Research Article Open Access
Statistical Evaluation of Ammonium Hydrofluoride Process for Beryllium Extraction from Indian Beryl Ore
B.M. Tripathi1*, Jyoti Prakash2, D.D. Thorat3 and D. Sathiyamoorthy41Powder Metallurgy Division, Bhabha Atomic Research Centre, Vashi Complex, Turbhe, Navi Mumbai 400705, India
2Powder Metallurgy Division, Bhabha Atomic Research Centre, Vashi Complex, Turbhe, Navi Mumbai 400705, India
3Powder Metallurgy Division, Bhabha Atomic Research Centre, Vashi Complex, Turbhe, Navi Mumbai 400705, India
4Powder Metallurgy Division, Bhabha Atomic Research Centre, Vashi Complex, Turbhe, Navi Mumbai 400705, India
- *Corresponding Author:
- D. Sathiyamoorthy
Head, Powder Metallurgy Division
Bhabha Atomic Research Centre, Vashi Complex
Turbhe, Navi Mumbai 400705, India
Tel: 25505010
E-mail: dsathiyamoorthy@gmail.com
Received Date: September 27, 2013; Accepted Date: November 20, 2013; Published Date: November 25, 2013
Citation: Tripathi BM, Prakash J, Thorat DD, Sathiyamoorthy D (2013) Statistical Evaluation of Ammonium Hydrofluoride Process for Beryllium Extraction from Indian Beryl Ore. J Powder Metall Min 2:118. doi: 10.4172/2168-9806.1000118
Copyright: © 2013 Tripathi BM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
Extraction of beryllium from Indian beryl ore using ammonium hydrofluoride as fluorinating agent was studied and optimized. This is process seems to be potentially promising for Indian beryl ore for extraction of beryllium. Four control factors, including first stage reaction temperature (T1), first stage reaction time (t1), second stage reaction temperature (T2) and second stage reaction time (t2), each in three levels have been anlysed using Taguchi technique. L9 orthogonal array and analysis of variance (ANOVA) were applied to determine the optimum conditions and the most significant factors affecting the overall beryllium recovery in the extraction process. The optimum extraction conditions to maximize beryllium recovery were T1=1500C, t1=60 min, T2=4000C and t2=60 min. In the verification extraction experiment, beryllium recovery was 97.7%. It was also found that first stage reaction temperature has the highest effect on beryllium recovery (75.15%). The other factors namely first stage reaction time, second stage reaction temperature and second stage reaction time could yield respectively 7.75%, 2.98% and 14.11% beryllium. The results were in close agreement with the prediction by the statistical model.
Keywords
Beryllium; Beryl ore; Beryllium extraction; Ammonium hydrofluoride; Optimization; Taguchi method
Introduction
The content of beryllium (Be) in the earth’s crust is ~2–6 μg g-1 [1]. There are about 30 minerals containing beryllium, the most significant of them being beryl (3BeO.Al2O3.6SiO2), phenacite (2BeO.SiO2), chrysoberyl (BeO.Al2O3) and bertrandite (4BeO.2SiO2.H2O) [2].
Properties such as high melting point (1285 ± 5°C) [2], low neutron absorption and high scattering cross sections make beryllium attractive for use in nuclear reactors as neutron reflector and moderator. Beryllium, because of its neutron multiplication properties by (n, 2n) reaction for high energy neutrons, coupled with low neutron damage in displacements per atom (dpa) [3], is considered as neutron multiplier material in the solid breeder blanket of the International Thermonuclear Experimental Reactor (ITER). Beryllium is also considered as plasma facing material (PFM) [3] in ITER.
Beryllium oxide (BeO) has highest thermal conductivity (300W/m-K) among the known ceramics, high melting point (2570°C) and high electrical resistivity. These properties make BeO a suitable crucible material for many melting and sintering operations [4].
