health-care facilities in low-and middle-income countries: A WHO practical toolkit, World Health Organization, 2019.
- Breijyeh Z, Jubeh B, Karaman R (2020) Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 25:1340.
[Crossref] [Google Scholar] [PubMed]
- Ramakrishna N, Jayaprada R, Sharma KK (2018) Significance of antimicrobial stewardship programme. J Clin Sci Res 7:179-183.
[Crossref]
- Grohmann R, Engel RR, Rüther E, Hippius H (2004) The AMSP drug safety program: Methods and global results. Pharmacopsychiatry 37:S4-S11.
[Crossref] [Google Scholar] [PubMed]
- Sakeena MHF, Bennett AA, McLachlan AJ (2018) Enhancing pharmacists’ role in developing countries to overcome the challenge of antimicrobial resistance: A narrative review. Antimicrob Resist Infect Control 7:63.
[Crossref] [Google Scholar] [PubMed]
- Purva M, Randeep G, Rajesh M, Mahesh CM, Sunil G, et al. Assessment of core capacities for antimicrobial stewardship practices in Indian hospitals: Report from a multicentric initiative of global health security agenda. Indian J Med Microbiol 37:309-317.
[Crossref] [Google Scholar] [PubMed]
- Shahrazad M, Alireza H, Hassan T, Gholami K, Javadi MR (2013) Carbapenem utilization in critically ill patients. J Pharmceut Care 1:141-144.
- Hawkey PM, Livermore DM (2012) Carbapenem antibiotics for serious infections. BMJ 344:e3236.
[Crossref] [Google Scholar] [PubMed]
- Malacarne P, Rossi C, Bertolini G, GiViTI group (2004) Antibiotic usage in intensive care units: A pharmaco-epidemiological multicentre study. J Antimicrob Chemother 54:221-224.
[Crossref] [Google Scholar] [PubMed]
- De Bus L, Gadeyne B, Steen J, Boelens J, Claeys G, et al. (2018) A complete and multifaceted overview of antibiotic use and infection diagnosis in the intensive care unit: Results from a prospective four-year registration. Crit Care 22:241.
[Crossref] [Google Scholar] [PubMed]
- Luyt CE, Bréchot N, Trouillet JL, Chastre J (2014) Antibiotic stewardship in the intensive care unit. Crit Care 18:480.
[Crossref] [Google Scholar] [PubMed]
- Perveen RA, Nasir M, Farha N, Islam MA (2018) Antibiotics in ICU: The challenges of use, cost and response in a tertiary care hospital. Int J Med Res Health Sci 7:94-99.
- Halstead DC, Gomez N, YS McCarter (2004) Reality of developing a community-wide antibiogram. J Clin Microbiol 42:1-6.
[Crossref] [Google Scholar] [PubMed]
- Moehring RW, Hazen KC, Hawkins MR, Drew RH, Sexton DJ, et al. (2015) Challenges in preparation of cumulative antibiogram reports for community hospitals. J Clin Microbiol 53:2977-2982.
[Crossref] [Google Scholar] [PubMed]
- Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, et al. (2016) Implementing an antibiotic stewardship program: Guidelines by the infectious diseases society of America and the society for healthcare epidemiology of America. Clin Infect Dis 62:e51-e77.
[Crossref] [Google Scholar] [PubMed]
- Khilnani GC, Zirpe K, Hadda V, Mehta Y, Madan K, et al. (2019) Guidelines for antibiotic prescription in intensive care unit. Indian J Crit Care Med 23:S1-S63.
[Crossref] [Google Scholar] [PubMed]
- Hanssens Y, Ismaeil BB, Kamha AA, Elshafie SS, Adheir FS, et al. (2005) Antibiotic prescription pattern in a medical intensive care unit in Qatar. Saudi Med J 26:1269-1276.
[Google Scholar] [PubMed]
- Naqvi M, Chiranjeevi U, Shobha JC (2014) Prescription patterns of antibiotics in acute medical care unit of a tertiary care hospital in India. Int J Curr Microbiol App Sci 3:673-679.
- Patel MK, Barvaliya MJ, Patel TK, Tripathi CB (2013) Drug utilization pattern in critical care unit in a tertiary care teaching hospital in India. Int J Crit Illn Inj Sci 3:250-255.
[Crossref] [Google Scholar] [PubMed]
- Smythe MA, Melendy S, Jahns B, Dmuchowski C (1993) An exploratory analysis of medication utilization in a medical intensive care unit. Crit Care Med 21:1319-1323.
[Crossref] [Google Scholar] [PubMed]
- Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities, World Health Organization, 2017.
- Salehifar E, Shiva A, Moshayedi M, Kashi TS, Chabra A (2015) Drug use evaluation of Meropenem at a tertiary care university hospital: A report from Northern Iran. J Res Pharm Pract 4:222-225.
[Crossref] [Google Scholar] [PubMed]
- Sanhoury OM, Eldalo AS (2016) Evaluation of meropenem utilization in intensive care unit in Sudan. Int J Clin Pharmacol Pharmacother 1:106.
- Katchanov J, Kreuels B, Maurer FP, Wöstmann K, Jochum J, et al. (2017) Risk factors for excessively prolonged meropenem use in the intensive care setting: A case-control study. BMC Infect Dis 17:131.
