Sprouty2 Inhibition Resolves Inflammation in Periodontal Disease and Creates a Suitable Environment for Periodontal Tissue Regeneration
Received: 28-Sep-2017 / Accepted Date: 13-Oct-2017 / Published Date: 16-Oct-2017
Abstract
Introduction: Periodontitis, a bacterial infection affecting periodontal tissues, results in the alveolar bone destruction. In particular, periodontitis is associated with Porphyromonas gingivalis (Pg)-induced inflammation and an increase of macrophages infiltrated into the gingiva. Sprouty (Spry) proteins function as negative regulators, which interfere with the activation of fibroblast growth factor (FGF) pathway by suppressing the mitogen-activated protein kinase (MAPK) signaling. We have previously showed that the suppression of Spry2 effectively induces the periodontal ligament migration along the root surface of the tooth and an increase of the alveolar bone, whereas impeding gingival epithelial down-growth toward bone defects.
Results: In the recent paper in our laboratory, we showed that Spry2 knockdown by Pg lipopolysaccharide (LPS) and interferon (IFN) γ stimulation converts macrophages from the M1 to M2 phenotype, and may effectively resolve inflammation by releasing anti-inflammatory cytokines in macrophages.
Conclusion: These studies show that the topical application of Spry2 inhibitors to bone loss may generate an appropriate environment for periodontal remodeling by inducing M2 macrophages, resolving inflammation in periodontitis, activating the periodontal ligament migration along the root surface of a tooth, promoting growth of the alveolar bone, and interfering with gingival epithelial down-growth toward bone defects. These findings thus provide a molecular basis for novel therapeutic targets in periodontal remodeling.
Short Communication
Periodontitis is bacterial infection affecting periodontal tissues, which results in the alveolar bone destruction, and is associated with bacteria-induced inflammation and an increase of macrophages infiltrated into the gingiva [1-3]. Porphyromonas gingivalis (Pg) is the most common causative bacterium causing periodontitis, in addition to other gram-negative anaerobic species [4,5]. Lipopolysaccharides (LPS) from Pg bind to Toll-like receptor (TLR) 2, inducing the activation of nuclear factor kappa B (NFκB) and producing pro-inflammatory cytokines such as interleukin (IL)-1β or tumor necrosis factor (TNF)-α [6,7]. Macrophages are of the classical activation (M1) and alternative activation (M2) phenotypes; the M1 phenotype activated by stimulation with the Th1 cytokine interferon (IFN) γ or LPS plays a crucial role in the onset and progression of inflammation [8,9]. On the contrary, the M2 phenotype is usually induced by Th2 cytokines IL-4 or IL-13, and plays roles in immune responses, production of anti-inflammatory cytokines, revascularization, and wound closure [10-13]. Therefore, macrophages are implicated in both tissue destruction and tissue remodeling and play crucial roles in the interface between inflammation and regeneration of tissue. Sprouty (Spry) proteins have been extensively studied as negative regulators that interfere with fibroblast growth factor (FGF) signaling by suppressing the mitogen-activated protein kinase (MAPK) pathway [14,15]. Four mammalian isoforms of Spry (Spry1, Spry2, Spry3, and Spry4) have been identified; Spry2 specifically attenuates the phosphorylation of the receptor tyrosine kinase (RTK) pathway in response to FGF, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF), or vascular endothelial growth factor (VEGF) [16-19]. In our earlier studies, we demonstrated that the sequestration of Spry2 enhances the extracellular signal-regulated kinase (ERK) activation, osteoblast proliferation, and osteoblastogenesis. On the other hand, Spry2 suppression showed decreased the activation of ERK, thereby inducing gingival epithelial cell proliferation [20]. Furthermore, we found showed that Spry2 depletion promoted cell migration and proliferation, whereas it interfered with differentiation to osteoblasts in periodontal ligament stem cell line [21]. Thus, Spry2 knockdown may effectively induce migration of the periodontal ligament along the root surface of a tooth, and the increase of alveolar bone, while impeding the down-growth of gingival epithelia in bone defects. These two previous studies indicated that Spry2 depletion may create favorable conditions in periodontal regeneration. The inflammation caused by Pg LPS must be resolved, and M2 alternative activated macrophages play an important role in periodontal wound healing during the start of periodontal remodeling. Accordingly, it is fundamental to examine the physiological mechanisms through which the downregulation of Spry2 by the stimulation with Pg LPS influences macrophage functions.