Beryllium oxide based novel enhanced thermal conductivity fuels are under development for nuclear reactors in order to address heat related issues of conventional fuels arises because of relatively low thermal conductivity of uranium oxide.
Beryllium intermetallics, (particularly Be12Ti) are advanced material under consideration as neutron multiplier for fusion demonstration blanket [5]. Aluminium matrix-beryllium composites can find special applications in avionics, space related optical systems, structural components for satellites, propellants etc. These composites combine the high modulus and low density of beryllium with the favorable fracture toughness, ductility and fabrication characteristics of aluminum.
These ever increasing numbers of applications of beryllium and its compounds in various domains of science and technology stimulated us to develop an efficient method of beryllium extraction from its ore. In the present work, a potentially promising method of beryllium extraction from Indian beryl ore using ammonium hydrofluoride was studied and optimized for beryllium production.
Background
In India, beryllium is extracted from beryl ore. In its pure form, beryl ore is a beryllium-aluminium silicate (3BeO.Al2O3.6SiO2) [6].
Though a large number of methods have been proposed, only two are currently used for beryllium extraction from the ore. These are the sulphate and the fluoride process [6]. In the sulphate process, either molten beryl is water quenched or beryl is fused with alkali in the presence of sodium or potassium carbonate and then digested in sulphuric acid, followed by leaching with water. The aqueous leach liquor contains sulphates of aluminium and beryllium with other impurities. Silica remains as insoluble residue. After aluminium is removed by precipitating as alum, this purified leach liquor is generally subjected to solvent extraction to obtain beryllium bearing solution.
The fluoride route, on the other hand, involves sintering of the beryl ore with sodium silicofluoride or sodium ferric fluoride. The reagent selectively converts the beryllium value of the ore into water soluble sodium beryllium fluoride.
We have been working on development of a new method of beryllium extraction from Indian beryl ore using ammonium hydrofluoride as fluorinating agent. By the virtue of strong fluorinating capability, ammonium hydrofluoride decomposes beryl ore at low temperature by fluorination process. Subsequently, the beryllium value of the ore is recovered from the decomposition products. We have already studied preliminary aspects of ammonium hydrofluoride process of beryllium extraction including process feasibility, decomposition mechanism, kinetics, process flow sheet and advantages/disadvantages [7]. Although, there has not been any study on the optimization of the ammonium hydrofluoride process of beryllium extraction. Therefore, the objective of present research work was to investigate the simultaneous effects of the operating factors including first stage reaction temperature; first stage reaction time, second stage reaction temperature and second stage reaction time on beryllium extraction in the ammonium hydrofluoride process and determine the optimum extraction conditions to maximize beryllium recovery. This study will be an important progress to achieve better results, especially beryllium recovery in the extraction process.
In order to study the factors affecting beryllium recovery, the traditional experimental technique is to vary one factor at a time while keeping others constant. This conventional parametric design of experiment approach is time consuming [8] and requires enormous resources. However, a quantitative estimation of the various parameters affecting the beryllium recovery in extraction process can be determined by an optimization approach using the Taguchi method [9-11].
The Taguchi method uses a special design of orthogonal arrays [12] to study the entire parameters space with a small number of experiments only [8,13]. Taguchi design can determine the effect of factors on characteristic properties and the optimal conditions of factors [13]. This method is a simple and systematic approach to optimize design for process performance, quality and cost of products [8,10]. Moreover, there are two differences of this method from other statistical experimental design methods. First, parameters affecting an experiment can be investigated as the controlling and non-controlling (noise factor). Second, this method can be used to investigate the parameters for more than two levels [12].
