Research Article Open Access
Standardization and Enrichment of Culture Medium Improve Detection of Group B Streptococci during Prepartum Screening
Leonardo Lodolo1, Cinzia Rossi1, Claudia Canale1, Michelangelo Barbaglia2, Giovanna Prandi3, Paola Ghiotti4, Iris Agreiter5, Leonardo Pagani5, Enrico Finale6,*, Nino Cappuccia1 and Andrea Guala21Department of Clinical Chemistry and Microbiology; Castelli Hospital, Verbania, Italy
2Department of Pediatrics, Castelli Hospital, Verbania, Italy
3Department of Neonatology, University of Turin, Italy
4Department of Health, Piedmont Region, Italy
5Division of Infectious Diseases, Bolzano Central Hospital, Italy
6Department of obstetrics and Gynecology, Castelli Hospital, Verbania, Italy
- *Corresponding Author:
- Enrico Finale
Department of Obstetrics and Gynaecology
“Giuseppe Castelli” Hospital
Via Crocetta 8, 28920, Verbania, Italy
Tel: +39-3930128648
Fax: +39-0323-541348
E-mail: enrico.finale@gmail.com
Received date August 27, 2014; Accepted date December 01, 2014; Published date December 03, 2014
Citation: Lodolo L, Rossi C, Canale C, Barbaglia M, Prandi G, et al. (2014) Standardization and Enrichment of Culture Medium Improve Detection of Group B Streptococci during Prepartum Screening. J Community Med Health Educ 4:319. doi: 10.4172/2161-0711.1000319
Copyright: © 2014 Lodolo L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
The identification and treatment of infections by the stereptococco group B (GBS), or Streptococcus agalactiae in pregnancy, are meant to prevent potential clinical disorders such as endometritis and neonatal sepsis is early onset (EOD) and late (LOD). The purpose of the study was to analyze the methods of collection and cultivation of GBS in all microbiology laboratories of two Italian regions, Piedmont and Valle d'Aosta, in search of a correlation that could explain the remarkable rate variability of outcomes of the test. The study team analyzed 28491 test from 70 public and private laboratories of microbiology, and crossing data with regional registries of births, there was a positive test by an average of 14.6%, range between 2.4% and 22.6%. Further analysis has revealed that the laboratories used an enrichment broth culture showed a positive test of the 15.49% of the cases (±95% CI: 14.78 to 16.23%; range 10.7 to 22.60%), while the samples analyzed without enrichment broth showed a positivity of 10.53% (±95% CI: 10 to 11.08%; range from 2.40 to 20%) (p<0.0000001). The results show that the breach of the guidelines of microbiology generate a high rate of false negatives and how the enrichment broth can be the gold standard for the culture of GBS during pregnancy.
Keywords
Group B streptococci; Prepartum Screening; Broth-enriched culture
Introduction
Many women are colonized in the genital and gastrointestinal tracts with Group B Streptococcus (GBS), or Streptococcus agalactiae, but remain free from symptoms [1]. However, pregnant women colonized with GBS are at increased risk to develop clinical disorders, such as urinary tract infections, endometritis and neonatal GBS sepsis, which occurs in two to four infants per 1000 live births [2,3]. The presentation of neonatal GBS diseases differs according to age at onset [4], with a bimodal distribution of cases by age, and two main syndromes have been recognized: early-onset disease (EOD) and late-onset disease (LOD) [5]. EOD usually occurs during labor or within the first few days after delivery and can affect the woman, the baby or both [1]. EOD is acquired from the mother by the ascending route in the uterus during labor or by direct contact at delivery. The clinical features are fulminant pneumonia, meningitis or sepsis. LOD can occur after the first week of life and may be acquired from the mother at birth or later from other individuals. It is characterized by bacteremia, meningitis and septic arthritis [6]. Krémery and Paradise observed GBS meningitis in LOD after 14 days of hospitalization in five of 101 cases [7].
