Review Article Open Access
Stem Cell Approaches for Treatment of Neurodegenerative Diseases
| Saurabh Anand and Kiminobu Sugaya* | |
| Burnett School of Biomedical Sciences, College of Medicine, University of Central Florida, Orlando, USA | |
| Corresponding Author : | Kiminobu Sugaya Burnett School of Biomedical Sciences College of Medicine, University of Central Florida Orlando, FL 32827, USA Tel: 407.266.7045 E-mail: ksugaya@ucf.edu |
| Received October 17, 2014; Accepted November 13, 2014; Published November 17, 2014 | |
| Citation: Anand S, Sugaya K (2014) Stem Cell Approaches for Treatment of Neurodegenerative Diseases. Clin Pharmacol Biopharm 3:126. doi:10.4172/2167-065X.1000126 | |
| Copyright: © 2014 Anand S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Neurodegenerative diseases are devastating age-related disorders severely affecting the patient, caregivers, and enormously increasing the financial burden of the nation. Despite decades of hard work both in laboratory and clinic, the effective treatment specifically designed for a patient is still far from reach. Stem cell therapy, though with several challenges including limited differentiation potential of adult stem cells, ethical issues with using embryonic and fetal stem cells, tumor formation upon transplantation of cells, etc., offers enormous potential for treatment of several neurodegenerative diseases. Pharmacological drugs currently available in the market on the other hand are mainly for alleviating the symptoms and not for treating the disease per se. The efficiency of drug delivery across the bloodbrain barrier, stability, efficacy, and side effects these drugs show on patients are some of the hurdles pharmacological approach has to overcome. A detailed understanding of these complicated diseases at molecular level followed by the right combination of specifically tailored stem cell therapy and/or effective drugs e.g. MS-818 used to increase the endogenous stem cell population might be the best course of action in coming years for patients with little time left after their diagnosis.
| Keywords |
| Pyrrolopyrimidine; Stem cell proliferator; Neurodegenerative diseases; Neural stem cells |
| Abbreviations |
| Aβ: Amyloid-β peptide; AD: Alzheimer Disease; ALS: Amyloid Lateral Sclerosis; APP: Amyloid Precursor Protein; (BBB): Blood-Brain Barrier; DA: Dopaminergic neurons; FGF: Fibroblasts Growth Factor; HD: Huntington Disease; L-DOPA: Laevo- 3,4 dihydroxyphenylalanine; MAPK: Mitogen Activated Protein Kinase; MS-818: 2-piperadino-6-methyl-5-oxo-5, 6-dihydro (7H) pyrrolo [2,3- d] pyrimidine maleate; NGF: Nerve Growth Factor; NMDA: N-methyl- D-aspartate receptors; NPC: Neuronal Precursor Cells; PD: Parkinson Disease; ZFX: Zinc Finger and X-linked transcription factor |
| Introduction |
| The nervous system and brain via a well-orchestrated network of electrical signals control the basic activities e.g. muscle movement, senses, speech, memories, thoughts, and emotions in humans. There are many forms of nerve diseases that in humans limit these functions in different ways. Neurodegenerative diseases are the result of the phenomenon of neurodegeneration which is an umbrella term used for the progressive structural and functional impairment of neurons eventually resulting in their death [1]. Acute neurodegeneration could result from stroke or trauma leading to damage and death of neurons at the site of injury, whereas chronic form develops over age and might result in loss of specific neuron subtype (e.g. in Parkinson Disease) or generalized population (e.g. in Alzheimer’s Disease and Huntington’s Disease) [2-5]. The different neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and Amyotrophic Lateral Sclerosis (ALS) etc. reveal similarities including accumulation of insoluble protein aggregates, apoptosis, compromised communication system etc. at the cellular level [6-10]. With high morbidity and mortality rates, these diseases have a huge social and economical impact [11]. According to Alzheimer’s association the number of people affected with Alzheimer’s disease in US alone is expected to triple in 2050 to a figure of ~16 million, costing $1.2 trillion in health care, long-term care and hospice care [12]. Not to mention that the emotional impact all of the aforementioned age-related disorders have on patients and their caregivers is hard to estimate and express in words. Unraveling the molecular mechanism underlying these complex diseases through scientific investigation would lay foundation for therapeutic intervention towards long-term goal to ameliorate and cure them [13]. We understand that these areas of research are vast and fast growing; the purpose of this review is to evaluate some of the currently available stem cell transplantation and pharmacological approaches and suggest a new class of compounds increasing stem cell populations as a safer and noninvasive approach for treatment of a few selected neurodegenerative diseases. |
| Conventional Pharmacological Approach |
| Alzheimer’s disease (AD) considered to be a protein misfolding disorder damages and kills neurons and is believed to result from a combination of genetic, lifestyle, and environmental factors [14]. The two hallmarks of Alzheimer’s: 1) accumulation of plaques made up of small peptides of beta-amyloid which is a fragment resulting from aberrant cleavage of amyloid precursor protein (APP) damages neurons by interfering with the communication system (8) 2) Neurofibrillary tangles which are made up of abnormally twisted hyperphosphorylated tau proteins causing damage and death of neurons by interfering with their transport system [3,14]. The common conventional therapeutic approaches include use of cholinesterase inhibitors by delaying the degradation of acetylcholine released from the synapse [15]. The N-methyl D-aspartate (NMDA) receptor antagonist named memantine or Namenda (generic) work on the glutamatergic system by blocking NMDA receptors and is another commonly used drug to alleviate the symptoms of AD [16]. Other conventional drugs are based on reducing Aβ production by modulating secretase activity, reducing Aβ aggregation, promoting Aβ clearance, targeting tau phosphorylation and assembly, immunotherapy against Aβ, altering metal ion’s interaction with beta-amyloid, oxidative stress etc. [17-27]. Cholinesterase inhibitors and NMDA receptor antagonists provide symptomatic relief to patients but fail in curing the underlying disease [28]. The conventional drugs targeting Aβ and tau failed in phase III clinical trials [23]. Parkinson’s disease (PD) results from the death of dopamine producing cells in substantia nigra region of the midbrain [29]. The damaged neurons have been found to abnormally accumulate alpha-synuclein bound to ubiquitin not allowing the complex to be directed to proteasome for degradation, eventually accumulating proteinaceous cytoplasmic Lewy bodies [30]. Carbidopa/Levodopa or L-Dopa (Sinemet) is the most potent and effective conventional medication for PD [31]. However, the use of L-DOPA was discontinued due to movement problems (motor fluctuations) including dyskinesias, wearing-off effect, distonias, etc. Many other dopamine agonists stimulating parts of the brain influenced by dopamine have also been used on patients [32,33]. Other anticholinergics decreasing the activity of acetylcholine regulating movement are helpful for tremors [34]. MAO-B inhibitors used for the treatment block enzymes in the brain that are responsible for breaking down dopamine [35]. They are used to make levodopa last longer or reduce the amount required. When selegiline (Eldepryl, Zelapar) is taken with levodopa, it results in dyskinesias, hallucinations etc. [36]. Rasagiline (Azilect) when taken without levodopa may result in headache, joint pain, indigestion, depression etc. [37]. COMT inhibitors on the other hand representing the newer class of drugs for PD inhibit the action of catechol-O-methyl transferase, an enzyme involved in degrading neurotransmitters. They have no direct effect on PD symptoms but have been found to prolong the effect of L-Dopa by blocking its metabolism [38]. Two of the pharmaceutical examples tolcapone and nicetapone were found to be toxic to the liver [39]. All of these drugs help alleviate the symptoms of PD but none of them have yet been able to cure or reverse the effects of the disease. Huntington’s disease (HD) is an inherited disease caused by a mutated form of the huntingtin gene where excessive CAG repeats result in formation of an unstable and aggregation-sensitive protein causing the progressive degeneration of GABAergic medium spiny neurons (MSNs) in the brain [5,40]. It affects patient’s abilities to perform basic functions usually resulting in movement, cognitive, and psychiatric disorders. The current drugs help manage the symptoms but cannot prevent the degeneration or cure the disease. Several drugs to treat movement disorders e.g. Tetrabenazine (Xenazine), antipsychotic drugs, antidepressants, and mood-stabilizing drugs are currently being used in patients with HD [41]. Amyotrophic lateral sclerosis (ALS) or Lou Gehrig’s disease specifically affects motor neurons eventually resulting in their death. As a result, the brain’s ability to initiate and control muscle movement is lost [42]. Riluzole (Rilutek) is the only FDA approved drug for ALS. It slows down the disease’s progression possibility by decreasing high levels of glutamate seen in ALS patients. This too causes side effects including dizziness, gastrointestinal complications, and abnormalities in liver function [43]. Another drug named Retigabine, an anticonvulsant, originally recommended for epilepsy, is a potassium channel opener, has recently been shown promising for ALS. Several adverse effects including drowsiness, dizziness, slurred speech etc. were noticed in phase II clinical trial for epilepsy; the side effects of this drug in ALS patients are currently under investigation [44]. |
| Stem Cell Transplantation Approach |
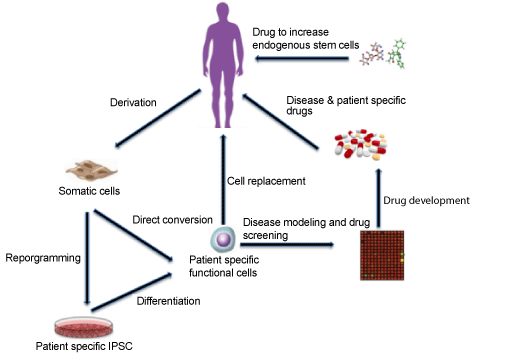
| Stem cell therapy has been proven to be promising for treatment of several human diseases [45,46]. The use of embryonic stem cells (ESC) and fetal stem cells (fetal proper stem cells and extra-embryonic fetal stem cells) for stem cell research faced several ethical issues in the past [47]. Induced pluripotent stem cells (iPSC) on the other hand are adult cells that have undergone reprogramming to an embryonic stem cell-like state by expression of genes responsible for maintaining the characteristics of ESCs [48]. IPS technology has broad scope of application possibilities as the adult cells can be isolated, differentiated into cells of interest in the plate, and injected back to the same patient, thus avoiding the complications of rejection post transplantation (Figure 1) [49]. Though adult stem cells are found in all the organs and theoretically can differentiate into different cell types, the differentiation potential exhibited by them is limited. Since patients suffering from neurodegenerative diseases exhibit different neuronal pathologies and complications, deciding on the course of treatment using stem cell therapy involve many considerations [46,50]. The treatment objectives in these cases have been centered on replacing damaged or dead neurons with new ones and/or augmenting the brain environment by using engineered stem cells expressing brain-derived growth factors [51]. Neural stem cells (NSCs) are a population of adult cells with selfrenewal and differentiation capacities. A major focus has been to use NSCs to combat age-related neurological disorders [52]. When NSCs were injected into the hippocampal area of the brain of transgenic AD mouse model, the cognitive function improved with no change in the Aβ plaques or neurofibrillary tangles [53]. Interestingly, in 3xTg-AD mice, which develop both amyloid and tangle pathology, the clearance of intraneuronal Aβ plaques by immunotherapy rescues the cognitive impairment. However, the reemergence Aβ pathology in antibodytreated mice again results in cognitive deficits [54]. |
