Stability Studies of Ternary Mixtures Containing Fosaprepitant, Dexamethasone, Ondansetron and Granisetron Used in Clinical Practice
Received: 03-Feb-2016 / Accepted Date: 03-Mar-2016 / Published Date: 10-Mar-2016 DOI: 10.4172/2155-9872.1000307
Abstract
The use of a combination of 5HT3 receptor antagonist, a NK-1 receptor antagonist and dexamethasone has been classified to be state of the art in patients receiving highly as well as moderately emetogenic chemotherapy like cisplatin and anthracyclines. The administration of the ad-hoc admixture of fosaprepitant, dexamethasone and ondansetron (FDO) or granisetron (FDG) in the same IV infusion solution will improve the management of ambulatory procedures related to reducing administration time and number of administered intravenous preparations. All this would improve patient safety and comfort. In order to guarantee security of patients and efficacy of treatment, information about physico-chemical stability of both ternary mixtures at concentrations used in routine clinical practice and at different conditions of storage is needed. In this study, physico-chemical stability of ternary mixtures of fosaprepitant (150 mg), dexamethasone (8 mg) and ondansetron (8 mg) or granisetron (3 mg) in 50,100 and 250 ml of 0.9 g/dl NaCl at room temperature/refrigerated and protective from/exposed to light has been evaluated. An HPLC method has been developed and validated according to International Conference on Harmonization guidelines to evaluate chemical stability of drugs in mixtures simultaneously. Physical stability study has been carried out by visual inspection, pH measure and gravimetry to control evaporation. The results shown in this paper represent the first evidence of the physico-chemical stability of both ternary mixtures used in clinical practice at different conditions of storage. The ternary mixtures of FDG in 100 and 250 ml of 0.9 g/dl NaCl are physico-chemical stable for 15 days at room temperature and refrigerated and exposed to and protected from light; mixtures in 50 ml are physico-chemical stable for 6 days. The ternary mixtures of FDO in 50, 100 and 250 ml of 0.9 g/dl NaCl are physico-chemical stable for 15 days at both conditions of temperature and light.
Keywords: Physico-chemical stability; Fosaprepitant; Dexamethasone; Ondansetron; Granisetron; HPLC
303482Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a frequent and potentially treatment-limiting complication of cancer therapy, which is associated with a significant deterioration in quality of life. The emetogenicity depends on factors related to the drug, the combination of antineoplastic drugs administered pharmacotherapy scheme, as well as factors related to the patient. The temporal pattern of appearance of emesis after chemotherapy can be acute or late. Acute emesis occurs within the first 24 hours after chemotherapy; it is the most intense emesis and is related to the release of serotonin and 5-HT3 receptors. The late emesis happens after the first 24 hours post-chemotherapy, there is evidence that the mechanisms involved begin at 8 hours and related to substance P and the NK1 receptors. With the correct use of antiemetic drugs, CINV can be prevented in almost 70%, and even up to 80% of patients [1].
5-hydroxytryptamine 3 (5-HT3)- receptor antagonists are now the standard therapy for preventing CINV, because emesis is caused by stimulation of 5-HT3 receptors located on vagal afferents by serotonin released from enterochromaffin cells in the small intestine. The first-generation 5-HT3-receptor antagonists, ondansetron (OND), granisetron (GRA), dolasetron, and tropisetron, show considerable efficacy in preventing acute CINV, with acute responses for single agents ranging from 50% to 70%. However, acute responses are further increased when used in combination with the glucocorticoid dexamethasone (DEX) [2]. More recently, understanding the importance of the neurokinin-1 (NK-1) receptor in the emetic pathway in late emesis has led to the development of a new class of effective antiemetics, the NK-1 receptor antagonist (aprepitant, fosaprepitant (FOS)) [3].
According to currently available Multinational Association of Supportive Care in Cancer (MASCC), European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) guidelines, the use of a combination of 5HT3 receptor antagonist, a NK-1 receptor antagonist and DEX has been classified to be state of the art in patients receiving highly as well as moderately emetogenic chemotherapy [4,5]. In everyday clinical practice, and adhoc admixture of antiemetic drugs in the same IV infusion solution is often highly preferred to accelerate the management of ambulatory procedures, related to reducing administration time and number of administered intravenous preparations. All this would improve patient safety and comfort. So, physico-chemical stability data of ternary mixtures of 5HT3 receptor antagonist/NK-1 receptor antagonist/ DEX are needed before to avoid unexpected drug loss or even precipitation.
In data base Stabilis [6], information about physico-chemical stability of single solutions of FOS, DEX, GRA or OND and the binary mixtures DEX/OND and DEX/GRA is available. Furthermore, Sun et al. evaluate physical compatibility of ternary mixtures containing FOS, DEX and GRA (FDG) and FOS, DEX and OND (FDO) [3]. However, until now and to our knowledge, there are no published articles that evaluate chemical and physical stability of both ternary mixtures.