[Crossref] [Google Scholar] [PubMed]
- Balkhy HH, El-Saed A, El-Metwally A, Arabi YM, Aljohany SM, et al. (2018) Antimicrobial consumption in five adult intensive care units: A 33-month surveillance study. Antimicrob Resist Infect Control 7:156.
[Crossref] [Google Scholar] [PubMed]
- Tenney J, Hudson N, Alnifaidy H, Li JTC, Fung KH (2018) Risk factors for aquiring multidrug-resistant organisms in urinary tract infections: A systematic literature review. Saudi Pharm J 26:678-684.
[Crossref] [Google Scholar] [PubMed]
- Aliberti S, Pasquale MD, Zanaboni AM, Cosentini R, Brambilla AM, et al. (2012) Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis 54:470-478.
[Crossref] [Google Scholar] [PubMed]
- Seligman R, Ramos-Lima LF, Oliveira VA, Sanvicente C, Sartori J, et al. (2013) Risk factors for infection with multidrug-resistant bacteria in non-ventilated patients with hospital-acquired pneumonia. J Bras Pneumol 39:339-348.
[Crossref] [Google Scholar] [PubMed]
- Ekren PK, Ranzani OT, Ceccato A, Bassi GL, Conejero EM, et al. Evaluation of the 2016 infectious diseases society of America/American thoracic society guideline criteria for risk of multidrug-resistant pathogens in patients with hospital-acquired and ventilator-associated pneumonia in the ICU. Am J Respir Crit Care Med 197:826-830.
[Crossref] [Google Scholar] [PubMed]
- Wilson HL, Daveson K, Del Mar CB (2019) Optimal antimicrobial duration for common bacterial infections. Aust Prescr 42:5-9.
[Crossref] [Google Scholar] [PubMed]
- Song YJ, Kim M, Huh S, Lee J, Lee E, et al. (2015) Impact of an antimicrobial stewardship program on unnecessary double anaerobic coverage prescription. Infect Chemother 47:111-116.
[Crossref] [Google Scholar] [PubMed]
- Huttner B, Jones M, Rubin MA, Madaras-Kelly K, Nielson C, et al. (2012) Double trouble: How big a problem is redundant anaerobic antibiotic coverage in veterans affairs medical centres?. J Antimicrob Chemother 67:1537-1539.
[Crossref] [Google Scholar] [PubMed]
- Beheshti M, Graber CJ, Goetz MB, Bluestone GL (2012) Clarifying the role of adjunctive metronidazole in the treatment of biliary infections. Clin Infect Dis 55:1583-1584.
[Crossref] [Google Scholar] [PubMed]
Citation: Raghavapuram N, Narmada T (2022) Stewardship Implementation in Intensive Care Unit in a Tertiary Care Unit and the Role of Clinical Pharmacist. J Infect Dis Ther S2:001. DOI: 10.4172/2332-0877.23.S2.001
Copyright: © 2022 Raghavapuram N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 862
- [From(publication date): 0-2023 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 785
- PDF downloads: 77

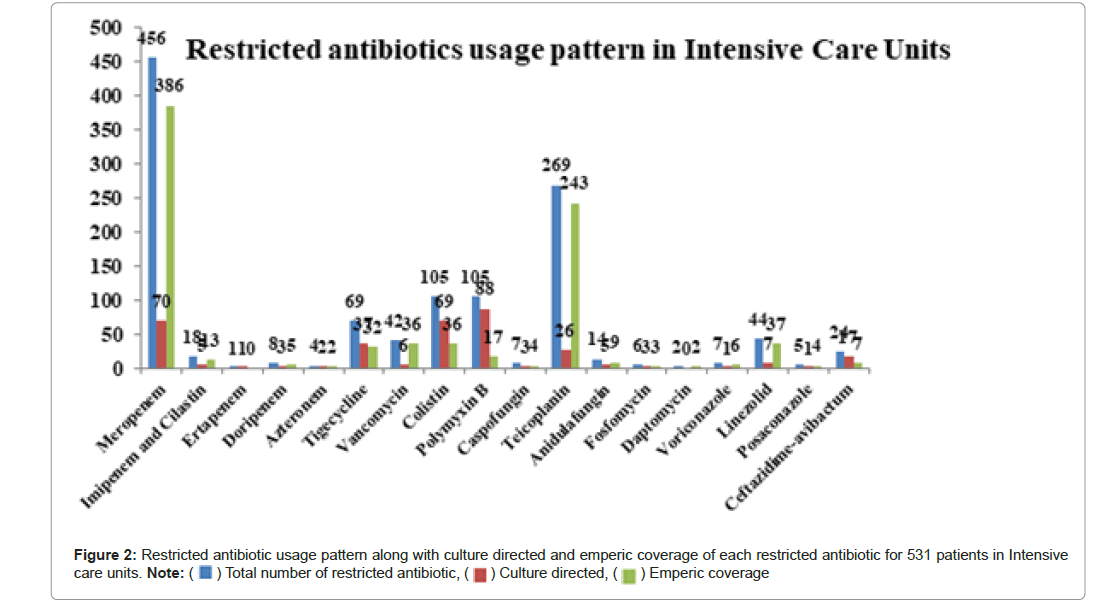
 ) Total number of restricted antibiotic, (
) Total number of restricted antibiotic, ( ) Culture directed, (
) Culture directed, ( ) Emperic coverage
) Emperic coverage