| It was also seen in the study that the improved cognition was a result of increased brain-derived neurotrophic factor (BDNF) [53]. The high levels of APP in the brain not only reduce NSCs, but also increase glial differentiation of transplanted stem cells, hence negatively affecting the outcome of therapy [55]. We previously showed significantly high levels of neurogenesis from transplanted NSCs in APP transgenic mice when APP level was reduced upon phenserine treatment [56]. Several research studies on expression of growth factors like vascular endothelial growth factors (VEGF), brain-derived neurotrophic factors (BDNF), nerve growth factor (NGF) etc. have shown these to have neuroprotective effects [57-60]. Since PD results from progressive death of dopaminergic neurons (DA) neurons, stem cell therapy is focused on replacing the same in substantia nigra of the brain. Studies show functional recovery upon grafting both embryonic stem (ES)- and mesenchymal stem cells (MSC)-derived DA neurons into rat PD model [61,62]. Transplantation of stem cells engineered to produce growth factors like BDNF, VEGF, GDNF etc. have been shown to protect DA neurons and functional recovery in transgenic PD models [62,63]. As HD results from loss of GABAnergic neurons, cellular therapies used are based on replacing dead neurons and also supplementing nerve growth factors in some cases. Transplantation of neural tissue and striatal grafts showed for the first time that MSNs could integrate and form circuitry in transgenic HD models [64]. NPCs engineered to overexpress GDNF protected neurons and promoted functional recovery in rodent models of HD [65]. In ALS that is currently considered incurable, the degradation of motor neurons that connect spinal cord to the muscles makes the life expectancy two to five years after diagnosis [66]. It has been shown that mouse embryonic stem cells can be differentiated into motor neurons and when transplanted into spinal cord of embryonic chick, motor neurons forms connections with skeletal muscles [61]. Thus in chick model replacement of dying motor neurons with healthy ones is a possibility but since body holds hundreds of different motor neurons, time will tell whether it will be practically feasible to convert stem cells into specific motor neuron and transplant to ALS patient in need [67]. |
| There are many published studies on efforts made using approaches of stem cell therapy and pharmacology to treat neurodegenerative diseases (Figure 1). However, both approaches have met with several challenges in the past [68,69]. Although stem cell therapy has met with some significant success when utilized for treatment of neurodegenerative disease, as mentioned above there are several important considerations and risk factors associated with this approach. The limited number of donors, intrinsic properties of the cell type used, difficulties in controlling the differentiation potential of iPSCs, immunorejection in case of allogeneic transplantation and subsequent use of immunosuppressants, growth of tumor resulting from injection of mixed populations of differentiated and undifferentiated cells etc. are some of the complications posed by this approach and ultimately putting patients at risk [70]. Recently, zinc finger and X-linked transcription factor (ZFX) was shown to play role in maintaining selfrenewal and tumorigenic potential of glioma stem cells (GSCs) through upregulation of c-Myc expression. Thus, targeting ZFX could represent one of the promising strategies to overcome the risk of tumor formation posed by injecting mixed population of stem cells through inducing apoptosis or differentiation of brain tumor [71]. The pharmacological treatments on the other hand are mostly limited by the low permeability of compounds to cross the blood brain barrier (BBB) along with the side effects these compounds have shown in patients undergoing clinical trials [72]. |
| Pharmacological Approach to Increase Stem Cell Number |
| Blood-brain barrier (BBB) which acquired selectivity during the course of evolution for the very purpose of protecting the brain against potentially harmful materials floating around in the blood supply actually makes pharmacological means of administering small molecular compounds to central nervous system more challenging [73,74]. Efforts are being made to increase the lipophilicity of these compounds to increase their chances of getting internalized in the brain. The use of nanotechnology to attach immunoglobulins, liposomes, and nanoparticles to these compounds for more efficient delivery across the blood-brain barrier has attracted many researchers in recent years [75]. Thus synthetic biology and precision nanomedicine aid in making drug delivery more effective which is one of the important criteria towards developing a safe and efficacious drug [76]. |
| Small molecules have also been routinely used as a tool in stem cell research laboratories for different purposes including but not limited to maintenance of stem cells in undifferentiated state in culture to increase proliferative potential of pluripotent cells, reprogramming, differentiation, and manipulation of different signaling cascades within the cells [77]. They offer several advantages such as high permeability, being chemically defined, high purity and stability, more economic in comparison to peptides and growth factors, and flexibility of playing around with concentration to alter the effect [78]. Many such useful compounds affecting Wnt signaling pathway by inhibiting glycogen synthase kinase-3 (GSK3-beta) are known to promote the selfrenewal of embryonic stem cells [79]. Inhibitors of TGF-β family type I receptor-like kinase (ALK5) preventing Smad2 phosphorylation and TGF-β pathway can increase the self-renewal of induced pluripotent stem cells [80]. Similar to other stem cell types, the addition of small molecules to culture media can enhance neuronal proliferation and differentiation [81]. Some of the other commercially available compounds can selectively inhibit p38 mitogen-activated protein kinase (MAPK), which may be an intrinsic negative regulator of NSC proliferation during early brain development resulting in neural stem cell proliferation [82]. Additionally, 1-Oleoyl lysophosphatidic acid (LPA) sodium salt, an agonist of LPA receptors, inhibits human ESCderived NSC differentiation [83]. Some of the P2Y receptor agonists maintaining neural stem cells in undifferentiated; proliferating, self-renewing state can be utilized for treatment of neurodegenerative diseases [84]. The list of such molecules utilized for maintaining pluripotency in laboratory is increasing, thus holding a lot of promise for these to be used in treatment of human diseases. |
