Stability Indicating Rp-Hplc Method for Estimation of Migalastatin Capsule Dosage Form
Received: 10-Jun-2021 / Accepted Date: 22-Jun-2021 / Published Date: 29-Jun-2021 DOI: 10.4172/2167-065X.1000225
Abstract
A stability-indicating reverse phase high performance liquid chromatography method was developed and validated for Migalastat. The wavelength selected for quantitation was 275 nm. The method has been validated for linearity, accuracy, precision, robustness, limit of detection and limit of quantitation. Linearity was observed in the concentration range of 10-50 μg/ml for Migalastat. For RP-HPLC, the separation was achieved by XTerra C 18 (150×4.6 mm) 5 μm column using 0.1% Tri fluro acetic acid: Methanol:Acetonitrile (20:40:40. v/v) as mobile phase with flow rate 0.7 ml/ min. The retention time of Migalastat was found to be 2.6 min respectively. During force degradation, drug product was exposed to hydrolysis (acid and base hydrolysis), H2O2, thermal degradation and photo degradation. In thermal degradation the highest amount of drug was found to be 4.69%. The developed methods were simple, specific and economic, which can be used for simultaneous estimation of Migalastat in capsule dosage form.
Aim and objective: Development of new RP-HPLC method for the estimation of Migalastat in capsule dosage form and then validation of the method.
Method: RP-Hplc Method, stability indicating RP-HPLC method for estimation of migalostat in capsule dosage form validation of the method developed using validation parameters.
Results: The assay of Migalastat was performed with Capsule and the % assay was found to be 100.25 which show that the method is useful for routine analysis.The linearity of Migalastat was found to be linear with a correlation coefficient of 0.999, which shows that the method is capable of producing good sensitivity. The acceptance criteria of precision is RSD should be not more than 2.0% and the method show precision 0.6 for Migalastat which shows that the method is precise. The acceptance criteria of intermediate precision is RSD should be not more than 2.0% and the method show precision 0.2 for Migalastat which shows that the method is repeatable when performed in different days also. The accuracy limit is the percentage recovery should be in the range of 98.0% - 102.0%. The total recovery was found to be 100.41% for Migalastat. The validation of developed method shows that the accuracy is well within the limit, which shows that the method is capable of showing good accuracy and reproducibility. The acceptance criteria for LOD and LOQ are 3 and 10. The LOD and LOQ for Migalastat was found to be 2.98 and 9.97.The robustness limit for mobile phase variation and flow rate variation are well within the limit, which shows that the method is having good system suitability and precision under given set of conditions. The acceptance criteria for degradation studies are less than 15%. The degradation results are within the limit.
Conclusion: The estimation of Migalastat was done by RP-HPLC.
Keywords: Migalostat; RP-HPLC method; Estimation and stability indicating studies; Method development and validation of developed method
Introduction
‘Health is wealth’. It is vital fact that a healthy body is desire of every human being. Good health is first condition to enjoy the life and all other things which mankind is having. Nowadays peoples are more concentrating towards health. Even governmental bodies of different countries and World health organization (WHO) are also focusing for health of human being. Health care is prevention, treatment and management of illness and preservation of mental and physical wellbeing. Health care embraces all the goods and services designed to promote health including preventive, curative and palliative in interventions. Due to rapid growth of pharmaceutical industry during last several years, number of pharmaceutical formulations are enter as a part of health care system and thus, there has been rapid progress in the field of pharmaceutical analysis. Developing analytical method for newly introduced pharmaceutical formulation is a matter of most importance because drug or drug combination may not be official in any pharmacopoeias and thus, no analytical method for quantification is available. To check the quality standards of the medicine various analytical methods are used. Modern analytical techniques are playing key role in assessing chemical quality standards of medicine [1]. Thus analytical techniques are required for fixing standards of medicines and its regular checking. Out of all analytical techniques, the technique which is widely used to check the quality of drug is known as ‘CHROMATOGRAPHY’.
HPLC is probably the most universal type of analytical procedure; its application areas include quality control, process control, forensic analysis, environmental monitoring and clinical testing. In addition HPLC also ranks as one of the most sensitive analytical procedures and is unique in that it easily copes with multi-component mixtures [2]. It has achieved this position as a result of the constant evolution of the equipment used in LC to provide higher and higher efficiencies at faster and faster analysis times with a constant incorporation of new highly selective column packings.
There are different modes of separation in HPLC. They are normal phase mode, reversed phase mode, reverse phase ion pair chromatography, affinity chromatography and size exclusion chromatography (gel permeation and gel filtration chromatography) [3].