Screening for GBS in pregnant women and targeted intrapartum antibiotic prophylaxis in whom identified as carriers are key elements to prevent EOD [8-10]. However, methods of specimen collection and analysis may affect the reliability of the results and subsequent treatment [11-13]. Too many cases of neonatal sepsis caused by GBS still occur in newborns of women not undergoing antibiotic prophylaxis: indeed, the lack of compliance with guidelines for specimen collection and culture may lead to false negative results.
In 2007, we performed GBS screening cultures on 28491 pregnant women of Piedmont and Aosta valley, and we found a rate of positive cultures which ranged between 2.4% and 22.6% in single laboratories. The purpose of the study was to investigate methods of GBS collection and culture in all the laboratories of these two Italian regions, in search of a correlation which could explain the considerable rate variability.
Material and Method
In 2007 all birth files were analyzed through a birth registration database. It was not necessary to seek the advice of the ethics committee because the study did not affect the normal care procedures.
Screening vaginal or vaginorectal swabs and culture results were performed at the 35th gestational week to all pregnant women who gave birth in Piedmont or Aosta Valley hospitals.
In spring 2008 we sent by post to all 70 public and private microbiological laboratories of Piedmont and Aosta Valley a file card regarding clinical tests performed in 2007 for GBS search in pregnancy and usually culture methods. The requested items for each laboratory were:
1.Sample technique and number of samples per year.
2.Typology of culture procedures: selective, chromogenic or broth.
3.Incubation atmosphere of cultures.
4.Identification systems.
5.Lay out of antibiotics sensitive tests.
6.Use of internal and external quality check.
7.Report standard: qualitative, half-quantitative, quantitative.
Four months after posting the file card, we performed a phone reminder to the laboratories which did not reply. A last reminder was posted two months later.
Results
We contacted 70 laboratories, 23 did not reply at all. Among the 47 responders, 16 provided partial results: five returned not interpretable data for the scope of research, four did not have a microbiology section, three laboratories sent all data except positivity percentage, and four only survey data, omitting answer to further inquiries; evaluable but partial data from these 16 labs were 6316.
Thirty-one labs provided all requested data, accounting for 22175 tests, 57.5% of the 38565 births recorded in Piedmont and Aosta Valley in 2007. Overall, 28491 (22175+6316) evaluable tests were collected: (24.36%) for vaginal, and (75.64%) for vaginorectal swabs. The average positivity percentage for GBS stands at 12.7% (CI ± 95%: 12.2-13.1) with a huge variability within each laboratory (range 2.4-22.6%). In 4183 cases (14.6%), GBS culture was positive.
During data analysis, a lack of homogeneity related to search methods for GBS in the different laboratories has been evidenced. In particular, reasons for such variability were:
1.Routine use of selective culture composed by Columbia agar + 5% blood + colistin-nalidixid acid (CNA), was related to 78% of the laboratories, accounting for 84% of swabs analyzed; 13% of laboratories utilized non selective culture with addition of 5% sheep blood (on 7% of total swabs); only 9% of the laboratories made use of chromogenic culture (9% of total swabs);
2.35% of the laboratories, which analyzed 44% of total swabs, resorted to broth enrichment over that time;
3.67% made use of CO2 to 10% in controlled atmosphere (on 75% of swabs); 24% of the laboratories (on 19% of swabs) utilized incubation in anaerobiosis; 9% of the laboratories used incubation in ambient atmosphere (on 6% of swabs).
The percentages of positivity detected, be compared with other data analyzed, showed significant variability, resulting in then use, or not, of the enrichment broth. This variability has allowed us to divide the laboratories, on the basis of three bands for positivity rate that are reported in the following three tables: (Tables 1-3).