Materials and Methods
Raw materials and apparatus
Ground beryl ore (-200 mesh) size was used in the extraction experiments. A typical chemical composition of Indian beryl ore [6] is given in Table 1. Ammonium hydrofluoride (NH4HF2), analytical grade was supplied by SD fine-chem Ltd., India. The samples for extraction experiments were prepared by mixing and grinding beryl ore and ammonium hydrofluoride using pestle and mortar in alumina crucibles. The samples were heated in an electrical furnace. After the extraction experiments, analysis of beryllium in the samples were carried out by JY-Horiba, France, ULTIMA 2C Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES).
| Component | (Wt.%) |
|---|---|
| BeO | 11-12 |
| Al2O3 | 19 |
| SiO2 | 64 |
| Alkalimetal oxides and minor amount of other oxides | 1-2 |
Table 1: A Typical composition of Indian beryl ore.
Experimental procedure
Extraction experiments in the ammonium hydrofluoride process were consisted of both pyrometallurgical and hydrometallurgical operations. In pyrometallurgical operation, all samples were prepared by thoroughly mixing beryl ore with ammonium hydrofluoride in alumina crucibles under identical conditions and the resulting mixture was heated in an electrical furnace. The quantity of NH4HF2 in the mixture was kept slightly above the stoichiometric value in all experiments. Heating of all the samples was performed in two steps. In the first step, the temperature and time was maintained in the range of 100-200°C and 30-90 min respectively. Similarly, in the second step, temperature and time was in the range of 300-500°C and 30-90 min respectively. During the heating process, beryl ore was sequentially decomposed by NH4HF2 and finally a mixture of beryllium fluoride (BeF2) and aluminium fluoride (AlF3) was obtained.
The BeF2 was separated from the final mixture by hydrometallurgical operation. In the hydrometallurgical operation, the final mixture (BeF2+AlF3) was subjected to water leaching at room temperature. By the virtue of high solubility of BeF2 and almost insolubility of AlF3 in water at room temperature, BeF2 was readily dissolved in water while AlF3 remained as residue during the leaching process.
Experimental design
Taguchi technique applies orthogonal arrays, to reduce the number of experiments and meanwhile obtaining statistically meaningful results. The selection of suitable orthogonal array depends on the number of control factors and their levels. By inspecting the results of our previous studies on ammonium hydrofluoride process of beryllium extraction, it was experienced that the recovery of beryllium in this process mainly depends on pyrometallurgical operation. The subsequent hydrometallurgical operation involving water leaching of BeF2 is relatively simple and straightforward because of high solubility of BeF2 and almost insoluble nature of AlF3 in water at room temperature. Therefore, only pyrometallurgical factors were selected for process optimization. The four selected control factors in three levels applied in this study are listed in Table 2. The aim was to maximize the beryllium recovery and also to determine the most significant factors affecting beryllium extraction. These are needed for the efficient beryllium recovery from beryl.
| Control factor | Units | Level 1 | Level 2 | Level 3 |
|---|---|---|---|---|
| (T1) First stage reaction temperature | °C | 100 | 150 | 200 |
| (t1) First stage reaction time | Min. | 30 | 60 | 90 |
| (T2) Second stage reaction temperature | °C | 300 | 400 | 500 |
| (t2) Second stage reaction time | Min. | 30 | 60 | 90 |
Table 2: Control factors and their values corresponding to their levels in extraction experiments.
In the classical experimental design using full factorial experimentation, the number of experiments to study the selected process space would be 34 = 81. With the selection of L9(34) orthogonal array the number of the required experiments can be drastically reduced to 9. The experimental plan and the results of beryllium extraction (%) in 9 extraction experiments are presented in Table 3.
| Control factors and their levels | Experimental Results | |||||
|---|---|---|---|---|---|---|
| Experiment No. | T1 | t1 | T2 | t2 | Beryllium extraction (%) | |
| 1 | 100 | 30 | 300 | 30 | 75.0 | |
| 2 | 100 | 60 | 400 | 60 | 84.5 | |
| 3 | 100 | 90 | 500 | 90 | 72.5 | |
| 4 | 150 | 30 | 400 | 90 | 88.5 | |
| 5 | 150 | 60 | 500 | 30 | 90.0 | |
| 6 | 150 | 90 | 300 | 60 | 94.4 | |
| 7 | 200 | 30 | 500 | 60 | 90.6 | |
| 8 | 200 | 60 | 300 | 90 | 91.7 | |
| 9 | 200 | 90 | 400 | 30 | 87.2 | |
Table 3: L9 (34) randomized experimental layout and experimental results.