In the normal phase mode, the stationary phase is polar and the mobile phase is non-polar in nature. In this technique, non-polar compounds travel faster and are eluted first. This is because of the lower affinity between the non-polar compounds and the stationary phase. Polar compounds are retained for longer times because of their higher affinity with the stationary phase. These compounds, therefore take more times to elute.
Normal phase mode of separation is therefore, not generally used for pharmaceutical applications because most of the drug molecules are polar in nature and hence takes longer time to elute.
Reversed phase mode is the most popular mode for analytical and preparative separations of compound of interest in chemical, biological, pharmaceutical, food and biomedical sciences. In this mode, the stationary phase is non-polar hydrophobic packing with octyl or octadecyl functional group bonded to silica gel and the mobile phase is polar solvent. An aqueous mobile phase allows the use of secondary solute chemical equilibrium (such as ionization control, ion suppression, ion pairing and complexation) to control retention and selectivity [4,5]. The polar compound gets eluted first in this mode and nonpolar compounds are retained for longer time. As most of the drugs and pharmaceuticals are polar in nature, they are not retained for longer times and hence elute faster. The different columns used are Octa Decyl Silane (ODS) or C18, C8, C4, etc. (in the order of increasing polarity of the stationary phase).
Optimization can be started only after a reasonable chromatogram has been obtained. A reasonable chromatogram means that more or less symmetrical peaks on the chromatogram detect all the compounds [6]. By sight change of the mobile phase composition, the position of the peaks can be predicted within the range of investigated changes. An optimized chromatogram is the one in which all the peaks are symmetrical and are well separated in less run time.
The peak resolution can be increased by using a more efficient column (column with higher theoretical plate number, N) which can be achieved by using a column of smaller particle size, or a longer column. These factors, however, will increase the analysis time. Flow rate does not influence resolution, but it has a strong effect on the analysis time.
In the present study estimation of mmigalostat in capsule dosage form by using Rp- HPLC method. An optimized method was developed and validated using validation parameters [7].
Materials and Methods
HPLC method development (Tables 1 & 2)
| Sl. No | Instrument | Model |
|---|---|---|
| 1 | HPLC | WATERS, software: Empower, 2695 separation module.2487 UV detector. |
| 2 | UV/VIS spectrophotometer |
LABINDIA UV 3000+ |
| 3 | pH meter | Adwa – AD 1020 |
| 4 | Weighing machine | Afcoset ER-200A |
| 5 | Pipettes and Burettes | Borosil |
| 6 | Beakers | Borosil |
Table 1: Instruments used.
| Sl. No | Chemical | Brand |
|---|---|---|
| 1 | Migalastat | Supplied by Pharmatrain |
| 2 | Tri fluoro acetic acid | FINAR chemical LTD |
| 3 | Water and Methanol for HPLC | Standard solutions Ltd |
| 4 | Acetonitrile for HPLC | Standard solutions Ltd |
| 5 | HCl, H2O2, NaOH | MERCK |
Table 2: Chemicals used.
Wave length selection: UV spectrum of 10 μg/ml Migalastat in diluents (mobile phase composition) was recorded by scanning in the range of 200 nm to 400 nm. From the UV spectrum wavelength selected as 275 nm. At this wavelength both the drugs show good absorbance.
Optimized chromatographic conditions: Instrument used- High performance liquid chromatography equipped with Auto Sampler and DAD or UV detector
Temperature: Ambient
Column: Xterra C18(150 x 4.6 mm, 5 μm)
Buffer:0.1% Tri fluoro acetic acid
Mobile phase: 0.1% Trifluoroacetic acid: Methanol: Acetonitrile (20%:40%:40%)
Flow rate:0.7 ml per min
Wavelength: 275 nm
Injection volume: 20 μl
Run time: 8 min
Prepartion of buffer and mobile phase
Preparation of 0.1% Tri fluoro acetic acid buffer: Take 1ml of Tri fluoro acetic acid in 1000 ml of water and sonicate for 15 minutes adjusted the PH for 3.0, Sonicate for 5 min.
Preparation of mobile phase: Mix a mixture of above buffer200 ml (20%) Methanol 400 ml (40%) and 400 ml Acetonitrile HPLC (40%) and degas in ultrasonic water bath for 5 minutes. Filter through 0.45 μ filter under vacuum filtration.
Diluent Preparation: Use the Mobile phase as Diluents.
Assay
Standard solution preparation: Accurately weigh and transfer 25 mg of Migalastat working standard into a 25 ml clean dry volumetric flask add Diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution).