| MS-818 as an Endogenous Stem Cell Proliferator |
| The currently available medications for neurodegenerative diseases only improve the symptoms and not treat the underlying disease. The fact that they show side effects in patients undergoing treatment calls for further refinement in the process of drug development. Different compounds including neurotransmission enhancers, anti-inflammation molecules, antioxidants, neurotropic factors, hormones have been utilized for developing promising therapies for neurodegenerative diseases [85]. Interestingly, different disease conditions, stressors, and the process of aging itself are known to negatively affect neurogenesis from endogenous NSCs highlighting the important roles played by endogenous factors [86]. So it is logical to target compounds resembling these factors for developing effective therapies. Several growth factors, peptides, and neurotransmitters including bFGF, TGFalpha, insulin-like growth factor-1, monoamine neurotransmitters, collagen peptides etc. have been shown to enhance neurogenesis [87-91]. However, their use as a pharmacological compound has always been limited by the stability and their ability to cross the BBB. This makes a case for developing compounds that are highly permeable to the BBB, stable, safe, and efficacious. Several small molecules including purine analogs, AMPA receptor potentiators, neuroleptins, P7C3 class of neuroprotective compounds, and lithium etc. have been reported to enhance neurogenesis in different animal models [92-96]. |
| We claimed in our patent (US 20110237574 A1), the application of MS-818’s (2-piperadino-6-methyl-5-oxo-5, 6-dihydro (7H) pyrrolo [2,3-d] pyrimidine maleate) ability to increase the stem cell number to be utilized for treatment of several human diseases. MS-818, a bicyclic pyrrolopyrimidine compound was originally synthesized in the late 1990’s by Awaya et al. [97] as an antimicrobial agent and later on shown to have neurotropic effects upon cultured human and mouse neuroblastoma cells, and upon nerve growth factor (NGF)-induced PC12 cell differentiation. Although initially thought to regulate neurite outgrowth, MS-818, since then has been shown to perform many important neural and non-neural functions in different animal models. |
| In our hands, MS-818 showed 7-fold increase in NSC population in the aged rats, which is much higher when compared with other aforementioned compounds [98]. To investigate the effect of MS-818 on proliferation of endogenous stem cells, MS-818 (3 mg/kg/day) was injected for 5 consecutive days into 27-month old rats, whereas control animals received the same amount of saline. Bromodeoxyuridine (BrdU) (100 mg/kg/day) was then injected for 3 days. The brains were removed after 24 hours of the last injection and fixed for immunohistochemical detection of the proliferating cells through BrdU staining. The number of BrdU positive cells increased more than seven folds in the cerebral cortices of MS-818-treated animals compared to controls, indicating an increased neural stem cell population in the brain. In the area of the subventricular zone, a significant increase not only in the proliferation but also in the migration of stem cells was found. |
| MS-818 plays a role in reduction of infarct size and amelioration of sensorimotor dysfunction as indicated by the results of forelimb and hindlimb placing tests following permanent focal cerebral ischemia in rats, thus making this small molecular compound a candidate for the treatment of focal cerebral ischemia [99]. It has been shown to enhance functional recovery of damaged sciatic nerves resulting from crush injury by promoting axonal sprouting through indirect activation of Schwann cells and possibly by activation of local production of nerve growth factors (NGFs) [100]. It promotes axonal survival by inhibiting cell death in a dose dependent manner and enhances the neurotrophic actions of bFGF through stimulation of signaling cascades that may increase MAPK levels within neurons [101]. MS-818 activates Schwann cells, which migrated from proximal stump inducing axonal elongation in vivo [102]. |
| Shimoda et al. [103] demonstrated dose-dependent suppression of UVB-induced increase in tumor necrosis factor alpha (TNF-alpha) that is one of the cytokines inducing apoptosis by MS-818. MS-818 protects epidermal cells from UVB-induced damage and also suppresses melanogenesis in B16 melanoma cells through downregulation of tyrosinase expression mediated by microphthalmia-associated transcription factor (MITF) and extracellular signal-regulated kinase (ERK) making it a strong contender for protective and whitening agent towards applications in cosmetics [103]. It has been shown to possess neurotrophic activity and to enhance basic fibroblast growth factor (bFGF)-induced angiogenesis in vivo [104]. MS-818 affects endothelial cells directly and induces migration of tubes and their formation by endothelial cells in vitro [105]. MS-818 also mobilizes endothelial progenitor cells from the bone marrow and potentiates their differentiation to endothelial cells. Thus, MS-818 promotes both angiogenesis and vasculogenesis [105]. Sugiyama et al. [106] explored MS-818’s effect on muscle regeneration and showed that the proliferation and differentiation of activated satellite cells and the fusion of myotubes to form immature myofibers were accelerated upon treatment with this compound [106]. MS-818 administered at a dose of 5 mg/kg intraperitoneally for 14 consecutive days promotes the fracture healing process in rat fracture model through enhancement of the effect of bFGF on endochondral ossification [107]. Its effect on in vivo gastric mucosal repair using a wound repair model with primary cultured gastric epithelial cells from rabbit was demonstrated by Watanabe et al. [108] MS-818 when administered alone had no effect but enhanced EGF-induced acceleration of gastric epithelial cell proliferation and migration at a dose of 10-100 μM [108]. |