So, the development of an appropriately designed stability study, following the Pharmacopoeia guidelines and the recommendations of the International Committee on Harmonization (ICH), including chemical and physical stability, will allow know stability data of ternary mixtures of FDG or FDO at the concentration levels and storage conditions used in clinical practice [7-9].
Physical stability of drugs in mixture is usually evaluated by measurement of pH of mixture, visual inspection of colour changes, cloudiness (turbidity) and/or precipitation and gravimetry to analyze the water loss measurement [10,11]. Evaluation of chemical stability consists in quantifying concentration of each drug in mixture at different time in order to detect degradation of drugs; in this sense, High Performance Liquid Chromatography (HPLC) has been widely employed due to its high-resolution capacity, sensitivity and specificity [9]. In case of mixtures of more than one drug, the development of a chromatographic method that allows simultaneously quantify all drugs in mixtures should be carried out.
Therefore, the aim of this study was to determine the physicochemical stability of the ternary mixtures FDG and FDO in 50, 100 and 250 ml of 0.9 g/dl sodium chloride (NaCl) under different storage conditions of light and temperature.
Experimental
Instrumentation and chromatographic conditions
An Agilent Technologies 1100 liquid chromatograph with a quaternary pump, a diode array detector (DAD), a thermostated column compartment, an autosampler and a HP Compaq computer equipped with Agilent-Chemstation software was used. 10 μL of each solution was injected, by duplicate, into the chromatograph through a Rheodyne valve (Cotati, CA), with a 20 μ loop. Kromasil® C18 column of 5 μm particle size (250 × 4.6 mm inner diameter, Análisis Vínicos, Spain) was used. Mobile phase was ortophosphoric acid (0.1%)-acetonitrile (50:50, v/v); the flow rate was set to 0.8 ml/min, temperature to 20°C and detection to 254 nm. The column was equilibrated with mobile phase for 30 min prior to injection of the drug solution. Ortophosphoric acid and acetonitrile solutions were previously vacuum-filtered through 0.45 μm nylon membranes (Micron Separations, Westboro, MA) and sonicated prior to HPLC analysis.
A pH meter (model 3510, Jenway, UK) connected to a glass pHelectrode and an analytical balance (GF-200, A&D Instruments Ltd, UK) were used to measure the pH and weight, respectively.
Chemicals
For the preparation of mixtures, FOS (Ivemend® 150 mg; Merck Sharp & Dohme, Spain), DEX (Fortecortin® 40 mg/5 ml; Merck, Spain), OND (8 mg/4 ml; Normon, Spain) and GRA (3 mg/3 ml; Genéricos Españoles Laboratorios, Spain) were used. 0.9 g/dl NaCl intravenous infusion BP Viaflo® 50, 100 and 250 ml were purchased from Baxter (Spain), too.
Acetonitrile (Scharlab SL, Spain), ortophosphoric acid (Fluka Analytical, Sweden) and sterile water for injection (Grifols, Spain) were used to prepare the mobile phase used in chromatographic analysis.
Solutions preparation for calibration curves
For each drug, calibration curves were done with six standards prepared by making serial dilutions with 0.9 g/dl NaCl from commercial formulations from DEX, OND, GRA and FOS solution obtained after reconstitution of drug powder content in commercially vial (Ivemend®) with 5 ml of 0.9 g/dl NaCl. The concentration range assayed for each drug was: FOS, 0.15-4.00 mg/ml; DEX and OND, 0.016-0.300 mg/ml; GRA, 0.005-0.100 mg/ml.
Mixtures preparation and storage conditions
24 mixtures were prepared in the same way as those prepared for hospital clinical practice following the guidelines of “Pharmaceutical Compounding: Sterile Preparations” of the United States Pharmacopeia (USP) [12-14]. 12 mixtures contained 150 mg of FOS, 8 mg of DEX and 8 mg of OND in 50 ml (mixtures 1-4), 100 ml (mixtures 5-8) and 250 ml (mixtures 9-12) of 0.9 g/dl NaCl. 12 mixtures contained 150 mg of FOS, 8 mg of DEX and 3 mg of GRA in 50 ml (mixtures 13-16), 100 ml (mixtures 17-20) and 250 ml (mixtures 21-24) of 0.9 g/dl NaCl.
For mixtures with the same volume of NaCl, two mixtures were introduced in protective bags for ambient light (PL); one of them was stored at room temperature (27.6°C, IC95% 26.6 to 28.7°C; RT) and the other at 5.8°C (IC95% 3.4 to 8.1°C; F). The other two mixtures were exposed to light (L) and one was stored at RT and the other at F.
Time of study was 15 days, and the assays were performed every 24 hours, and in the first 24 hours were performed at 3 hours, 6 hours and 12 hours, too Tables 1 and 2 summarizes the storage conditions of each mixture assayed.