| Lab. n° | (1) tipo | % vag. swabs | %vag./rect. swabsor vag+rectswabs | Total investig. | %pos | (2)Medium | Enrichm. broth | (3) Incubation atmosphere | (4)ID |
|---|---|---|---|---|---|---|---|---|---|
| 10 | a | 20% | 80% | 1731 | 4.39% | a + c | no | a | a |
| 18 | c | 0% | 100% | 945 | 6.90% | a | no | a | b |
| 24 | c | 81% | 19% | 420 | 7.38% | c + d | no | c | b |
| 30 | c | 32% | 68% | 296 | 5.84% | a | no | Data not received | |
| 31 | c | 32% | 68% | 135 | 6.30% | a | no | a | b |
| 37 | c | 81% | 19% | 415 | 6.40% | a | no | c | b |
| 43* | c | 0% | 100% | 415 | 2.40% | a + b | no | a | a + b |
| 57* | c | 97% | 3% | 667 | 2.00% | a + c | no | a | a + b |
| Tot. e% | 36% | 64% | 5024 | 4.95% | no 100% | ||||
| a: S.O.C. (Operational Structure Complex); b: S.O.S.(Operational Structure Simple) c: Sector of the general Lab. of Clinical pathology |
|||||||||
Table 1: Laboratories with positivity <10% in the investigation of screening.
| Lab. No. | (1) tipo | % vag. swabs | %vag./rect. swabsor vag+rect swabs | Total investig. | %pos | (2)Medium | Enricm. broth | (3) Incubation atmosphere | (4)ID |
|---|---|---|---|---|---|---|---|---|---|
| 1 | c | 95% | 5% | 1147 | 11.43% | a | yes | a | a |
| 4 | a | 75% | 25% | 1717 | 10.07% | a + c | yes | b | a + b |
| 8 | b | 0% | 100% | 23 | 14.60% | a | no | b | a + b |
| 9 | c | 0% | 100% | 2668 | 14.20% | a + c | no | a | a |
| 12 | b | 0% | 100% | 1009 | 12.80% | a | no | a | b |
| 15 | b | 0% | 100% | 876 | 14.27% | a + c | no | a | b |
| 17 | c | 0% | 100% | 946 | 14.00% | a | yes | a | b |
| 19 | c | 0% | 100% | 180 | 12.20% | a + c | no | a | a |
| 28 | b | 20% | 80% | 158 | 13.80% | a + c | yes | a | b |
| 38 | a | 0% | 100% | 800 | 11.00% | b | no | c | b |
| 41 | b | 46% | 54% | 435 | 12.00% | a | yes | a | a + b |
| 48* | c | 94% | 6% | 237 | 14.35% | b | yes | a | b |
| 49* | c | 75% | 25% | 112 | 12.00% | b | no | b | b |
| 67* | c | 91% | 9% | 110 | 10.00% | a | no | b | b |
| Tot. e% | 29% | 71% | 10418 | 12.61% | no 55.46% | ||||
| a: Blood agar Columbia CNA ; b: Chromogenic media c: Blood agar; d: other culture media |
|||||||||
Table 2: Laboratories with positivity between 10% and 15% in the investigation of screening.
| Lab. no | (1) tipo | % vag. swabs | %vag./rect. swabsor vag+rect swabs | Total investig. | % | Lab. no | (1) tipo | % vag. swabs | %vag./rect. swabs or vag+rect swabs |
|---|---|---|---|---|---|---|---|---|---|
| 6 | b | 99% | 1% | 683 | 15.60% | a | no | b | b |
| 11* | c | 0% | 100% | 200 | 20.00% | a | yes | a | a |
| 14 | b | 0% | 100% | 602 | 16.80% | a + c | no | b | a + b |
| 25 | c | 19% | 81% | 1263 | 19.00% | c | yes | a | a |
| 34 | b | 80% | 20% | 842 | 17.30% | c + d | yes | a | b |
| 42 | b | 6% | 94% | 1534 | 19.20% | a | yes | a | b |
| 55* | c | 0% | 100% | 490 | 20.00% | a | no | a | b |
| 61* | c | 87% | 13% | 145 | 22.60% | a | yes | a | a + b |
| 70 | c | 1% | 99% | 974 | 19.42% | a | yes | b | b |
| Tot. e% | 27% | 73% | 6733 | 20.05% | no 26.30% | ||||
| a-18-24 h incubation time at 35-37° C in CO2; b-18-24 h incubation time at 35-37° anaerobically c-18-24 h incubation time at 35-37° aerobically |
|||||||||
Table 3: Laboratories with positivity >15% in the investigation of screening.