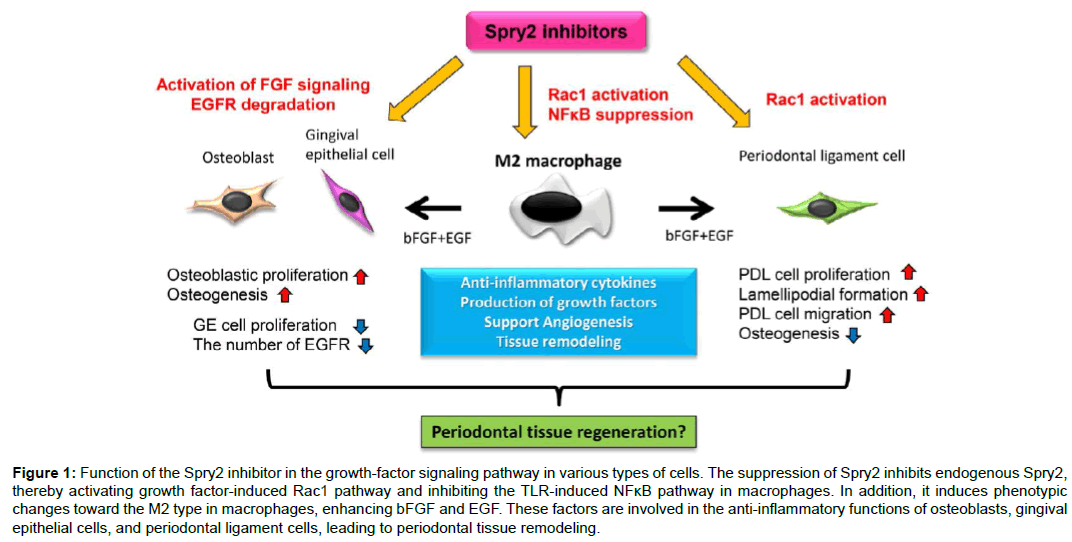
In the recent paper in our laboratory entitled “Inhibition of Sprouty2 polarizes macrophages toward an M2 phenotype by stimulation with interferon γ and Porphyromonas gingivalis lipopolysaccharide”, we found that inhibition of Spry2 in combination with Pg LPS and IFNγ converts macrophages to the M2 phenotype, and may effectively resolve inflammation by producing anti-inflammatory cytokines such as IL-10 and various types of growth factors in macrophages [22]. First, Spry2 knockdown promoted the activation of growth factor-induced AKT/phosphoinositide 3-kinase (PI3K) and Rho family GTPases, specifically Rac1, in macrophages, thereby increasing the efferocytosis of apoptotic cells after Pg LPS and IFNγ stimulation. In addition, we demonstrated that the recognition and engulfment of apoptotic cells, called “efferocytosis”, result in the release of anti-inflammatory cytokines, including IL-10 by the suppression of Spry2 [22]. Second, AKT/PI3K signaling can activate TLR pathway induced by LPS in a feedback loop which suppresses the phosphorylation of TLR activators. Our experiments suggested that AKT/PI3K suppressed by Spry2 promoted the activation of NFκB p65 and IκB degradation. These interactions between TLR and RTK pathway such as growth factor signaling decreased interference with polarization toward M1 macrophages [22].
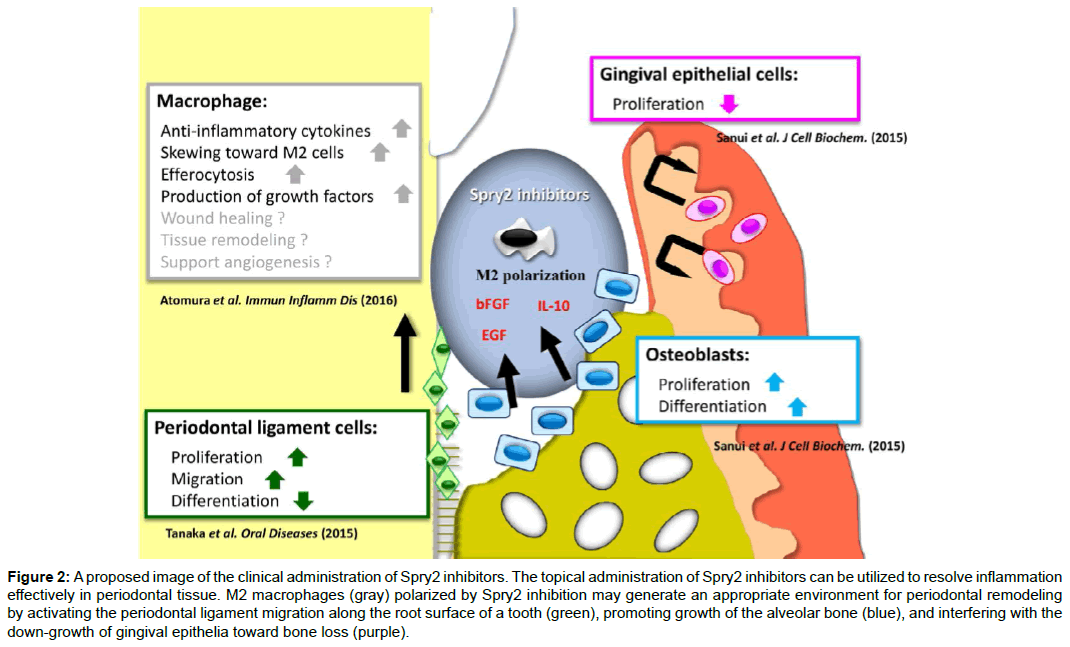
Consequently, our results demonstrate that Spry2 inhibitors suppress endogenous Spry2, thereby inducing the activation of growth factor-induced Rac1 and AKT/PI3K, and inhibiting the TLR-induced NFκB pathway in macrophages. Moreover, Spry2 suppression induces change of the macrophage phenotype toward the M2 type, and enhances the production of bFGF and EGF, which are involved in the cell functions between osteoblasts, gingival epithelial cells, and periodontal ligament cells (Figure 1). Taken together, these studies show that the topical application of Spry2 inhibitors to bone loss may generate an appropriate environment for periodontal remodeling by inducing M2 macrophages, resolving inflammation in periodontitis, activating the periodontal ligament migration along the root surface of a tooth, promoting growth of the alveolar bone, and interfering with gingival epithelial down-growth toward bone defects (Figure 2). These findings thus provide a molecular basis for novel therapeutic approaches in periodontal tissue regeneration.