Results and Discussion
Analysis of variance
Statistical analysis of the results obtained from 9 experiments, was performed to see whether the effect of process parameters are statistically significant or not as well as to justify the adequacy of the models. The relative effect of different factors can be obtained by the decomposition of total variation into its appropriate components, which is commonly called analysis of variance (ANOVA). The results of ANOVA analysis for beryllium extraction are presented in Tables 4,5. According to the percent contribution of factors, only first stage reaction temperature (75.74%) was the most significant effective parameter. The effects of other parameters including, first stage reaction time (7.18%) and second stage reaction time (14.26%) were marginal on beryllium extraction. The effect of second stage reaction temperature (2.82%) was almost negligible on beryllium extraction.
| Source | DOF | Sum of Squares | Variance | F Value | P Value | Contribution of factor (%) |
|---|---|---|---|---|---|---|
| Model | 8 | 453.38 | 56.67 | - | - | 100 |
| (T1) First stage reaction temperature | 2 | 343.40 | 171.7 | - | - | 75.74 |
| (t1) First stage reaction time | 2 | 32.53 | 16.27 | - | - | 7.18 |
| (T2) Second stage reaction temperature | 2 | 12.80 | 6.40 | - | - | 2.82 |
| (t2) Second stage reaction time | 2 | 64.64 | 32.32 | - | - | 14.26 |
| Error | 0 | 0.00 | - | - | - | |
| Total | 8 | 453.38 | 100 |
Table 4: Results of the analysis of variance (ANOVA) for the beryllium extraction.
| Source | DOF | Sum of Squares | Variance | F Value | P Value | Pure sum | Contribution of factor (%) |
|---|---|---|---|---|---|---|---|
| Model | 6 | 440.58 | 73.43 | 11.47 | 0.0824 | 402.18 | 88.70 |
| (T1) First stage reaction temperature | 2 | 343.4 | 171.7 | 26.83 | 0.0359 | 330.6 | 72.92 |
| (t1) First stage reaction time | 2 | 32.53 | 16.27 | 2.54 | 0.2825 | 19.73 | 4.35 |
| (T2) Second stage reaction temperature | (2) | (12.8) | - | Pooled | Pooled | - | - |
| (t2) Second stage reaction time | 2 | 64.64 | 32.32 | 5.05 | 0.1653 | 51.84 | 11.43 |
| (Error) | 2 | 12.8 | 6.4 | 11.30 | |||
| Total | 8 | 453.38 |
Table 5: Results of the pooled ANOVA for the beryllium extraction.
F value is a criterion for comparing model (or factors) variance with residual (error) variance (Table 4). If the variances are close to the same, the ratio will be close to 1and it is less likely that any of the factors have a significant effect on the response. F value is calculated by model variance divided by error variance. According to Table 4, error degree of freedom (DOF) is zero and the error variance could not be calculated. Consequently it was not possible to calculate the F value. In order to eliminate the zero DOF from the error term, a pooled ANOVA was applied. The values of the ANOVA analysis for beryllium extraction after pooling of the second stage reaction temperature from the model are given in Table 5. The model F value 11.47 implies the model was significant and there was only 8.24% chance that a model F value is greater than or equal to 11.47 and this large could occur due to noise. P values less than 0.1 indicate that the effect of model factors are significant within the 90% confidence interval. Therefore, only first stage reaction temperature was significant model term. The percent contribution reveals that the second stage reaction temperature is not statistically significant, at least in the range under study. The first stage reaction time (P = 0.2825 in Table 5) and second stage reaction time are also effective factors on beryllium extraction, but their statistical significance is marginal in 90% confidence interval (P = 0.1653 in Table 5). Meanwhile, Table 5 indicates that 88.7% of the total variation in the beryllium extraction was attributed to the studied experimental variables. Comparing the ANOVA result and pooled ANOVA result, indicated that after pooling the factor with least variance, the percent contributions of the remaining factors decreased slightly, but the ranking of factor effects still remained the same.