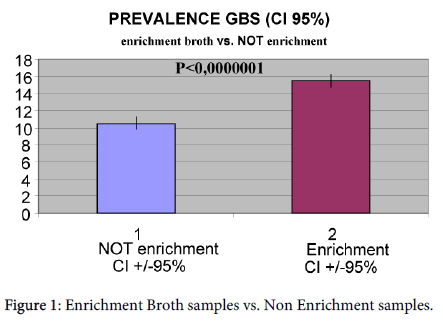
GBS cultures of laboratories which made use of broth enrichment had a GBS positivity of 15.49% (CI ± 95%: 14.78-16.23%; range 10.7-22.60%). Analyzed samples without broth enrichment showed a positivity of 10.53% (CI ± 95%: 10-11.08%; range 2.40-20%) (Figure 1).
The difference between the two methods was statistically significant (p<0.0000001) with χ2 test.
No significant statistical evidence was noted for other investigated indicators.
Conclusion
Prevention of EOD is primarily focused on the identification of risk factors and carrying out of cultural screening of women in childbirth [1] and on the efficacy of intrapartum antimicrobial prophylaxis thereafter [10].
The Centers for Disease Control and Prevention (CDC) developed consensus guidelines to reduce the rate of GBS neonatal disease [11]. These guidelines suggested two alternative approaches: the first concerned antibiotic prophylaxis for patients with one or more risk factors for GBS neonatal transmission; the second was based on universal screening of all pregnant women between 35 and 37 gestation weeks through vaginorectal cultures. The key element of a screening-based prevention strategy is a careful identification of colonized parturients who should then be targeted with intrapartum antimicrobial prophylaxis. Infants born to women prenatally identified as GBS carriers, indeed, had 29 times the risk of EOD than infants born to women with negative cultures. Prematurity, rupture of the membranes ≥18 hours before delivery and intrapartum fever were about seven times more likely to be complicated by EOD. The guidelines also suggested some modifications encouraging the use of antibiotics with narrower spectrum to reduce induction of resistance and to improve the predictive value of screening cultures.
To detect the incidence and existing policies for prevention of GBS infection in Europe, a questionnaire was sent to all members of the European Society for Paediatric Infectious Diseases and to all delegates of the European Association of Perinatal Medicine in 1999. The reported incidence of GBS colonization among pregnant women in Europe ranged from 1.5 to 30% [12].
The efficacy of cultural methods for GBS search is often dependent on several variables of accuracy operations which include
1.Period in which the survey was conducted. The most suitable to provide sensibility and specificity in detection women, who remain colonized until delivery is between 35th and 37th gestation week.
2.A preanalytical phase characterized by accuracy of sample collection. This can be carried out with only one swab, taking together vaginal and rectal specimens, or with two distinct swabs. Double swabbing improves the efficacy of microbiological survey. Such swabs, put in non-nutritive culture (Amies or Stuart’s) keep viability of GBS for four days at room temperature. Badri et al. demonstrated that the higher incidence of positive rectal swabs in comparison with vaginal cultures suggests that the gastrointestinal tract is the primary site of GBS colonization and vaginal colonization may represent a contamination [14].
3.Analytical phase, whose efficacy, in part conditioned by used materials, depends particularly on adherence to protocols, as well as accuracy of operators who conduct the microbiological survey. Utilizing one swab for both sample taking, or two swabs, one for each place, does not influence diagnostic efficacy of the survey. The site of GBS isolation does not represent significance for clinical management of an eventually positivity. For both swabs is it recommendable utilizing a single enrichment broth and selective culture, as both sites present a resident microbial population, whose growth could inhibit GBS, with consequent false negative results.
4.Post analytical phase, consisting in submitting GBS isolated strains to antimicrobial susceptibility testing.