Further pipette 0.3ml of Migalastat of the above stock solution into a 10ml volumetric flask and dilute up to the mark with Diluents [8].
Sample solution preparation: Accurately weigh and transfer equivalent to 25 mg of Migalastat equivalent weight of the sample into a 25 ml clean dry volumetric flask add Diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent. (Stock solution).
Further pipette 0.3 ml of Migalastat of the above stock solution into a 10ml volumetric flask and dilute up to the mark with Diluents.
Procedure: Inject 20 μL of the standard, sample into the chromatographic system and measure the areas for the Migalastat peaks and calculate the %Assay by using the formulae.
Validation parameters
Linearity: Preparation of stock solution- Accurately weigh and transfer 25 mg of Migalastat working standard into a 25 ml clean dry volumetric flask add Diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent. (Stock solution).
Preparation of Level - I (10 ppm of Migalastat)- 0.1 ml of stock solution has taken in 10ml of volumetric flask dilute up to the mark with Diluents.
Preparation of Level - II (20 ppm of Migalastat)- 0.2 ml of stock solution has taken in 10ml of volumetric flask dilute up to the mark with Diluents.
Preparation of Level - III (30 ppm of Migalastat)- 0.3 ml of stock solution has taken in 10ml of volumetric flask dilute up to the mark with Diluents.
Preparation of Level - IV (40 ppm of Migalastat)- 0.4 ml of stock solution has taken in 10ml of volumetric flask dilute up to the mark with Diluents.
Preparation of Level – V (50 ppm of Migalastat)- 0.5 ml of stock solution has taken in 10ml of volumetric flask dilute up to the mark with Diluents.
Procedure: Inject each level into the chromatographic system and measure the peak area. Plot a graph of peak area versus concentration (on X- axis concentration and on Y-axis Peak area) and calculate the correlation coefficient.
Precision: Preparation of stock Solution- Accurately weigh and transfer 25 mg of Migalastat working standard into a 25 ml clean dry volumetric flask add Diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution).
Further pipette 0.3 ml of Migalastat the above stock solution into a10ml volumetric flask and dilute up to the mark with Diluents [9].
Procedure: The standard solution was injected for six times and measured the area for all six injections in HPLC. The %RSD for the area of six replicate injections was found to be within the specified limits.
Intermediate precision/ruggedness: To evaluate the intermediate precision (also known as Ruggedness) of the method, Precision was performed on different day within the laboratory.
Preparation of stock solution: Accurately weigh and transfer 25 mg of Migalastat working standard into a 25 ml clean dry volumetric flask add Diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution).
Further pipette 0.3 ml of Migalastat the above stock solution into a10ml volumetric flask and dilute up to the mark with Diluents.
Procedure: The standard solution was injected for six times and measured the area for all six injections in HPLC. The %RSD for the area of six replicate injections was found to be within the specified limits [10].
Accuracy: For accuracy determination, three different concentrations were prepared separately i.e. 50%, 100% and 150% for the analyte and chromatograms are recorded for the same.
Preparation of Standard stock solution: Accurately weigh and transfer 25 mg of Migalastat working standard into a 25ml clean dry volumetric flask add Diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution).
Further pipette 0.3 ml of Migalastat the above stock solution into a10ml volumetric flask and dilute up to the mark with Diluents.
Preparation Sample solutions: For preparation of 50% solution (With respect to target Assay concentration).
Accurately weigh and transfer 12.5 mg of Migalastat equivalent weight of tablet powder into a 25 ml clean dry volumetric flask add and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution) [11].
Further pipette 0.3ml of Migalastat the above stock solution into a10ml volumetric flask and dilute up to the mark with Diluents.
For preparation of 100% solution (With respect to target Assay concentration): Accurately weigh and transfer 25 mg of Migalastat equivalent weight of tablet powder into a 25 ml clean dry volumetric flask add and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution).
Further pipette 0.3ml of Migalastat the above stock solution into a10ml volumetric flask and dilute up to the mark with Diluents.
For preparation of 150% solution (With respect to target Assay concentration): Accurately weigh and transfer 37.5 mg of Migalastat equivalent weight of tablet powder into a 25 ml clean dry volumetric flask add and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution).
Further pipette 0.3 ml of Migalastat the above stock solution into a10ml volumetric flask and dilute up to the mark with Diluents.
Procedure: Inject the standard solution, Accuracy-50%, Accuracy-100% and Accuracy-150% solutions.