Chromatographic method validation
| Mixture | Storage condition | VNaCl (ml) | Concentration (mg/ml) | |||
|---|---|---|---|---|---|---|
| Light | T | FOS | DEX | OND | ||
| 1 | L | F | 50 | 3.000 | 0.160 | 0.160 |
| 2 | L | RT | ||||
| 3 | PL | F | ||||
| 4 | PL | RT | ||||
| 5 | L | F | 100 | 1.500 | 0.080 | 0.080 |
| 6 | L | RT | ||||
| 7 | PL | F | ||||
| 8 | PL | RT | ||||
| 9 | L | F | 250 | 0.600 | 0.032 | 0.032 |
| 10 | L | RT | ||||
| 11 | PL | F | ||||
| 12 | PL | RT | ||||
FOS: Fosaprepitant DEX: Dexamethasone OND: Ondansetron
T: Temperature L: Exposition to ambient light PL: Protection from light
F: Refrigerated RT: Room temperature
VNaCl: Volume of 0.9 g/dl NaCl
Table 1: Concentration and conditions of storage of mixtures of FDO.
| Mixture | Storage condition | VNaCl (ml) | Concentration (mg/ml) | |||
|---|---|---|---|---|---|---|
| Light | T | FOS | DEX | GRA | ||
| 13 | L | F | 50 | 3.000 | 0.160 | 0.060 |
| 14 | L | RT | ||||
| 15 | PL | F | ||||
| 16 | PL | RT | ||||
| 17 | L | F | 100 | 1.500 | 0.080 | 0.030 |
| 18 | L | RT | ||||
| 19 | PL | F | ||||
| 20 | PL | RT | ||||
| 21 | L | F | 250 | 0.600 | 0.032 | 0.012 |
| 22 | L | RT | ||||
| 23 | PL | F | ||||
| 24 | PL | RT | ||||
FOS: Fosaprepitant DEX: Dexamethasone GRA: Granisetron
T: Temperature L: Exposition to ambient light PL: Protection from light
F: Refrigerated RT: Room temperature
VNaCl: Volume of 0.9 g/dl NaCl
Table 2: Concentration and conditions of storage of mixtures of FDG.
The developed chromatographic method was validated with each drug for linearity, specificity, accuracy, precision, limit of detection and limit of quantification, in accordance with ICH guidelines [15]. The chromatograms were evaluated on the basis of the peak area of each drug. So, for each drug were evaluated the following parameters:
Linearity: The graph mean absorbance (y-axis) versus concentration (x-axis) was plotted and correlation coefficient (r), y-intercept and slope of regression line were estimated.
Specificity: The specificity of the method was ascertained by evaluating the presence of interferences at the retention time of drug.
Accuracy (% Recovery): The accuracy of the method was determined by calculating recoveries by method of standard additions; known amount of drug (0%, 50%, 100%, 150%) were added to a prequantified sample solution and was determined.
Method precision (Repeatability): Three standards of drug were analyzed six times and relative standard deviation (%RSD) was calculated for each concentration level.
Intermediate precision (Reproducibility): Intra-day precision was determined by analyzing three standards for three times in the same day and inter-day precision, by analyzing three standards daily for five days.
Limit of Detection (LOD) and Limit of Quantification (LOQ): LOD and LOQ were calculated using following equation: LOD=3·σ/S, LOQ=10·σ/S; where σ is the standard deviation of y-intercepts of regression lines and S is the slope of the calibration curve.
Physical and chemical stability assessment
Physical compatibility was evaluated daily by: (1) visual inspection of the mixtures for colour changes, cloudiness (turbidity) and/or precipitation. Incompatibility: appearance of some parameter; (2) loss of volume due to evaporation by gravimetry, weighting each mixture before and after extracting aliquot to HPLC analysis. Incompatibility: loss of weight ≥ 5%; (3) pH of mixtures, measured each two days in an aliquot of 2.5 ml removed from each mixture by inserting into the bag injection port, previous homogenization by double inversion. Incompatibility: variation of pH>20%.
Chemical stability was evaluated daily determining the concentration of each drug in the mixture by HPLC. For this purpose, each day, an aliquot of 150 μl was removed from each mixture, in the same way that for the analysis of pH. For each drug, the pair data concentration and time were adjusted, if it was possible, to a zero- (equation 1) or first-order kinetic equation (equation 2):
C = C0 – k0·t (equation 1)
ln C = ln C0 - k1·t (equation 2)
being C, drug concentration at a specific time; C0, drug concentration at t=0; k0, the zero-order degradation rate constant and k1, the first-order degradation rate constant.
For each drug in mixture, data were reported as a percentage compared with 100% (concentration determined just after preparation); the parameter T90 (time at witch remaining drug concentration is of 90%) was calculated by: (1) using equation 3 and 4, depending on the order of reaction or (2) if adjust to kinetic equation was not possible, it was considered the maximum time at which remaining drug concentration was ≥ 90%. Caducity of mixture was established by considering the lowest T90 value of the three drugs in the mixture, maximum study time (15 days).