| We demonstrated in our patent (US 20110237574 A1) MS-818’s role in increasing corneal and retinal stem cell number. Ophthalmic formulations when applied upon removal of lachrymal gland resulted in increased stem cell population in cornea in rat model. When 10 μg as one time injection of MS-818 was injected directly into the vitreous cavity of rat followed by BrDU administration, a dramatic increase in the number of BrdU-positive cells was seen in the retinal ciliary marginal zone after three days. Thus, MS-818 might find application in treatment of eye diseases, which affects millions in US and cost a fortune. The formulations we used for exploring MS-818’s role in endogenous stem cell proliferation in eye were found to be slightly acidic. We are currently investigating whether increasing the pH of formulation to physiological range by changing the salt would improve its permeability across BBB, eventually resulting in higher stem cell proliferation potential. Different biochemical and biophysical assays will be used to screen these formulations for their permeability, solubility, and stability over time. The exact mechanism of action is not yet known for MS-818; however, there are some reports on it acting through MAPK [105]. Table 1 summarizes its functions, model systems used, and possible mechanisms of action. The quick biological response shown by MS-818, and its IC50 being in nanomolar range possibly suggests some other mechanism of action. It could possibly be working through purinergic receptors, which play roles in proliferation and migration of neural stem cells, vascular reactivity, cytokine release, and apoptosis etc. It is also possible that MS-818 works through cytokine receptors, or through altering the gene expression. |
| Not only MS-818 but also any molecule that acts through any of the above mentioned mechanisms to proliferate the endogenous stem cell population could be a good candidate for a generalized stem cell proliferator to be utilized for treatment of neurodegenerative diseases. The studies performed in our laboratory show that MS-818 is highly permeable to the BBB and nontoxic even at very high dose. Our longterm goal is to develop oral pills of MS-818 as a stem cell proliferator. However, towards that goal its stability in highly acidic environment and adsorption in stomach have to be nailed down first. |
| Conclusions |
| The absence of effective treatment for neurodegenerative diseases put enormous monetary and emotional burden on the nation. A lot of progress has been made in the last few decades utilizing stem cell therapy and pharmacology towards that goal. Although these endeavors have been successful in improving the symptoms of these diseases; designing an effective stem cell therapy or medication specifically tailored for each patient taking in consideration his age, hereditary, stage of illness, and other complications will certainly keep scientists and physicians busy for quite some time. As the patients suffering from neurodegenerative diseases have not much time left after their diagnosis, finding the right cure for them would certainly require collaboration among people from different walks of life e.g. scientists, physicians, clinicians, medicinal chemists, pharmacologists etc. As a means to increase stem cell number, treatment with MS-818 would serve as a powerful tool of regenerative medicine. Increasing endogenous stem cell population through drug treatment can become the future treatment of choice. As different disease conditions affect the differentiation pattern of newly formed stem cells, this demands for a more in-depth monitoring of how the patient’s brain environment is affecting their neurogenesis. |
| Competing Interests |
| The authors declare that they have no competing interests. |
| Authors’ Contributions |
| SA contributed to the framework, composition, and writing of the review. KS contributed to the conception, composition and editing of the review. |
| Acknowledgements |
| We thank Stephanie Merchant for editing of the review. |
| References |
References
- Skovronsky DM, Lee VM, Trojanowski JQ (2006) Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol 1: 151-170.
- Bigler ED (2013) Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci 7: 395.
- De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV (2012) Alzheimer's disease. Subcell Biochem 65: 329-352.
- Shulman JM, De Jager PL, Feany MB (2011) Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol 6: 193-222.
- Labbadia J, Morimoto RI (2013) Huntington's disease: underlying molecular mechanisms and emerging concepts. Trends Biochem Sci 38: 378-385.
- Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10: S10-S17.
- Radi E, Formichi P, Battisti C, Federico A (2014) Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis 42: S125-S152.
- Berry MD, Boulton AA (2003) Apoptosis and human neurodegenerative diseases. Prog Neuropsychopharmacol Biol Psychiatry 27: 197-198.
- Cardinale A, Chiesa R, Sierks M (2014) Protein misfolding and neurodegenerative diseases. Int J Cell Biol 2014: 217371.
- Soto C (2003) Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4: 49-60.
- Uryu K, Haddix T, Robinson J, Nakashima-Yasuda H, Lee VM, et al. (2010) Burden of neurodegenerative diseases in a cohort of medical examiner subjects. J Forensic Sci 55: 642-645.
- Hebert LE, Beckett LA, Scherr PA, Evans DA (2001) Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord 15: 169-173.
- Crews L, Masliah E (2010) Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol Genet 19: R12-20.
- Querfurth HW, LaFerla FM (2010) Alzheimer's disease. N Engl J Med 362: 329-344.