Calculate the Amount found and Amount added for Migalastat and calculate the individual recovery and mean recovery values [12].
Limit of detection: Preparation of Migalastat solution
Preparation of 0.36μg/ml solution: Accurately weigh and transfer 25 mg of Migalastat working standard into a 25 ml clean dry volumetric flask add Diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution).
Further pipette 0.3 ml Migalastat the above stock solution into a10 ml volumetric flask and dilute up to the mark with Diluent.
Further pipette 1ml of the above stock solution into a 10 ml volumetric flask and dilute up to the mark with Diluent.
Further pipette 1.2ml of the above stock solution into a 10 ml volumetric flask and dilute up to the mark with Diluent.
Limit of quantification: Preparation of Migalastat solution Preparation of 1.20 μg/ml solution: Accurately weigh and transfer 25 mg of Migalastat working standard into a 25 ml clean dry volumetric flask add Diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution).
Further pipette 0.3 ml Migalastat the above stock solution into a10ml volumetric flask and dilute up to the mark with Diluent.
Further pipette 1ml of the above stock solution into a 10ml volumetric flask and dilute up to the mark with Diluent.
Further pipette 4ml of the above stock solution into a 10ml volumetric flask and dilute up to the mark with Diluent.
Robustness: As part of the Robustness, deliberate change in the Flow rate, Mobile Phase composition, Temperature Variation was made to evaluate the impact on the method.
The flow rate was varied at 0.9 ml/min to 1.1 ml/min.
Standard solution 30 μg/ml of Migalastat prepared and analysed using the varied flow rates along with method flow rate.
The Organic composition in the Mobile phase was varied from 54% to 66%.
Standard solution 30 μg/ml of Migalastat was prepared and analysed using the varied Mobile phase composition along with the actual mobile phase composition in the method.
Degradation studies
The International Conference on Harmonization (ICH) guideline entitled stability testing of new drug substances and products requires that stress testing be carried out to elucidate the inherent stability characteristics of the active substance. The aim of this work was to perform the stress degradation studies on the Migalastat using the proposed method [13-15].
Preparation of stock: Accurately weigh and transfer 25 mg of Migalastat equivalent weight of tablet powder into a 25 ml clean dry volumetric flask add and sonicate to dissolve it completely and make volume up to the mark with the same solvent. (Stock solution).
Hydrolytic degradation under acidic condition: Pipette 0.3 ml of above solution into a 10 ml volumetric flask and 3 ml of 0.1N HCl was added. Then, the volumetric flask was kept at 60ºC for 6 hours and then neutralized with 0.1 N NaOH and make up to 10ml with diluent. Filter the solution with0.22 microns syringe filters and place in vials[16].
Hydrolytic degradation under alkaline condition: Pipette 0.3 ml of above solution into a 10ml volumetric flask into a 10 ml volumetric flask and add 3ml of 0.1N NaOH was added in 10ml of volumetric flask. Then, the volumetric flask was kept at 60ºC for 6 hours and then neutralized with 0.1N HCl and make up to 10 ml with diluent. Filter the solution with 0.22 microns syringe filters and place in vials.
Oxidative degradation: Pipette 0.3 ml above stock solution into a 10 ml volumetric flask solution into a 10 ml volumetric flask 1 ml of 3% w/v of hydrogen peroxide added in 10 ml of volumetric flask and the volume was made up to the mark with diluent. The volumetric flask was then kept at room temperature for 15 min. Filter the solution with 0.45 microns syringe filters and place in vials.
Thermal induced degradation: Migalastat sample was taken in petridish and kept in Hot air oven at 1100 C for 24 hours. Then the sample was taken and diluted with diluents and injected into HPLC and analyzed.
Photo degradation: Pipette 0.3 ml above stock solution into a 10 ml volumetric flask and expose to sunlight for 24hrs and the volume was made up to the mark with diluent. Filter the solution with 0.45 microns syringe filters and place in vials.