T90 = 0.1·C0/ k0 (equation 3)
T90 = 0.105/ k1 (equation 4)
Results and Discussion
The present study evaluated the effects of concomitant dilution and storage of FOS with DEX as corticosteroid and OND or GRA as 5-HT3 antagonists. The reason for evaluating physical and chemical stability of the ternary mixtures FDO and FDG was based on the fact that: (1) ad hoc admixtures of these antiemetic drugs in the same IV infusion solution could alleviate the everyday clinical practice particularly in ambulatory settings; (2) both ternary mixtures may represent two potent antiemetic regimens for first-line treatment in case of highly emetogenic chemotherapy as well as salvage regimens in moderately emetogenic chemotherapy; (3) only compatibility information of both mixtures was publicated.
Optimization and validation of the chromatographic method
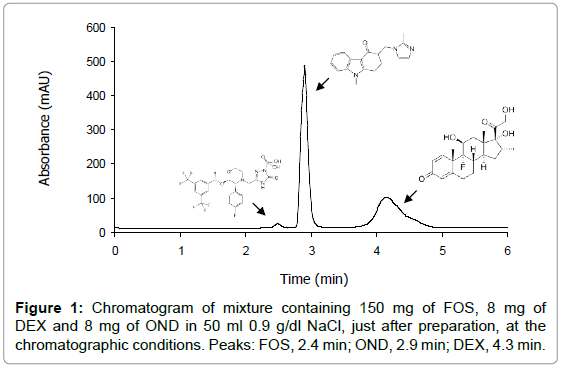
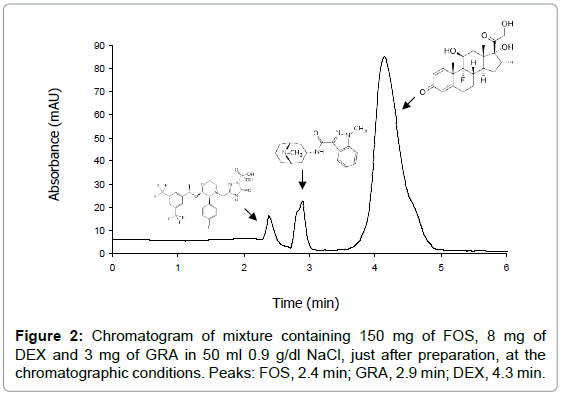
To optimize the chromatographic conditions a C18 HPLC column, ortophosphoric acid solution (0.1%) and acetonitrile mixture were found to be the best stationary phase and mobile phase combination to have symmetrical and well-resolved peaks of FOS, DEX and OND or GRA, simultaneously, in 0.9 g/dl NaCl mixtures. The same method was used to analyze both ternary mixtures. The total runtime for the analysis was 5 min and the retention times of drugs were: FOS, 2.4 min; OND, 2.9 min; GRA, 2.9 min and DEX, 4.3 min.
Chromatographic method validation
In the experimental conditions indicated, the analytical performance parameters suggested by ICH guidelines were evaluated: linearity, specificity, accuracy, method precision, intermediate precision, LOD and LOQ. Because of the simplicity of the procedure, no internal standard was needed. Table 3 shows the values of some of the parameters obtained for each drug. Furthermore, specificity was adequate since no interferences were observed at retention time of drugs. So, since all the criteria were acceptable according to ICH guidelines, the proposed chromatographic method was adequate to determine simultaneously FOS, DEX and OND or GRA in mixtures. Figures 1 and 2 show the chromatograms of mixtures of FDO and FDG in 50 ml of 0.9 g/dl NaCl, just after preparation.
| Parameters | FOS | DEX | OND | GRA |
|---|---|---|---|---|
| r | 0.9907 | 0.9984 | 0.9991 | 0.9999 |
| Accuracy (%) | 95.1-103.0 | 101.0-103.1 | 101.7-102.7 | 99.3-103.5 |
| Repeatability (%) | ≤ 1.9 | ≤ 6.0 | ≤ 3.2 | ≤ 4.1 |
| Intra-day precision (%) | ≤ 1.9 | ≤ 7.0 | ≤ 2.9 | ≤ 3.1 |
| Inter-day precision (%) | ≤ 4.0 | ≤ 6.0 | ≤ 5.6 | ≤ 6.2 |
| LOD (mg/ml) | 0.097 | 0.011 | 0.009 | 0.002 |
| LOQ (mg/ml) | 0.323 | 0.040 | 0.030 | 0.006 |
FOS:Fosaprepitant DEX: Dexamethasone GRA:Granisetron
OND:Ondansetron r: correlation coefficient
LOD: Limit of Detection
LOQ: Limit of Quantification
Table 3: Validation parameters of chromatographic method.
Physico-chemical stability assessment of mixtures containing FDO
At the end of the study, none of the mixtures (mixtures 1-12) showed changes in colour, precipitation or measurable losses of volume at the different storage conditions. So, all mixtures were physically stable and so, compatible, during the time of storage.