- Tabet N (2006) Acetylcholinesterase inhibitors for Alzheimer's disease: anti-inflammatories in acetylcholine clothing! Age Ageing 35: 336-338.
- Robinson DM, Keating GM (2006) Memantine: a review of its use in Alzheimer's disease. Drugs 66: 1515-1534.
- Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, et al. (2001) A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis 8: 890-899.
- Obregon D, Hou H, Deng J, Giunta B, Tian J, et al. (2012) Soluble amyloid precursor protein-α modulates β-secretase activity and amyloid-β generation. Nat Commun 3: 777.
- Rogers K, Felsenstein KM, Hrdlicka L, Tu Z, Albayya F, et al. (2012) Modulation of γ-secretase by EVP-0015962 reduces amyloid deposition and behavioral deficits in Tg2576 mice. Mol Neurodegener 7: 61.
- Jia Q, Deng Y, Qing H (2014) Potential therapeutic strategies for Alzheimer's disease targeting or beyond β-amyloid: insights from clinical trials. Biomed Res Int 2014: 837157.
- Steele JW, Gandy S (2011) Apomorphine and Alzheimer Aβ: roles for regulated α cleavage, autophagy, and antioxidation? Ann Neurol 69: 221-225.
- Brunden KR, Trojanowski JQ, Lee VM (2009) Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nat Rev Drug Discov 8: 783-793.
- Brunden KR, Ballatore C, Crowe A, Smith AB 3rd, Lee VM, et al. (2010) Tau-directed drug discovery for Alzheimer's disease and related tauopathies: a focus on tau assembly inhibitors. Exp Neurol 223: 304-310.
- Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, et al. (2004) Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation 1: 24.
- Maynard CJ, Bush AI, Masters CL, Cappai R, Li QX (2005) Metals and amyloid-beta in Alzheimer's disease. Int J Exp Pathol 86: 147-159.
- Behl C (1999) Alzheimer's disease and oxidative stress: implications for novel therapeutic approaches. Prog Neurobiol 57: 301-323.
- Perry G, Cash AD, Smith MA (2002) Alzheimer Disease and Oxidative Stress. J Biomed Biotechnol 2: 120-123.
- Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F (2012) New pharmacological strategies for treatment of Alzheimer's disease: focus on disease modifying drugs. Br J Clin Pharmacol 73: 504-517.
- Beitz JM (2014) Parkinson's disease: a review. Front Biosci (Schol Ed) 6: 65-74.
- Zhang NY, Tang Z, Liu CW (2008) Alpha-Synuclein protofibrils inhibit 26 S proteasome-mediated protein degradation: understanding the cytotoxicity of protein protofibrils in neurodegenerative disease pathogenesis. J Biol Chem 283: 20288-20298.
- Connolly BS, Lang AE (2014) Pharmacological treatment of Parkinson disease: a review. JAMA 311: 1670-1683.
- Peppe A, Dambrosia JM, Chase TN (1993) Risk factors for motor response complications in L-dopa-treated parkinsonian patients. Adv Neurol 60: 698-702.
- Contin M, Riva R, Martinelli P, Albani F, Baruzzi A (1997) Relationship between levodopa concentration, dyskinesias, and motor effect in parkinsonian patients: a 3-year follow-up study. Clin Neuropharmacol 20: 409-418.
- Katzenschlager R, Sampaio C, Costa J, Lees A (2003) Anticholinergics for symptomatic management of Parkinson's disease. Cochrane Database Syst Rev CD003735.
- Teo KC, Ho SL (2013) Monoamine oxidase-B (MAO-B) inhibitors: implications for disease-modification in Parkinson's disease. Transl Neurodegener 2: 19.
- Khan TS (2012) Off spells and dyskinesias: pharmacologic management of motor complications. Cleve Clin J Med 79: S8-S13.
- Gaines KD, Hinson VK (2012) Adjunctive therapy in Parkinson's disease: the role of rasagiline. Neuropsychiatr Dis Treat 8: 285-294.
- Ruottinen HM, Rinne UK (1998) COMT inhibition in the treatment of Parkinson's disease. J Neurol 245: P25-P34.
- Haasio K (2010) Toxicology and safety of COMT inhibitors. Int Rev Neurobiol 95: 163-189.
- Ross CA, Tabrizi SJ (2011) Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 10: 83-98.
- Videnovic A (2013) Treatment of huntington disease. Curr Treat Options Neurol 15: 424-438.
- Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ (2011) Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol 7: 616-630.
- Roch-Torreilles I, Camu W, Hillaire-Buys D (2000) [Adverse efects of riluzole (Rilutek) in the treatment of amyotrophic lateral sclerosis]. Therapie 55: 303-312.
- Harris JA, Murphy JA (2011) Retigabine (ezogabine) as add-on therapy for partial-onset seizures: an update for clinicians. Ther Adv Chronic Dis 2: 371-376.
- Lindvall O, Kokaia Z, Martinez-Serrano A (2004) Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med 10: S42-S50.
- Dantuma E, Merchant S, Sugaya K (2010) Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res Ther 1: 37.
- Robertson JA (2001) Human embryonic stem cell research: ethical and legal issues. Nat Rev Genet 2: 74-78.
- Stadtfeld M, Hochedlinger K (2010) Induced pluripotency: history, mechanisms, and applications. Genes Dev 24: 2239-2263.
- Webb S (2009) iPS cell technology gains momentum in drug discovery. Nat Rev Drug Discov 8: 263-264.