Results and Discussion
Optimized chromatogram chromatographic conditions
Column :Xterra C18(150 x 4.6 mm, 5 μm)
Mobile phase ratio : 0.1% Tri fluoro acetic acid : Methanol :
Acetonitrile (20:40:40)
Detectionwavelength : 275 nm
Flow rate : 0.7 ml/min
Injection volume : 20 μl
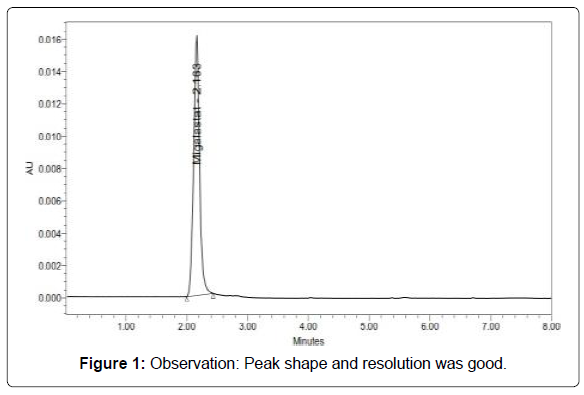
Run time :8min (Table 3, Figure 1)
| S. No. |
Name | RT (min) |
Area (µV sec) | Height (µV) | USP tailing |
USP plate count |
|---|---|---|---|---|---|---|
| 1 | Migal astat | 2.163 | 108579 | 15979 | 1.07 | 2310. 79 |
Table 3: Results of system suitability parameters.
System suitability (Figures 2 and 3)
Acceptance criteria:
• Theoretical plates must be not less than 2000.
• Tailing factor must be not more than 2.
• It was found from above data that all the system suitability parameters for developed method were within the limit.
Assay
Standard and sample solution injected as described under experimental work. The corresponding chromatograms and results are shown below (Figures 4 and 5, Table 4).
| Label Claim (mg) | % Assay | |
|---|---|---|
| Migalastat | 75 | 100.25 |
Table 4: Results of assay for Migalastat.
Validation parameters
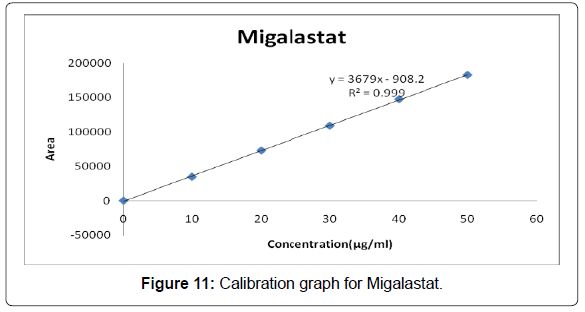
Linearity: The linearity range was found to lie from 10μg/ml to 50μg/ml of Migalastat and chromatograms are shown below (Figures 6-11, Tables 5 and 6).
| S. No |
Migalastat | |
|---|---|---|
| Concentration (µg/ml) | Area | |
| 1 | 0 | 0 |
| 2 | 10 | 34608 |
| 3 | 20 | 72855 |
| 4 | 30 | 108936 |
| 5 | 40 | 147300 |
| 6 | 50 | 182696 |
Table 5: Area of different concentration of Migalastat.
| Parameters | Migalastat |
|---|---|
| Slope (m) | 3679 |
| Intercept (c) | 908.2 |
| Correlation coefficient (R2) | 0.999 |
Table 6: Analytical performance parameters of Migalastat.
Acceptance criteria:Correlation coefficient (R2) should not be less than 0.999
The correlation coefficient obtained was 0.999 which is in the acceptance limit [17,18].
Precision: Precision of the method was carried out for both sample solutions as described under experimental work. The corresponding chromatograms and results are shown below (Figures 12-17, Table 7).
Acceptance criteria:
• %RSD for sample should be NMT 2.
• The %RSD for the standard solution is below 1, which is within the limits hence method is precise.
Intermediate precision (ruggedness): There was no significant change in assay content and system suitability parameters at different conditions of ruggedness like day to day and system to system variation (Figures 18-23, Table 8).
| Injection | Area |
|---|---|
| Injection-1 | 108897 |
| Injection-2 | 108982 |
| Injection-3 | 108500 |
| Injection-4 | 110188 |
| Injection-5 | 108944 |
| Injection-6 | 108537 |
| Average | 109008.0 |
| Standard Deviation | 614.7 |
| %RSD | 0.6 |
Table 7: Results of Precision for Migalastat.
Acceptance criteria:
• %RSD of six different sample solutions should not more than 2
• The %RSD obtained is within the limit, hence the method is rugged.