Several studies have documented that the accuracy of prenatal screening cultures in identifying intrapartum colonization status can be enhanced by collection of cultures between 35 and 37 gestation weeks, swabbing the lower vagina and rectum and using selective enrichment broth to maximize the isolation of GBS [11].
A previous study by Vergani et al. [8] demonstrated that the optimal strategy to prevent GBS disease could not be established. They compared neonatal mortality and morbidity rates associated with EOD in three periods characterized by different prevention strategies. From 1/1987 to 12/1990, no screening for GBS during pregnancy, nor standardized chemoprophylaxis; from 1/1991 to 12/1994, antibiotic prophylaxis only with risk factors for GBS; from 1/1995 to 12/1999, universal screening for GBS with vaginorectal cultures and chemoprophylaxis for women with positive results or risk factors. In fact, the adoption of universal screening is associated with a reduction in rate of EOD compared with the risk factor policy. The results of this study show that the wide difference of results can be attributed to GBS culture technique, particularly to non-enriched broth. A false negative vaginal swab, therefore, can be misleading in intrapartum antibiotic prophylaxis decision making. Pinto et al. reported that from 92 infants with EOD, prenatal culture for GBS colonization was performed only in 22 women [15]. Of the 18 negative cultures for GBS, appropriate selective culture media had been used in only six patients and only three of these were obtained within six weeks before delivery. In 15 cases, GBS swabs were collected more than six weeks before delivery or obtained using suboptimal culture technique. Both the timing of specimen collection and a correct analytical process according to guidelines appear mandatory to improve the reliability of screening test.
A recent Italian experience by Berardi et al. pointed out that during the study period, 2003-2005, confirmed EOD occurred 30 times [16]. Maternal cultures were available for 22 cases, 17 screened mothers were GBS negative and five positive. During 2003, optimal culture methods were available from only one of 26 laboratories, but at the end of the study, the number raised to 12/26. It’s important to emphasize that among 17 screened negative mothers, culture methods were optimal in only one of 17 subjects.
In a recent study, Pulver et al. concluded that out of 45 women who delivered infants with EOD, 79% cultures obtained during routine prenatal care were negative [17]. The possible explanations for negative screens are attributable to inappropriately collection technique, but microbiological culture methods were not described. Many factors, such as improper conservation, culture technique, sample transportation and intermittent elimination of bacterium in carriers can influence the negativity of a recto-vaginal swab [18]. Van Dyke et al. highlighted that screening more than five weeks before childbirth, collection of specimens, processing of cultures, transient colonization of GBS, reporting and recording of screening results are part of factors which may contribute to false negative results [19].
Our results show how most of the “false negative” cultures are related to non-observance of microbiological guidelines, particularly as far as broth enrichment is concerned. Standardization of microbiological methods in the different realities is warranted, in order to get more comparable data, and to improve reliability of results.
New scenarios are now opening through the availability of rapid screening test based on NAAT (nucleic acid amplification test), whose preliminary results are very encouraging [20,21]. At this moment, however, broth-enriched culture of vaginorectal specimens as prepartum screening is still the golden standard for the prevention of neonatal GBS infections. Rapid NAAT methods can be an alternative during peripartum for women who did not undergo such a screening [22].
However, to date, it is not possible to come to a definitive conclusion, as you will need to perform a new study to demonstrate the difference in GBS culture positive by both methods in the same settings for the same laboratory conditions, as the same methodology of sample collection and the simultaneous treatment of the same.
It is also important to remember that, the development of vaccines against GBS in the next future could essentially modify the epidemiology of GBS carriers and the clinical approach to pregnant women and newborns.
References
- Schuchat A (1999) Group B streptococcus.Lancet 353: 51-56.
- Yancey MK, Duff P, Clark P, Kurtzer T, Frentzen BH, et al. (1994) Peripartum infection associated with vaginal group B streptococcal colonization.ObstetGynecol 84: 816-819.
- Regan JA, Klebanoff MA, Nugent RP, Eschenbach DA, Blackwelder WC, et al. (1996) Colonization with group B streptococci in pregnancy and adverse outcome. VIP Study Group.Am J ObstetGynecol 174: 1354-1360.