Level average analysis
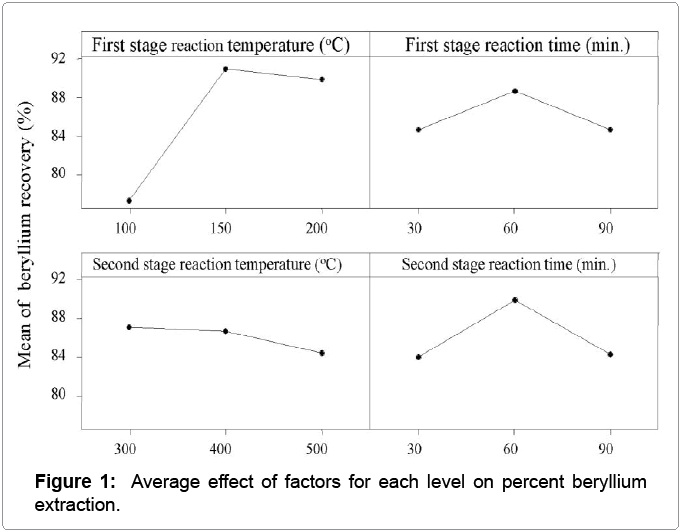
The degrees of influence of the parameters on the mean responses under study are given in Table 6 and also depicted in the main effect graph in Figure 1. The optimal level of a process parameter is the level with the highest mean. In terms of maximizing beryllium extraction in ammonium hydrofluoride process, the first stage reaction temperature of 150°C (Level 2), first stage reaction time of 60 min (Level 2), second stage reaction temperature of 400°C (Level 2) and second stage reaction time of 60 min (Level 2) were chosen.
| Process parameters | Beryllium recovery (%) | |||
|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | (Maximum-Minimum) | |
| (T1) First stage reaction temperature | 77.33 | 90.97 | 89.83 | 13.64 |
| (t1) First stage reaction time | 84.70 | 88.73 | 84.70 | 4.03 |
| (T2) Second stage reaction temperature | 87.03 | 86.73 | 84.37 | 2.67 |
| (t2) Second stage reaction time | 84.07 | 89.83 | 84.23 | 5.76 |
Table 6: Mean of beryllium recovery (%).
Confirmation experiment
The optimal levels of parameters for maximizing beryllium extraction are presented in Table 6. It can be seen that the experiments corresponding to optimum conditions for beryllium extraction is not included in L9 experimental plan given in Table 3. Therefore, another extraction experiment was carried out separately including the combination of parameter levels in which maximum amount of beryllium extraction (%) is proposed by the model. Based on last row of Table 7, the percent beryllium extraction under these conditions was 97.7%. The predicted amount of beryllium extraction proposed by the model under these conditions was 97.4%. As can be seen there is close agreement between the predicted and the observed results.
| Parameters | Units | Optimal levels of factor and verification experiment results |
|---|---|---|
| First stage reaction temperature | °C | 150 |
| First stage reaction time | min. | 60 |
| Second stage reaction temperature | °C | 400 |
| Second stage reaction time | min. | 60 |
| Predicted Be extraction | (%) | 97.4 |
| Observed Be extraction | (%) | 97.7 |
| Predicted confidence interval for Be extraction | (%) | 90.93-100 |
Table 7: Optimal levels of factors predicted by the model and verification experiment results.
