Status of Groundwater Quality by Analyses of Physico-Chemical Parameters: A Case Study of Tehsil-Pampore (J and K, India)
Received: 02-Feb-2023 / Manuscript No. JEE-23-88517 / Editor assigned: 06-Feb-2023 / PreQC No. JEE-23-88517 (PQ) / Reviewed: 20-Feb-2023 / QC No. JEE-23-88517 / Revised: 02-May-2024 / Manuscript No. JEE-23-88517 (R) / Published Date: 09-May-2024
Abstract
Water is an indispensable source for all living creatures whose quality depends on values of physicochemical parameters. Water is found in several forms, among which one of the most readily available is the underground water. But unfortunately the quality of same source is being deteriorated day by day because of the accelerated rate of dumping of several solid and liquid industrial, institutional, domestic, residential, agricultural and biomedical wastes inside the earth. The present paper briefs about the fifteen parameters of groundwater of three famous villages namely Ladhu, Khrew and Shar of Tehsil Pampore, district Pulwama (J and K, India) to find out its suitability for drinking and domestic purposes. The analysis of the parameters were done according to the prescribed standard procedure to reveal the values of pH, electrical conductivity, DO, free CO2, Ca2+ hardness, total hardness, alkalinity, chloride, TDS, Mg2+, nitrite-nitrogen, orthophosphate etc. The analysis of the water samples have shown that the average value of maximum parameters were lying within the maximum permissible limit of ISI and WHO. The soil of the study areas is loamy with little clay content but rich in lime with a high content of magnesia. The soil around the study sites also receives a good content of chemical fertilizers in addition to green manure and legume. During winter the strong winds bring snow and rain from Mediterranean depression.
Keywords: Physico-chemical; Groundwater; Water quality; Pampore; Chemical fertilizers
Introduction
Water is one of the most abundant resources on earth, covering three fourths of the planet’s surface. About 97% of the earth’s water is saline and is present in the oceans and 3% is fresh water contained in the poles (in the form of ice), ground water, lakes and rivers, which supply most of human and animal needs. Nearly, 70% of this tiny 3% of the world’s fresh water is frozen in glaciers, permanent snow cover, ice and permafrost. The other 30% of all fresh water is ground, most of it in deep, hard to reach aquifers. Lakes and rivers together contain just a little more than 0.25% of all freshwater where lakes contain most of it [1-3].
Fresh water is constantly recharged through a process known as the hydrological cycle. Ground water recharge is the process by which water percolates down the soil and reaches the water table, either by natural or artificial methods. The quantity and the rate of ground water recharge depend on efficiency of ground water resource management [4]. Rainfall is the principal source of groundwater recharge. The amount of moisture that will eventually reach the water table is defined as natural ground water recharge, which depends on the rate and duration of rainfall, the subsequent conditions at the upper boundary, the antecedent soil moisture conditions, the water table depth and the soil type [5].
Ground water is less susceptible to bacterial pollution than surface water because the soil and rocks through which ground water flows screen out most of the bacteria. Dumping of wastes near road sides, flood due to heavy rains, improper waste management in hospitals, markets, flats etc. increases the pollution of ground water [6].
Polluted groundwater is the major causes for the spread of diseases like typhoid, jaundice, dysentery, diarrheic, tuberculosis and hepatitis. In India almost 90% of the rural population depends on untreated ground water for potable water supplies. The quality of ground water is highly related with the local environmental and geological conditions [7].
Materials and Methods
Brief description about study sites
For the present work the groundwater samples from four villages of Pampore namely Ladhu, Khrew and Shar (district Pulwama, J and K India) have been taken for physico-chemical analysis. Bore well was selected as the source of sample from all study sites with depth of 15.24 m, 21.34 m and 18.29 m.
Ladhu: Lies at an elevation of 1590 m. The village is gifted with a lot of groundwater in the form of springs, bore wells and open wells. Due to open cast and underground mining the groundwater quality has been lowered to some extent.
Khrew: Lies at an elevation of 1607 m. The village also has good quantity of groundwater especially in the form of springs like Naarbal. Actually the village is known as Naag Shahar (Spring village) as there are almost 15 springs. The quality and flow of groundwater has been found impaired due to miming and water use in factories [8,9].
Shar: The average elevation is 1595 m. The groundwater sprouts at many places of the area due to the presence of aquifers, which have given birth to number of springs like Sonaraz. Groundwater is the main source of water supply for the area as at one site (Vastoorvan mountain) water sprinkles out without any machinery and technology and is supplied to the whole area for drinking and other purposes.
Sample collection: For the present work water samples were collected on monthly basis from January to March 2011. The collected samples thus put under analysis for different physicochemical parameters noteworthy electrical conductivity, free CO2, Ca++ hardness, alkalinity, chloride, TDS, Mg++ hardness, nitrite-nitrogen, orthophosphate etc. by adopting standard methods [10,11].
The analyses were done with the help of instruments such as graduated Celsius thermometer, digital pH meter, digital conductivity meter. Besides these some well-known methods such as Winkler, Golterman, Clymo methods have also been followed in addition to some well-defined procedures.