- Heath PT, Balfour G, Weisner AM, Efstratiou A, Lamagni TL, et al. (2004) Group B streptococcal disease in UK and Irish infants younger than 90 days.Lancet 363: 292-294.
- Edwards MS, Baker CJ (2005) Streptococcus agalactiae (group B streptococcus). Principles and practice of infectious diseases. (6th edn) Philadelphia Elsevier
- Sensini A, Tissi L, Verducci N, Orofino M, von Hunolstein C, et al. (1997) Carriage of group B Streptococcus in pregnant women and newborns: a 2-year study at Perugia General Hospital.ClinMicrobiol Infect 3: 324-328.
- Krcméry V, Paradisi F (2000) Nosocomial bacterial and fungal meningitis in children; an eight year national survey reporting 101 cases. Pediatric Nosocomial Meningitis Study Group.Int J Antimicrob Agents 15: 143-147.
- Vergani P, Patanè L, Colombo C, Borroni C, Giltri G, et al. (2002) Impact of different prevention strategies on neonatal group B streptococcal disease.Am J Perinatol 19: 341-348.
- Yancey MK, Schuchat A, Brown LK, Ventura VL, Markenson GR (1996) The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery.ObstetGynecol 88: 811-815.
- Baltimore RS (2007) Consequences of prophylaxis for group B streptococcal infections of the neonate.SeminPerinatol 31: 33-38.
- Centers for Disease Control and Prevention (2002) Prevention of perinatal group B streptococcal disease. Morbidity and Mortality Weekly Report 51: 1-22.
- Trijbels-Smeulders M, Smeulders M, De Jonge GA, Pasker-Dejonge PCM, Gerards LJ et al. (2004) Neonatal group B streptococcal infection: incidence and strategies for prevention in Europe. Pediatric Infectious Disease Journal 23: 172-173
- Centers for Disease Control and Prevention (1999). Laboratory practices for prenatal group B streptococcal screening and reporting. Connecticut, Georgia and Minnesota, 1997-1998. Morbidity and Mortality Weekly Report 48: 426-428
- Badri MS, Zawaneh S, Cruz AC, Mantilla G, Baer H, et al. (1977) Rectal colonization with group B streptococcus: relation to vaginal colonization of pregnant women.J Infect Dis 135: 308-312.
- Pinto NM, Soskolne EI, Pearlman MD, Faix RG (2003) Neonatal early-onset group B streptococcal disease in the era of intrapartum chemoprophylaxis: residual problems.J Perinatol 23: 265-271.
- Berardi A, Lugli L, Baronciani D, Creti R, Rossi K, et al. (2007) Group B streptococcal infections in a northern region of Italy.Pediatrics 120: e487-493.
- Pulver LS, Hopfenbeck MM, Young PC, Stoddard GJ, Korgenski K, et al. (2009) Continued early onset group B streptococcal infections in the era of intrapartum prophylaxis.J Perinatol 29: 20-25.
- Hansen SM, Uldbjerg N, Kilian M, Sørensen UB (2004) Dynamics of Streptococcus agalactiae colonization in women during and after pregnancy and in their infants.J ClinMicrobiol 42: 83-89.
- Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, et al. (2009) Evaluation of universal antenatal screening for group B streptococcus.N Engl J Med 360: 2626-2636.
- Edwards RK, Novak-Weekley SM, Koty PP, Davis T, Leeds LJ, et al. (2008) Rapid group B streptococci screening using a real-time polymerase chain reaction assay.ObstetGynecol 111: 1335-1341.
- Alfa MJ, Sepehri S, De Gagne P, Helawa M, Sandhu G, et al. (2010) Real-time PCR assay provides reliable assessment of intrapartum carriage of group B Streptococcus.J ClinMicrobiol 48: 3095-3099.
- Centers for Disease Control and Prevention(2010) Provisional recommendations for the prevention of perinatal group B Streptococcal disease.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 15282
- [From(publication date):
December-2014 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10746
- PDF downloads : 4536