- Sugaya K (2003) Stem cell strategies, future and beyond. Seishin Shinkeigaku Zasshi 105: 68-80.
- Björklund A, Lindvall O (2000) Cell replacement therapies for central nervous system disorders. Nat Neurosci 3: 537-544.
- Dietrich J, Kempermann G (2006) Role of endogenous neural stem cells in neurological disease and brain repair. Adv Exp Med Biol 557: 191-220.
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, et al. (2009) Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A 106: 13594-13599.
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM (2005) Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron 45: 675-688.
- Kwak YD, Brannen CL, Qu T, Kim HM, Dong X, et al. (2006) Amyloid precursor protein regulates differentiation of human neural stem cells. Stem Cells Dev 15: 381-389.
- Marutle A, Ohmitsu M, Nilbratt M, Greig NH, Nordberg A, et al. (2007) Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proc Natl Acad Sci U S A 104: 12506-12511.
- Sondell M, Lundborg G, Kanje M (1999) Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 19: 5731-5740.
- Binder DK, Scharfman HE (2004) Brain-derived neurotrophic factor. Growth Factors 22: 123-131.
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, et al. (2009) Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med 15: 331-337.
- Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24: 1217-1281.
- Bjorklund LM, Sánchez-Pernaute R, Chung S, Andersson T, Chen IY, et al. (2002) Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A 99: 2344-2349.
- Kitada M, Dezawa M (2012) Parkinson's disease and mesenchymal stem cells: potential for cell-based therapy. Parkinsons Dis 2012: 873706.
- Cai J, Hua F, Yuan L, Tang W, Lu J, et al. (2014) Potential therapeutic effects of neurotrophins for acute and chronic neurological diseases. Biomed Res Int 2014: 601084.
- Benraiss A, Goldman SA (2011) Cellular therapy and induced neuronal replacement for Huntington's disease. Neurotherapeutics 8: 577-590.
- Lunn JS, Sakowski SA, Hur J, Feldman EL (2011) Stem cell technology for neurodegenerative diseases. Ann Neurol 70: 353-361.
- Boillée S, Vande Velde C, Cleveland DW (2006) ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52: 39-59.
- Thonhoff JR, Ojeda L, Wu P (2009) Stem cell-derived motor neurons: applications and challenges in amyotrophic lateral sclerosis. Curr Stem Cell Res Ther 4: 178-199.
- Feng Z, Gao F (2012) Stem cell challenges in the treatment of neurodegenerative disease. CNS Neurosci Ther 18: 142-148.
- Barchet TM, Amiji MM (2009) Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert Opin Drug Deliv 6: 211-225.
- Lindvall O, Kokaia Z (2010) Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest 120: 29-40.
- Fang X, Huang Z, Zhou W, Wu Q, Sloan AE, et al. (2014) The zinc finger transcription factor ZFX is required for maintaining the tumorigenic potential of glioblastoma stem cells. Stem Cells 32: 2033-2047.
- Shineman DW, Fillit HM (2009) Meeting the unique challenges of drug discovery for neurodegenerative diseases. BMC Neurol 9: I1.
- Abbott NJ (2013) Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36: 437-449.
- Krol S (2012) Challenges in drug delivery to the brain: nature is against us. J Control Release 164: 145-155.
- Park K (2007) Nanotechnology: What it can do for drug delivery. J Control Release 120: 1-3.
- Neumann H, Neumann-Staubitz P (2010) Synthetic biology approaches in drug discovery and pharmaceutical biotechnology. Appl Microbiol Biotechnol 87: 75-86.
- Jung DW, Kim WH, Williams DR (2014) Reprogram or reboot: small molecule approaches for the production of induced pluripotent stem cells and direct cell reprogramming. ACS Chem Biol 9: 80-95.
- Pardridge WM (2012) Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 32: 1959-1972.
- Kirby LA, Schott JT, Noble BL, Mendez DC, Caseley PS, et al. (2012) Glycogen synthase kinase 3 (GSK3) inhibitor, SB-216763, promotes pluripotency in mouse embryonic stem cells. PLoS One 7: e39329.
- Hao J, Sawyer DB, Hatzopoulos AK, Hong CC (2011) Recent Progress on Chemical Biology of Pluripotent Stem Cell Self-renewal, Reprogramming and Cardiomyogenesis. Recent Pat Regen Med 1: 263-274.
- Lowry ER, Henderson CE (2014) Stem cell differentiation: yielding substrates for neurons. Nat Mater 13: 543-544.
- Graichen R, Xu X, Braam SR, Balakrishnan T, Norfiza S, et al. (2008) Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation 76: 357-370.
- Dottori M, Leung J, Turnley AM, Pébay A (2008) Lysophosphatidic acid inhibits neuronal differentiation of neural stem/progenitor cells derived from human embryonic stem cells. Stem Cells 26: 1146-1154.
- Lin JH, Takano T, Arcuino G, Wang X, Hu F, et al. (2007) Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol 302: 356-366.
- Rasool M, Malik A, Qureshi MS, Manan A, Pushparaj PN, et al. (2014) Recent updates in the treatment of neurodegenerative disorders using natural compounds. Evid Based Complement Alternat Med 2014: 979730.
- Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H (2010) When neurogenesis encounters aging and disease. Trends Neurosci 33: 569-579.
- Sun D, Bullock MR, McGinn MJ, Zhou Z, Altememi N, et al. (2009) Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol 216: 56-65.