Results and Discussion
Analysis of physical and chemical characteristics of water helps us to predict the quality of water. It also determines the biological composition of an aquatic ecosystem [12,13]. The lower value of pH (6.94 units) was recorded at Ladhu in January that may be attributed to decomposition of organic matter which releases CO2 and other acidic compounds whereas the maximum value (8.1 units) was observed at Khrew in March that could be low rate of decomposition and presence of appreciable amounts of carbonates of calcium and magnesium. Similar results were observed by Brose SK and Bhave PV [14]. The minimum water temperature fluctuates from 8.1°C at Shar in January to a maximum of 10.2°C in both Khrew and Shar in March. The water conductivity varies from 210 μS cm-1 at Shar in February and March to a maximum of 430 μS cm-1 in Ladhu during January. Water attains conductivity due to the presence of ions like calcium ions whose fluctuation could be related with the biological decomposition of organic matter, influx of seepage and nutrients from the drainage basin etc. The current research shows the conformity with the observations recorded by Kumar SK, et al. [15].
The dissolved oxygen concentration values from 5.2 mg/l at Shar in March to 12.4 mg/l at Khrew in January. Since DO value was found in a narrow range for all sites, the lower value of DO at all three sites could be attributed mainly to the presence of turbidity of water and input of allochthonous materials in addition to the interception of sunlight by ground thus, limits the growth of phytoplanktons in wells and subsequently production of oxygen by autotrophs. The release of CO2 by organic matter seems to be the next reason for lower value of DO. The mean value of free CO2 in Ladhu, Khrew and Shar was found to be 19.3 mg/l, 222.66 mg/l and 24 mg/l respectively. The lower values were registered due to the presence of higher values of hydrogen ion concentration in the ground water. The CO2 react with water to form carbonic acid and thus results in the reduction of free CO2.
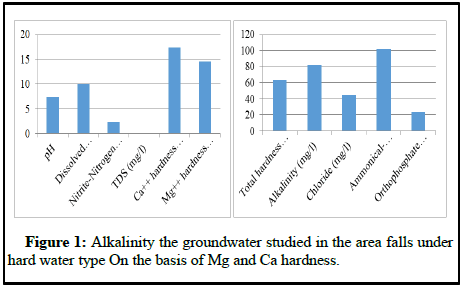
Maximum value of total alkalinity (224 mg/l) was recorded for Shar during the month of January whereas minimum value (18 mg/l) was observed from the same site during March. The higher value of pH could be the driving force for higher alkalinity in addition to the presence of carbonates and bicarbonates in ground water plus washing of excreta in the groundwater. The possible factors behind the higher values of total hardness could be mining of limestone and the richness of bedrock with lime. Other studies have shown likewise results, that calcium, magnesium in combination with carbonates, bicarbonates, sulphates and sulphites and other anions are responsible for total hardness of water. The maximum values of Mg hardness (16.65 mg/l) reported at Shar site owes its origin to the lacustrine deposits in the state. On the basis of Mg hardness, Ca hardness and total alkalinity the groundwater studied in the area falls under hard water type (Figure 1).
The chloride content ranges from 12.98 mg/l at Shar and Khrew to 66.99 mg/l at Ladhu. The lower and higher values could be attributed to the low and high level of animal origin pollution. The nitrite-nitrogen level showed a little variation on temporal and spatial basis. The low level may be due to tendency of rapid transition between nitrite ammonia and molecular nitrogen. Ammonical nitrogen concentration varies from 90 mg/l at Khrew to 105 mg/l at Ladhu which could be the result of decomposition of organic matter and drainage of agricultural wastes into the groundwater aquifers (Table 1). Orthophosphate concentration of maximum 50 mg/l was recorded at Khrew that might be the reason of drainage of agricultural wastes beneath the ground through rains.