Table 4 shows initial medium pH values (pH0) of mixtures and variations of pH after 7 and 15 days of storage. In mixtures PL (mixtures 3-4, 7-8, 11-12) mean of pH variations were 0.28% (IC95%, -1.02% to 1.59%) at 7th day and 0.017% (IC95%, -1.404% to 1.438%) at 15th day; however, in mixtures exposed to L (mixtures 1-2, 5-6, 9-10), mean of pH variations were -6.22% (IC95%, -6.87% to -5.56%) at 7th day and -6.07% (IC95%, -6.74% to -5.40%) at 15th day, being the lowest pH achieved 7.1 what means a reduction of 3.4% respect to blood pH (7.35-7.45). In a previous study carried out by us about physico-chemical stability of FOS in mixtures [11], a maximum decrease in pH after 15 days of storage of 13.6% was observed, being the lowest pH achieved 7.0, too; it was explained by considering the flow of CO2 through polyolefin bag and the consequent acidification of solutions and no FOS degradation.
| Mixture | VNaCl (ml) | Storage condition | pH0 | Variation of pH (%) | ||
|---|---|---|---|---|---|---|
| Light | T | t7 | t15 | |||
| 1 | 50 | L | F | 7.39 ± 0.27 | -5.7 | -5.1 |
| 2 | L | RT | -6.9 | -5.7 | ||
| 3 | PL | F | +1.4 | +2.1 | ||
| 4 | PL | RT | +2.4 | +2.5 | ||
| 5 | 100 | L | F | 7.32 ± 0.24 | -4.9 | -5.6 |
| 6 | L | RT | -6.1 | -5.9 | ||
| 7 | PL | F | +1.3 | -1.1 | ||
| 8 | PL | RT | -1.0 | -1.0 | ||
| 9 | 250 | L | F | 7.16 ± 0.22 | -6.7 | -7.4 |
| 10 | L | RT | -7.0 | -6.7 | ||
| 11 | PL | F | -0.7 | -1.1 | ||
| 12 | PL | RT | -1.7 | -1.3 | ||
| 13 | 50 | L | F | 8.94 ± 0.05 | -7.9 | -8.1 |
| 14 | L | RT | -9.2 | -8.8 | ||
| 15 | PL | F | -8.3 | -7.9 | ||
| 16 | PL | RT | -7.8 | -7.9 | ||
| 17 | 100 | L | F | 8.89 ± 0.04 | -10.3 | -10.3 |
| 18 | L | RT | -10.9 | -11.0 | ||
| 19 | PL | F | -10.7 | -9.6 | ||
| 20 | PL | RT | -11.5 | -12.6 | ||
| 21 | 250 | L | F | 8.55 ± 0.04 | -14.2 | -16.6 |
| 22 | L | RT | -16.1 | -16.5 | ||
| 23 | PL | F | -15.7 | -16.1 | ||
| 24 | PL | RT | -17.8 | -16.6 | ||
pH0: mean initial pH ± standard deviation
t7: 7 days of storage
t15: 15 days of storage
Table 4: pH0 and variation of pH of mixtures after 7 and 15 days of storage.
Table 5 shows the percentage of remaining drugs concentration (%RC) at the different conditions of storage at day 7 and 15. As can be observed, in all mixtures, %RC of FDO were ≥ 90% after 15 days of storage; so, it seems to be that temperature, light and drug concentration do not affect FDO chemical stability. Table 6 shows the T90 values obtained from kinetic adjust and experimental value, being the last one the time at which %RC of all three drugs in the mixture were ≥ 90%; experimental value was in all cases of 15 days.