Accuracy: Sample solutions at different concentrations (50%, 100%, and 150%) were prepared and the % recovery was calculated (Figures 24-32, Table 9).
| Injection | Area |
|---|---|
| Injection-1 | 109237 |
| Injection-2 | 108617 |
| Injection-3 | 108805 |
| Injection-4 | 108943 |
| Injection-5 | 109173 |
| Injection-6 | 108915 |
| Average | 108948.3 |
| Standard Deviation | 230.3 |
| %RSD | 0.2 |
Table 8: Results of Intermediate precision for Migalastat.
| %Concen tration (at specificati on Level) |
Area * |
Amo unt Add ed (mg) | Amo unt Fou nd (mg) |
% Reco very |
Mean Reco very |
|---|---|---|---|---|---|
| 50% | 5457 5.7 |
12.5 | 12.5 | 100.0 9 |
100.4 1 |
| 100% | 1097 49 |
25 | 25.1 6 |
100.6 3 |
|
| 150% | 1644 33.7 |
37.5 | 37.6 9 |
100.5 2 |
|
| *Average of three determinations | |||||
Table 9: Accuracy (recovery) data for Migalastat.
Acceptance Criteria:The percentage recovery was found to be within the limit (98-102%) [19].
The results obtained for recovery at 50%, 100%, 150% are within the limits. Hence method is accurate.
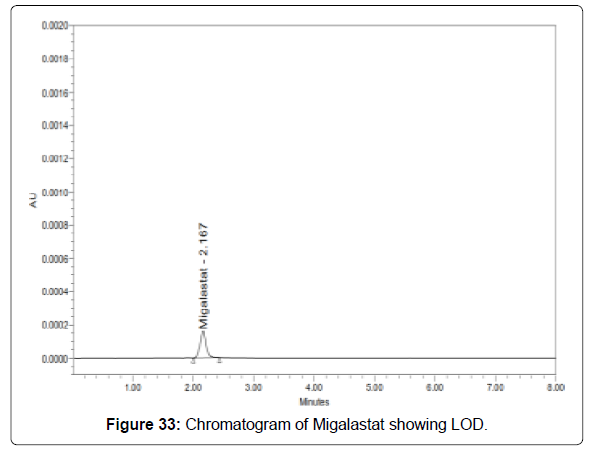
Limit of detection for migalastat: The lowest concentration of the sample was prepared with respect to the base line noise and measured the signal to noise ratio (Figure 33 and Table 10) [20-21].
| Drug name | Baseline noise (µV) | Signal obtained (µV) | S/N ratio |
|---|---|---|---|
| Migalastat | 64 | 191 | 2.98 |
Table 10: Results of LOD.
• Signal to noise ratio shall be 3 for LOD solution.
• The result obtained is within the limit.
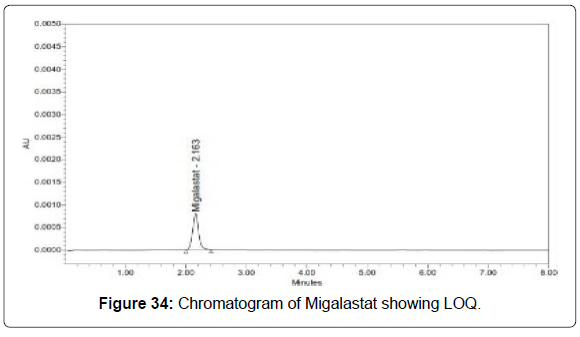
Limit of quantification for migalastat: The lowest concentration of the sample was prepared with respect to the base line noise and measured the signal to noise ratio (Figure 34 and Table 11).
| Drug name | Baseline noise (µV) | Signal obtained (µV) | S/N ratio |
|---|---|---|---|
| Migalastat | 64 | 638 | 9.97 |
Table 11: Results of LOQ.
• Signal to noise ratio shall be 10 for LOQ solution.
• The result obtained is within the limit.
Robustness: The standard and samples of Migalastat were injected by changing the conditions of chromatography. There was no significant change in the parameters like resolution, tailing factor, asymmetric factor, and plate count.
Variation in flow: Variation of mobile phase organic composition (Figures 35-38, Table 12 and 13) [22-23].
| S. No | Flow Rate (ml/min) | System Suitability Results | |
|---|---|---|---|
| USP Tailing |
USP plate count | ||
| 1 | 0.9 | 1.05 | 2252.22 |
| 2 | 1.0 | 1.07 | 2334.25 |
| 3 | 1.1 | 1.06 | 2925.12 |
* Results for actual flow (1.0ml/min) have been considered from Assay standard
Table 12: Results for variation in flow for Migalastat.
| . No | Change in Organic Composition in the Mobile Phase | ystem Suitability Results | |
|---|---|---|---|
| USP Tailing |
USP Plate count | ||
| 1 | 10% less | 1.15 | 3005.27 |
| 2 | *Actual | 1.07 | 2334.25 |
| 3 | 10% more | 1.11 | 3384.13 |
* Results for actual Mobile phase composition have been considered from Accuracy standard
Table 13: Results for variation in mobile phase composition for Migalastat.