| Sr. No. | Parameter | Observed range of samples | Site 1 (Ladhu) Mean ± SD | Site 2 (Khrew) Mean ± SD | Site 3 (Shar) Mean ± SD |
|---|---|---|---|---|---|
| 1 | pH (NTU) | 6.94-8.1 | 7.28 ± 0.37 | 7.6 ± 0.37 | 7.5 ± 0.13 |
| 2 | E. Conductivity (µScm-1) | 210-430 | 413.3 ± 12.47 | 224.3 ± 9.88 | 210.6 ± 0.94 |
| 3 | Depth (m) | 15.24-21.34 | 15.24 ± 0 | 21.34 ± 0 | 18.29 ± 0 |
| 4 | Temperature (°C) | 8.1-10.2 | 9.13 ± 0.77 | 9.0 ± 0.85 | 9.1 ± 0.86 |
| 5 | Dissolved Oxygen (mg/l) | 5.2-12.4 | 10.0 ± 0.33 | 9.7 ± 2.29 | 7.2 ± 1.42 |
| 6 | Free CO2 (mg/l) | 16-32 | 19.3 ± 0.94 | 22.6 ± 66.8 | 24 ± 3.26 |
| 7 | Ca2+ hardness (mg/l) | 8.41-42.89 | 17.37 ± 10.33 | 21.58 ± 12.33 | 21.58 ± 15.2 |
| 8 | Total hardness (mg/l) | 20-116 | 63.3 ± 26.20 | 50 ± 23.73 | 73.3 ± 38.86 |
| 9 | Alkalinity (mg/l) | 18-224 | 82.0 ± 80.65 | 71.3 ± 60.16 | 91.3 ± 93.98 |
| 10 | Chloride (mg/l) | 12.98-66.99 | 44.98 ± 21.40 | 14.98 ± 2.83 | 37.65 ± 20.42 |
| 11 | TDS (mg/l) | 0.04-0.12 | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.08 ± 0.03 |
| 12 | Mg2+ hardness (mg/l) | 2.40-16.65 | 14.49 ± 0.53 | 6.9 ± 3.2 | 10.9 ± 5.61 |
| 13 | Nitrite-nitrogen (µg/l) | 2.0-24 | 2.4 ± 0.32 | 4.5 ± 0.52 | 2.96 ± 0.2 |
| 14 | Ammonical-nitrogen (µg/l) | 90.0-105 | 102.3 ± 2.05 | 95.6 ± 64.2 | 95.43 ± 0.38 |
| 15 | Orthophosphate (µg/l) | 20-50 | 23.33 ± 2.36 | 44.33 ± 6.65 | 31.66 ± 2.35 |
Table 1: Parameter and observed range of samples are showing in Ladhu, Khrew and Shar sites.
Conclusion
From the above results and discussion, it can be concluded that the physical and chemical characteristic values of groundwater of the selected study sites lies within the prescribed standard limits of threshold level for portable water. So the water can be put to use for domestic and agricultural purposes.
References
- El-Dessouki HT, Ettouney HM (2002) Fundamentals of salt water desalination. 1st edition, Elsevier, Amsterdam, Netherland, 690.
- Eltawil MA, Zhengming Z, Yuan L (2009) A review of renewable energy technologies integrated with desalination systems. Renew Sust Energy Rev 13: 2245-2262.
- Kalogirou SA (2005) Seawater desalination using renewable energy sources. Prog Energy Combust Sci 31: 242-281.
- Mishra SP, Pandey SN (2008) Essential of environmental studies. 2nd edition, Ane Books Pvt. Ltd, New Delhi, India, 82-83.
- Garg RK, Rao RJ, Uchchariya D, Shukla G, Saksena DN (2010) Seasonal variations in water quality and major threats to Ramsagar reservoir, India. Afr J Environ Sci Technol 4: 61-76.
- Juahir H, Zain SM, Yusoff MK, Hanidza TT, Armi AM, et al. (2011) Spatial water quality assessment of Langat river basin (Malaysia) using environmetric techniques. Environmental Monit Assess 173: 625-641.
[Crossref] [Google Scholar] [PubMed]
- Golterman HL, Clymo RS (1969) Methods for physical and chemical analysis of freshwaters. Blackwell Scientific Publications, Oxford, UK, 166.
- American Public Health Association (1998) Standard methods for the examination of water and wastewater. 20th edition, American Public Health Association, Washington, USA.
- Bhardwaj V, Singh DS (2011) Surface and groundwater quality characterization of Deoria district, Ganga Plain, India. J Environ Earth Sci 63: 383-395.
- Gundogdu KS, Guney I (2007) Spatial analysis of groundwater levels using universal Kriging. J Earth Syst Sci 116: 49-55.
- Kumar SK, Rammohan V, Sahayam JD, Jeevanandam M (2009) Assessment of groundwater quality and hydrochemistry of Manimuktha river basin, Tamil Nadu, India. Environ Monit Assess 159: 341-351
[Crossref] [Google Scholar] [PubMed]
- Tadesse N, Bairu A, Bheemalingeswara K (2011) Suitability of groundwater quality for irrigation with reference to hand dug wells, Hantebet catchment, Tigray Northern Ethiopia. Momona Ethiop J Sci 3: 31-47.
- Zutshi DP, Subla BA, Khan MA, Wanganeo A (1980) Comparative limnology of nine lakes of Jammu and Kashmir, Himalaya. Hydrobiologia 72: 101-112.
- Solanki VR, Hussain MM, Raja SS (2010) Water quality assessment of Lake Pandu Bodhan, Andhra Pradesh state, India. Environ Monit Assess 163: 411-419.
[Crossref] [Google Scholar] [PubMed]
- Pradhan SK, Patnaik D, Rout SP (2001) Water quality index for the ground water around a phosphate fertilizer plant. Indian J Environ Prot 21: 355-358.
Citation: Bhat GR, Gul I (2024) Status of Groundwater Quality by Analyses of Physico-Chemical Parameters: A Case Study of Tehsil-Pampore (J and K, India). J Ecosyst Ecography 14: 516.
Copyright: © 2024 Bhat GR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 631
- [From(publication date): 0-2024 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 430
- PDF downloads: 201