| Mixture | %RC | |||||
|---|---|---|---|---|---|---|
| FOS | DEX | OND | ||||
| t7 | t15 | t7 | t15 | t7 | t15 | |
| 1 | 99.00 ± 3.00 | 99.00 ± 0.70 | 102.50 ± 0.50 | 100.00 ± 0.06 | 95.10 ± 0.30 | 96.00 ± 0.90 |
| 2 | 100.10 ± 0.30 | 97.80 ± 1.70 | 102.50 ± 1.50 | 100.50 ± 0.13 | 98.00 ± 1.30 | 97.00 ± 0.90 |
| 3 | 98.30 ± 0.60 | 96.40 ± 0.90 | 96.06 ± 1.22 | 98.00 ± 0.50 | 92.50 ± 0.23 | 95.20 ± 0.90 |
| 4 | 93.10 ± 0.50 | 92.30 ± 0.90 | 94.00 ± 5.00 | 98.30 ± 2.50 | 96.70 ± 0.30 | 99.60 ± 1.90 |
| 5 | 100.20 ± 0.60 | 97.60 ± 1.70 | 97.30 ± 2.50 | 99.70 ± 0.14 | 99.20 ± 1.02 | 99.10 ± 0.21 |
| 6 | 97.00 ± 5.00 | 97.50 ± 1.40 | 100.70 ± 0.30 | 99.90 ± 0.30 | 99.30 ± 0.50 | 99.40 ± 1.00 |
| 7 | 97.00 ± 6.00 | 95.00 ± 6.00 | 98.00 ± 0.03 | 96.30 ± 0.40 | 98.70 ± 0.90 | 98.10 ± 0.40 |
| 8 | 99.40 ± 0.15 | 99.30 ± 0.50 | 98.60 ± 0.90 | 97.80 ± 0.50 | 98.00 ± 3.00 | 95.70 ± 0.20 |
| 9 | 98.00 ± 3.00 | 99.10 ± 1.09 | 99.10 ± 0.80 | 98.70 ± 0.90 | 99.10 ± 1.50 | 96.90 ± 1.30 |
| 10 | 100.70 ± 1.00 | 90.00 ± 3.00 | 97.50 ± 0.10 | 97.70 ± 0.30 | 99.70 ± 0.50 | 99.60 ± 0.60 |
| 11 | 96.50 ± 0.90 | 96.60 ± 0.80 | 98.10 ± 1.12 | 96.80 ± 0.80 | 94.80 ± 0.40 | 93.60 ± 0.30 |
| 12 | 96.40 ± 1.15 | 96.30 ± 0.30 | 93.00 ± 1.19 | 94.30 ± 1.17 | 92.00 ± 5.00 | 91.90 ± 0.90 |
FOS: Fosaprepitant DEX: Dexamethasone OND: Ondansetron
%RC: Percentage of remaining drug concentration ± standard deviation
t7: 7 days of storage
t15: 15 days of storage
Table 5: Percentage of remaining concentrations of FDO in mixtures at day 7 and 15 of storage.
| Mixture | T90 (days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| FOS | DEX | OND | |||||||
| Adj | Exp | Adj | Exp | Adj | Exp | ||||
| O0 | O1 | O0 | O1 | O0 | O1 | ||||
| 1 | - | - | 15 | - | - | 15 | 27 | 27 | 15 |
| 2 | - | - | 15 | - | - | 15 | 53 | 55 | 15 |
| 3 | 28 | 29 | 15 | 45 | 70 | 15 | 15 | 15 | 15 |
| 4 | 12 | 12 | 15 | - | - | 15 | - | - | 15 |
| 5 | - | - | 15 | - | - | 15 | - | - | 15 |
| 6 | - | - | 15 | - | - | 15 | - | - | 15 |
| 7 | - | - | 15 | - | - | 15 | - | - | 15 |
| 8 | - | - | 15 | - | - | 15 | 22 | 22 | 15 |
| 9 | - | - | 15 | - | - | 15 | - | - | 15 |
| 10 | 19 | 20 | 15 | 51 | 53 | 15 | - | - | 15 |
| 11 | - | - | 15 | - | - | 15 | - | - | 15 |
| 12 | - | - | 15 | 17 | 17 | 15 | - | - | 15 |
FOS: Fosaprepitant DEX: Dexamethasone OND: Ondansetron
Adj: value obtained from kinetic adjust
Exp: experimental time at which %RC was ≥ 90%
O0: zero-order kinetic adjust
O1: first-order kinetic adjust
%RC: Percentage of remaining drug concentration
Table 6: T90 values for mixtures containing FDO assayed.
To sum up, mixtures containing 150 mg of FOS, 8 mg of DEX and 8 mg of OND in 50, 100 and 250 ml of 0.9 g/dl NaCl were physicochemical stable for 15 days at different conditions of light (PL and L) and temperature (RT and F).
Physico-chemical stability assessment of mixtures containing FDG
At the end of the study, none of the mixtures (mixtures 13-24) showed changes in colour or measurable losses of volume at the different storage conditions. Only in mixtures 13 and 16, precipitate was observed by visual inspection after 7 days of storage. So, mixture 13 and 16 were physically compatible for 6 days while the rest of mixtures were compatible for 15 days.
As can be observed in Table 4, pH0 mean values were higher than pH of mixtures containing FDO. Furthermore, variation of pH with time was highest and increased with increasing 0.9 g/dl NaCl volume (Table 7), being the lowest pH achieved 7.0. As has been commented for mixture FDO, acidification could be a consequence of the flow of CO2 through polyolefin bag and in mixtures with highest drugs concentrations (mixtures 13-16) it could have provoked precipitation.