Acceptance criteria: The Retention time, USP plate count, USP tailing factor obtained for change of flow rate, variation in mobile phase was found to be within the acceptance criteria. Hence the method is robust.
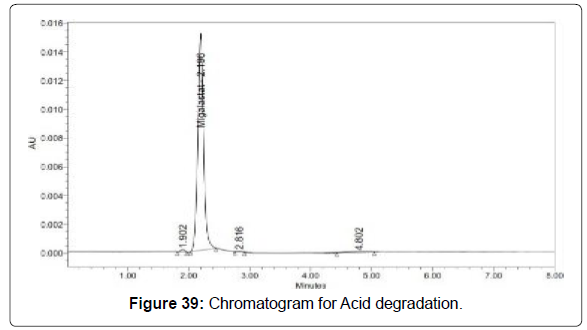
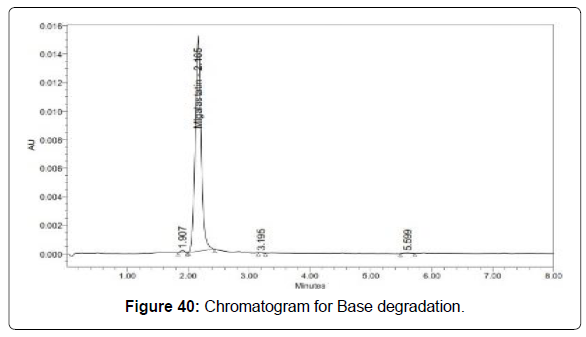
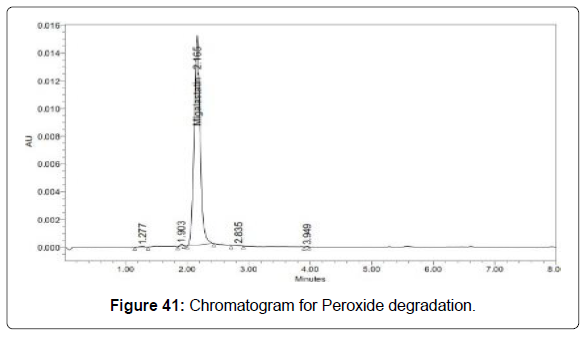
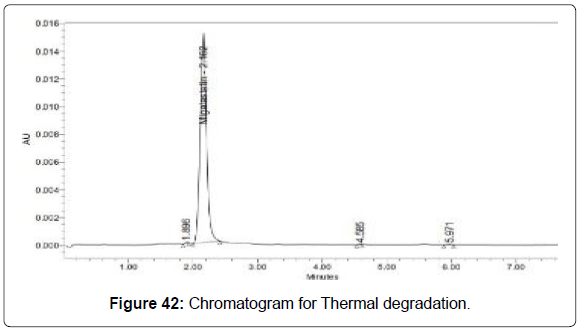
Degradation studies (Figures 39-43, Table 14)
| Sample Name | Migalastat | |
|---|---|---|
| Area | % Degraded | |
| Standard | 108838.7 | |
| Acid | 106936 | 1.75 |
| Base | 106497 | 2.15 |
| Peroxide | 107083 | 1.61 |
| Thermal | 103735 | 4.69 |
| Photo | 105639 | 2.94 |
Table 14: Degradation results for Migalastat.
Conclusion
The estimation of Migalastat was done by RP-HPLC.
The assay of Migalastat was performed with Capsule and the % assay was found to be 100.25 which show that the method is useful for routine analysis.
The linearity of Migalastat was found to be linear with a correlation coefficient of 0.999, which shows that the method is capable of producing good sensitivity.
The acceptance criteria of precision is RSD should be not more than 2.0% and the method show precision 0.6 for Migalastat which shows that the method is precise.
The acceptance criteria of intermediate precision is RSD should be not more than 2.0% and the method show precision 0.2 for Migalastat which shows that the method is repeatable when performed in different days also.
The accuracy limit is the percentage recovery should be in the range of 98.0% - 102.0%. The total recovery was found to be 100.41% for Migalastat. The validation of developed method shows that the accuracy is well within the limit, which shows that the method is capable of showing good accuracy and reproducibility.
The acceptance criteria for LOD and LOQ are 3 and 10. The LOD and LOQ for Migalastat was found to be 2.98 and 9.97.
The robustness limit for mobile phase variation and flow rate variation are well within the limit, which shows that the method is having good system suitability and precision under given set of conditions.