- Guerra-Crespo M, Gleason D, Sistos A, Toosky T, Solaroglu I, et al. (2009) Transforming growth factor-alpha induces neurogenesis and behavioral improvement in a chronic stroke model. Neuroscience 160: 470-483.
- Carlson SW, Madathil SK, Sama DM, Gao X, Chen J, et al. (2014) Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. J Neuropathol Exp Neurol 73: 734-746.
- Berg DA, Belnoue L, Song H, Simon A (2013) Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 140: 2548-2561.
- Kakoi C, Udo H, Matsukawa T, Ohnuki K (2012) Collagen peptides enhance hippocampal neurogenesis and reduce anxiety related behavior in mice. Biomed Res 33: 273-279.
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, et al. (2010) Discovery of a proneurogenic, neuroprotective chemical. Cell 142: 39-51.
- Gysbers JW, Rathbone MP (1992) Guanosine enhances NGF-stimulated neurite outgrowth in PC12 cells. Neuroreport 3: 997-1000.
- O'Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES (2004) AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord 3: 181-194.
- Wakade CG, Mahadik SP, Waller JL, Chiu FC (2002) Atypical neuroleptics stimulate neurogenesis in adult rat brain. J Neurosci Res 69: 72-79.
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK (2000) Enhancement of hippocampal neurogenesis by lithium. J Neurochem 75: 1729-1734.
- Awaya A, Kobayashi H, Horikomi K, Tanaka S, Kabir AM, et al. (1993) Neurotropic pyrimidine heterocyclic compounds. I. The newly synthesized pyrimidine compounds promote neurite outgrowth of GOTO and neuro 2a neuroblastoma cell lines, and potentiate nerve growth factor (NGF)-induced neurite sprouting of PC 12 cells. Biol Pharm Bull 16: 248-253.
- Sugaya K and T Qu (2010) Use of modified pyrimidine compounds to promote stem cell migration and proliferation. US 7687505 B2.
- Mitsuyama T, Kawamata T, Yamane F, Awaya A, Hori T (2002) Role of a synthetic pyrimidine compound, MS-818, in reduction of infarct size and amelioration of sensorimotor dysfunction following permanent focal cerebral ischemia in rats. J Neurosurg 96: 1072-1076.
- Fukuyama R, Ohta M, Ohta K, Salwaki T, Fushiki S, et al. (2000) A synthesized pyrimidine compound, MS-818, promotes walking function recovery from crush injury of the sciatic nerve through its indirect stimulation of Schwann cells. Restor Neurol Neurosci 17: 9-16.
- Sanjo N, Owada K, Kobayashi T, Mizusawa H, Awaya A, et al. (1998) A novel neurotrophic pyrimidine compound MS-818 enhances neurotrophic effects of basic fibroblast growth factor. J Neurosci Res 54: 604-612.
- Torigoe K, Awaya A (1998) A newly synthesized neurotropic pyrimidine compound, MS-818, may activate migratory schwann cells in peripheral nerve regeneration. Brain Res 787: 337-340.
- Shimoda N, Mutou Y, Shimura N, Tsukimoto M, Awaya A, et al. (2010) Effect of heterocyclic pyrimidine compounds on UVB-induced cell damage in human keratinocytes and on melanogenesis in mouse B16 cells. Biol Pharm Bull 33: 862-868.
- Yasuhara S, Kashiwagi S, Ito H, Awaya A (1995) The neurotrophic pyrimidine heterocyclic compound MS-818 promotes the angiogenesis induced by basic FGF. Int J Clin Pharmacol Res 15: 167-174.
- Kanemura M, Abe M, Ueda M, Ueki M, Awaya A, et al. (2004) MS-818 accelerates mobilization of endothelial progenitor cells and differentiation to endothelial cells. Endothelium 11: 221-230.
- Sugiyama N, Yoshimura A, Fujitsuka C, Iwata H, Awaya A, et al. (2002) Acceleration by MS-818 of early muscle regeneration and enhanced muscle recovery after surgical transection. Muscle Nerve 25: 218-229.
- Yoshikawa M, Saura R, Mizuno K, Awaya A (2000) The effect of MS-818, newly synthesized pyrimidine compound, on fracture repair. Kobe J Med Sci 46: 265-282.
- Watanabe S, Wang XE, Hirose M, Osada T, Yoshizawa T, et al. (1998) A neurotrophic pyrimidine compound, MS-818, enhances EGF-induced restoration of gastric epithelial wounds in vitro. J Clin Gastroenterol 27: S105-S109.
- Jiang XM, Ohnishi A, Yamamoto T, Murai Y, Awaya A, et al. (1995) The effect of MS-818, a pyrimidine compound, on the regeneration of peripheral nerve fibers of mice after a crush injury. Acta Neuropathol 90: 130-134.
- Ohbayashi K, Inoue HK, Awaya A, Kobayashi S, Kohga H, et al. (1996) Peripheral nerve regeneration in a silicone tube: effect of collagen sponge prosthesis, laminin, and pyrimidine compound administration. Neurol Med Chir (Tokyo) 36: 428-433.
- Itoh S, Samejima H, Shinomiya K, Awaya A (1999) The effect of neurotrophic pyrimidine heterocyclic compounds, MS-818 and MS-430, on the regeneration of injured peripheral nerves. Restor Neurol Neurosci 14: 265-273.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 16481
- [From(publication date):
November-2014 - Jul 14, 2025] - Breakdown by view type
- HTML page views : 11687
- PDF downloads : 4794