| Mixture | T90 (days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FOS | DEX | GRA | |||||||||
| Adj | Exp | Adj | Exp | Adj | Exp | ||||||
| O0 | O1 | O0 | O1 | O0 | O1 | ||||||
| 13 | - | - | 15 | - | - | 15 | - | - | 15 | ||
| 14 | - | - | 15 | - | - | 15 | - | - | 15 | ||
| 15 | - | - | 15 | - | - | 15 | - | - | 15 | ||
| 16 | - | - | 15 | - | - | 15 | - | - | 15 | ||
| 17 | - | - | 15 | - | - | 15 | - | - | 15 | ||
| 18 | - | - | 15 | - | - | 15 | - | - | 15 | ||
| 19 | - | - | 15 | - | - | 15 | - | - | 15 | ||
| 20 | - | - | - | - | - | 15 | - | - | 15 | ||
| 21 | - | - | 15 | - | - | 15 | - | - | 15 | ||
| 22 | - | - | 15 | - | - | 15 | - | - | 15 | ||
| 23 | - | - | 15 | - | - | 15 | - | - | 15 | ||
| 24 | - | - | 15 | - | - | 15 | - | - | 15 | ||
FOS:Fosaprepitant DEX: Dexamethasone GRA: Granisetron
Adj: value obtained from kinetic adjust
Exp: experimental time at which %RC was ≥ 90%
O0: zero-order kinetic adjust
O1: first-order kinetic adjust
%RC: Percentage of remaining drug concentration
Table 7: T90 values for mixtures containing FDG assayed.
Regards chemical stability, after 15 days of storage, %RC of all drugs were ≥ 93.5% for all mixtures (Table 8). Kinetic adjust was not possible in mixtures and experimental T90 values was in all cases of 15 days (Table 9).
| Time | Mean variation pH (%) (IC95%) | ||
|---|---|---|---|
| Mixtures 13-16 | Mixtures 17-20 | Mixtures 21-24 | |
| t7 | -8.30 (-8.92 to -7.68) | -10.85 (-11.34 to -10.36) | -15.95 (-17.40 to -14.50) |
| t15 | -8.18 (-8.59 to -7.76) | -10.88 (-12.13 to -9.62) | -16.45 (-16.68 to -16.22) |
t7: 7 days of storage
t15: 15 days of storage
IC95%: Confidence interval at 95%
Table 8: Mean variation of pH for mixtures containing FDG after 7 and 15 days of storage.
| Mixture | %RC | |||||
|---|---|---|---|---|---|---|
| FOS | DEX | GRA | ||||
| t7 | t15 | t7 | t15 | t7 | t15 | |
| 13 | 98.9 ± 1.30 | 99.7 ± 0.40 | 99.7 ± 0.30 | 97.8 ± 2.40 | 99.8 ± 0.16 | 98.9 ± 0.90 |
| 14 | 98.4 ± 0.50 | 98.8 ± 1.00 | 100.3 ± 0.80 | 99.8 ± 0.13 | 98.7 ± 0.30 | 98.8 ± 0.70 |
| 15 | 99.4 ± 0.70 | 93.5 ± 0.50 | 99.5 ± 1.21 | 98.7 ± 0.10 | 98.2 ± 2.00 | 98.7 ± 2.30 |
| 16 | 97.1 ± 1.80 | 98.3 ± 0.80 | 102.2 ± 0.70 | 101. ± 0.10 | 100.3 ± 0.70 | 100.5 ± 0.60 |
| 17 | 98.6 ± 0.04 | 99.8 ± 0.30 | 99.8 ± 1.40 | 99.6 ± 0.30 | 98.9 ± 0.70 | 98.5 ± 0.40 |
| 18 | 98.4 ± 0.00 | 97.3 ± 1.50 | 99.7 ± 0.05 | 98.6 ± 0.50 | 98.7 ± 0.30 | 99.5 ± 0.19 |
| 19 | 99.1 ± 1.09 | 99.2 ± 0.21 | 99.4 ± 1.60 | 98.9 ± 0.40 | 94.1 ± 1.30 | 94.8 ± 1.60 |
| 20 | 102.0 ± 3.00 | 98.0 ± 1.02 | 101.4 ± 0.30 | 99.4 ± 1.00 | 100.0 ± 4.00 | 94.2 ± 0.12 |
| 21 | 97.0 ± 6.00 | 99.6 ± 1.50 | 99.8 ± 1.40 | 99.6 ± 0.30 | 98.6 ± 1.70 | 97.9 ± 1.30 |
| 22 | 100.2 ± 0.30 | 99.4 ± 2.00 | 98.0 ± 40 | 101.0 ± 4.0 | 96.0 ± 3.00 | 98.9 ± 1.08 |
| 23 | 95.1 ± 1.20 | 99.7 ± 1.50 | 99.4 ± 0.06 | 99.9 ± 0.30 | 99.9 ± 1.30 | 97.8 ± 0.70 |
| 24 | 100.6 ± 0.05 | 99.6 ± 1.20 | 99.4 ± 0.17 | 99.9 ± 0.20 | 98.0 ± 4.00 | 95.5 ± 0.40 |
FOS: Fosaprepitant
DEX: Dexamethasone
GRA: Granisetron
%RC: Percentage of remaining drug concentration (%)
t7: 7 days of storage
t15: 15 days of storage
Table 9: Percentage of remaining concentrations of FDG in mixtures at day 7 and 15 of storage.