The acceptance criteria for degradation studies are less than 15%. The degradation results are within the limit.
References
- Brahmankar DM, Jaiswal SB (2005) Biopharmaceutics and Pharmakokinetics, A Treatise. 1st edn, Vallabh Prakasan, Delhi 27:5-6.
- Swarbrick J, Boylan JC (2002) Encyclopedia of Pharmaceutical Technology, 2nd edition. 1:8.
- Leuner C, Dressmann J (2000) Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm 50:47-48.
- Shargel L, Andrew BC (1985) Applied Biopharmaceutics and Pharmacokinetics. Appleton-Century-Crofts p:134.
- Garekani HA, Sadeghi F, Badiee A, Mostafa SA, Rajabisiahboomi AR (2001) Crystal habit modifications of Ibuprofen and their Physicochemical Characteristics. Drug Dev Ind Pharm 27:803-809.
- Shargel L, Andrew BC (1985) Applied Biopharmaceutics and Pharmacokinetics.Appleton-Century-Crofts, 4th Ed p:135.
- Brahmankar DM, Jaiswal SB (2005) Biopharmaceutics and Pharmakokinetics, A Treatise. 1st edn, Vallabh Prakasan, Delhi 27:29-30.
- Das NG, Das SK (2006) Formulation of Poorly Soluble Drugs. Drug Delivery Report Spring/Summer pp:52-55.
- Chiou WL, Riegelman SJ (1971) Pharmaceutical applications of solid dispersion systems. J Pharm Sci 60:1283-1297.
- Swarbrick J, Boylan JC (2004) Encyclopedia of Pharmaceutical Technology, 2nd edn. 1:641-647.
- Leuner C, Dressman J (2000) Improving Drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm 50:48-51.
- Modi A, Tayade P (2006) Enhancement of dissolution profile of solid dispersion (Kneading) technique. AAPS Pharm Sci Tech 7:1-13.
- Devarajan, Padma V, Gore SP (2000) Melt-in-mouth tablets- innovative oral drug delivery systems. Express Pharma Pulse 7:16-18.
- Reddy LH, Ghosh BR (2002) Fast dissolving drug delivery systems: A Review of Literature. Indian J Pharm Sci 64:331-336.
- Kuchekar BS, Bhise SB, Armugam V (2001) Design of fast dissolving tablets. Ind J Pharm Edu35:150-152.
- Ozeki T, Yasuzawa Y, Katsuyama H, Takashima Y, Kasai T (2003) Design of rapidly disintegrating oral tablets using acid-treated yeast cell wall: A Technical note. AAPS PharmSciTech 4:561-564.
- Beatrice P, Rubessa F, Moneghini M, Voinovich D (2003) Formulation design of carbamazepine fast-release tablets prepared by melt granulation technique. Int J Pharma 256:53-63.
- Abdelbary G, Prinderre P, Eouani C, Joachim J, Reynier JP, et al. (2004) The preparation of orally disintegrating tablets using a hydrophilic waxy binder. Int J Pharma 278:423-433.
- Okuda Y, Irisawa Y, Okimoto K, Osawa T, Yamashita S (2009) A new formulation for orally disintegrating tablets using a suspension spray- coating method. Int J Pharm 382:80-87.
- Sastry SV, Nyshadham JR, Fix JA (2000) Recent technological advances in oral drug delivery- a review.Pharm Sci Technol Today 3.
- Bandari S, Mittapalli RK, Gannu R, Rao YM (2008) Orodispersible tablets: an overview. Asian J Pharm 2.
- Giugliani R, Waldek S, Germain DP, Nicholls K, Bichet DG, et al. (2013) A Phase 2 study of migalastat hydrochloride in females with Fabry disease: Selection of population, safety and pharmacodynamic effects. Molecular Genetics and Metabolism 109:86-92.
- Shin SH, Park MH, Byeon JJ, Lee BI, Park Y, et al. (2018) A Liquid Chromatography-Quadrupole- Timeof-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse. Pharmaceutics 10:69.
Citation: Pavan Kumar S, Swaroopa Rani K, Sathya Sowmya P, Somsekhar G (2021) Stability Indicating Rp-Hplc Method for Estimation of Migalastatin Capsule Dosage Form. Clin Pharmacol Biopharm, 10: 225. DOI: 10.4172/2167-065X.1000225
Copyright: © 2021 Pavan Kumar S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4685
- [From(publication date): 0-2021 - Dec 17, 2025]
- Breakdown by view type
- HTML page views: 3684
- PDF downloads: 1001