Conclusion
The ammonium hydrofluoride process of beryllium extraction from Indian beryl ore was investigated over a range of experimental conditions. The effect of process parameters including first stage reaction temperature (T1), first stage reaction time (t1), second stage reaction temperature (T2) and second stage reaction time (t2) each in three levels, was studied with the Taguchi technique using an L9(34) orthogonal array. The percentage of beryllium extraction was optimized. Based on experimental results and their presented analysis, the following conclusions can be made:
• The first stage reaction temperature was the most significant parameter affecting the beryllium extraction from bery in the ammonium hydrofluoride process. Two factors including second stage reaction time and first stage reaction time, which are 2nd and 3rd ranking factors respectively, have marginal effect on beryllium extraction. The effect of second stage reaction temperature on beryllium extraction was almost negligible.
• In optimum conditions of operating parameters, second stage reaction temperature was not a statistically significant factor in the ammonium hydrofluoride process of beryllium extraction from beryl. This means that good amount of beryllium extraction could be achieved using optimum combination of other factors.
• The optimum extraction conditions for maximizing beryllium extraction were T1 = 150°C, t1 = 60 min, t2 = 60 min. Beryllium extraction of 97.4% was proposed as the optimum result by the statistical model. The result of the confirmation extraction experiment, 97.7% was in close agreement with the predicted results in the confidence interval of 90%.
• It was shown that the ammonium hydrofluoride process of beryllium extraction is an efficient method to recover berylliym from Indian beryl ore.
• It was concluded that Taguchi method can successfully be applied to the beryllium extraction from beryllium concentrates for maximizing beryllium recovery. The statistical model developed using the experimental results, was effective to navigate the design space of the process.
References
- Yamini Y, Hassan J, Mohandesi R, Bahramifar N (2002) Preconcentration of trace amounts of beryllium in water samples on octadecyl silica cartridges modified by quinalizarine and its determination with atomic absorption spectrometry. Talanta 56: 375-381.
- Zaki EE, Ismail ZH, Daoud JA, Aly HF (2005) Extraction equilibrium of beryllium and aluminium and recovery of beryllium from Egyptian beryl solution using CYANEX 921. Hydrometallurgy 80: 221–231.
- Argentina FS, Longhurst GR, Shestakov V, Kawamura H (2000) Beryllium R&D for fusion applications. Fusion Engineering and Design 51: 23–41.
- Saha S (1994a) Preparation of high purity beryllia and synthesis of alexandrite. Mineral Processing and Extractive Metallurgy Review 13: 69-75.
- Kawamura H, Takahashi H, Yoshida N, Shestakov V, Ito Y, et al. (2002) Application of beryllium intermetallic compounds to neutron multiplier of fusion blanket. Fusion Engineering and Design 61: 391-397.
- Saha S (1994b) Hydrometallurgy of beryllium in the Indian perspective. Mineral Processing and Extractive Metallurgy Review 13: 43–52.
- Thorat DD, Tripathi BM, Sathiyamoorthy D (2011) Extraction of beryllium from Indian beryl by ammonium hydrofluoride. Hydrometallurgy 109: 18-22.
- Demir F, Dönmez B (2008) Optimization of the dissolution of magnesite in citric acid solutions. Int J Miner Process 87: 60–64.
- Phadke, Madhav S (1989) Quality Engineering Using Robust Design, P T R Prentice-Hall, New Jersey.
- Roy RK (1995) A-Primer-on-the-Taguchi-Method, Van Nostrand Reinhold, New York.
- Roy RK (2003) Design of Experiments Using the Taguchi Approach- 16 Steps to Product and Process Improvement. Elsevier Science & Technology Books, New York.
- Abali Y, Copur M, Yavuz M (2006) Determination of the optimum conditions for dissolution of magnesite with H2SO4 solutions. Indian Journal of Chemical Technology 13: 391-397.
- Dehghan R, Noaparast M, Kolahdoozan M, Mousavi SM (2008) Statistical evaluation and optimization of factors affecting the leaching performance of a sphalerite concentrate. Int J Miner Process 89: 9-16.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 14430
- [From(publication date):
November-2013 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9824
- PDF downloads : 4606