So, mixtures containing 150 mg of FOS, 8 mg of DEX and 3 mg of GRA in 100 and 250 ml of 0.9 g/dl NaCl were physico-chemical stable for 15 days at different conditions of light (PL and L) and temperature (RT and F). Mixtures 13 and 16, in 50 ml of 0.9 g/dl NaCl, were physicochemical stable for 6 days due to appearance of precipitate after day 7.
Conclusion
The caducity of all-in-one admixtures containing fosaprepitant 150 mg, dexamethasone 8 mg and ondansetron 8 mg or granisetron 3 mg in 50, 100 and 250 ml of 0.9 g/dl NaCl was established at different conditions of light and temperature. The results from this paper represent the first evidence of the physico-chemical stability of both ternary mixtures FDO and FDG used in clinical practice at different drugs concentrations and conditions of storage. These results indicate that advance preparation of these ternary mixtures is possible, reducing waiting times for patients and that their administration simplify the management of these treatments in terms of reducing number of preparations and improving patient safety and comfort.
Acknowledgements
The authors acknowledge FISABIO for the financial support.
References
- Jordan K,Gralla R,Jahn F,Molassiotis A (2014) International antiemetic guidelines on chemo therapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol 722: 197-202.
- Saito M,Aogi K, Sekine I, Yoshizawa H, Yanagita Y, et al. (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasoneforprevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparativephase III trial. LancetOncol 10: 115-124.
- Sun S,Schaller J, Placek J, Duersch B (2013) Compatibility of intravenous fosaprepitant with intravenous 5-HT3 antagonists and corticosteroids. Cancer Chemother Pharmacol 72: 509-513.
- MASCC/ESMO (2013) Antiemetic Guideline[http://www.mascc.org/assets/Guidelines-Tools/mascc_antiemetic_english_2014.pdf]MASCC/ESMO. Accessedon:July 2016.
- Basch E,Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, et al. (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29: 4189-4198.
- Stabilis (2015) Stability and compatibility of drugs[http://www.stabilis.org]Stabilis. Accessedon:July 2016.
- US Department of Health and Human Services FDA Center forDrugEvaluation and Research; US Department of Health and Human Services FDA Center forBiologicsEvaluation and Research; US Department of Health and Human Services FDA Center forDevices and Radiological Health (2006) Guidance for industry: patient-reported outcomemeasures: use in medical productdevelopmenttosupportlabelingclaims: draftguidance. HealthQualLifeOutcomes 4: 79.
- International ConferenceonHarmonisation of Technical Requirements for Registration of Pharmaceuticalfor Human Use (ICH) (2003) ICH Harmonised Tripartite Guideline StabilityTesting of New DrugSubstances and Products (Q1A-R2) [http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf] International ConferenceonHarmonisation of TechnicalRequirementsforRegistration of Pharmaceuticalfor Human Use. Accessedon: February 2016.
- Bakshi M, Singh S (2002) Development of validatedstability-indicatingassaymethods--criticalreview. J PharmBiomed Anal 28: 1011-1040.
- Gómez MA, Arenas VJ, Sanjuán MM, Hernández MJ, Almenar CB, et al. (2007) Stabilitystudies of binary mixtures of haloperidol and/ormidazolamwithotherdrugsfor parenteral administration. J PalliatMed 10: 1306-1311.
- Martinez MA, Moya A, Porta B, Climente M (2014) Physico-ChemicalStability of Mixtures of Fosaprepitantused in ClinicalPractice. J Anal BioanalTech 5: 2-6.
- The United States Pharmacopeia (USP) (2015)Pharmaceutical compounding: sterile preparations[http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/usp-gc-797-proposed-revisions-sep-2015.pdf]TheUnited States Pharmacopeia (USP).Accessedon:February 2016.
- The Spanish National Health Service, Ministry of Health, Social Services and Equality (2014) Guide togoodpractices in preparing medicines in hospital pharmacy services. [http://www.msssi.gob.es/profesionales/farmacia/ documentacion.htm]The Spanish National Health Service.Accessedon: June 2014.
- Pharmacopoeia Official(2004) Rules of Good Preparation of Medicines in Pharmacy[http://www.fog.it/fogliani/giancarlo/normebp.htm] Pharmacopoeia Official. Accessedon:February 2016.
- ICH (2005)Harmonised Tripartite Guideline onValidation of analytical procedures: text and methodology ICH Q2(R1)[http://www.ich.org] ICH.Accessedon: June 2014.
Citation: Moya-Gil A, Martínez-Gómez MA, Gras-Colomer E, Porta-Oltra B, Climente-Martí M (2016) Stability Studies of Ternary Mixtures Containing Fosaprepitant, Dexamethasone, Ondansetron and Granisetron Used in Clinical Practice. J Anal Bioanal Tech 7:307. DOI: 10.4172/2155-9872.1000307
Copyright: © 2016 Moya-Gil A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 12362
- [From(publication date): 4-2016 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 11443
- PDF downloads: 919