Figure 1: Function of the Spry2 inhibitor in the growth-factor signaling pathway in various types of cells. The suppression of Spry2 inhibits endogenous Spry2, thereby activating growth factor-induced Rac1 pathway and inhibiting the TLR-induced NFκB pathway in macrophages. In addition, it induces phenotypic changes toward the M2 type in macrophages, enhancing bFGF and EGF. These factors are involved in the anti-inflammatory functions of osteoblasts, gingival epithelial cells, and periodontal ligament cells, leading to periodontal tissue remodeling.
Figure 2: A proposed image of the clinical administration of Spry2 inhibitors. The topical administration of Spry2 inhibitors can be utilized to resolve inflammation effectively in periodontal tissue. M2 macrophages (gray) polarized by Spry2 inhibition may generate an appropriate environment for periodontal remodeling by activating the periodontal ligament migration along the root surface of a tooth (green), promoting growth of the alveolar bone (blue), and interfering with the down-growth of gingival epithelia toward bone loss (purple).
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research C (17K11986) from the Japan Society for the Promotion of Science, and Takeda Science Foundation. Technical support was provided from the Research Support Center, Graduate School of Medical Sciences, Kyushu University.
Author Disclosure Statement
The authors have declared that no conflicts of interest exist.
References
- Oliver RC, Brown LJ (2000) Periodontal diseases and tooth loss. Periodontol 2: 117-127.
- Darveau RP (2010) Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8: 481-490.
- Moskow BS, Polson AM (1991) Histologic studies on the extension of the inflammatory infiltrate in human periodontitis. J Clin Periodontol 18: 534-542.
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL (1998) Microbial complexes in subgingival plaque. J Clin Periodontol 25: 134-144.
- Hajishengallis G, Darveau RP, Curtis MA (2012) The keystone-pathogen hypothesis. Nat Rev Microbial 10: 717-725.
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, et al. (1999) Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11: 443-451.
- Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, et al. (2004) Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both Toll-like receptors 2 and 4. Infect Immun 72: 5041-5051.
- Dalton DK, Pitts-Keek S, Keshav S, Figari IS, Bradley A, et al. (1993) Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259: 1739-1742.
- Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 23-25.
- Montaner LJ, da Silva RP, Sun J, Sutterwala S, Hollinshead M, et al. (1999) Type 1 and type 2 cytekine reglation of macrophage endocytosis: differential activation by IL-4/IL-13 as opposed to IFN-γ or IL-10. J Immunol 162: 4606-4613.
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE (1999) The IL-4 receptor: Signaling mechanisms and biologic functions. Annu Rev Immunol 17: 701-738.
- Mantovani A, Allavena P, Sica A (2004) Tumour-associated macrophages as a prototypic type II polarized phagocyte population: role in tumour progression. Eur J Cancer 40: 1660-1667.
- Stout RD, Suttles J (2004) Functional plasticity of macrophages: reversible adaption to changing microenvironments. J Leukoc Biol 76: 509-513.
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA (1998) Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell 92: 253-263.
- Kramer S, Okabe M, Hacohen N, Krasnow MA, Hiromi Y (1999) Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Dev 126: 2515-2525.
- Gross I, Bassit B, Benezra M, Licht JD (2001) Mammalian sprout proteins inhibit cell growth and differentiation by preventing ras activation. J Biol Chem 276: 46460-46468.
- Impagntiello MA, Weitzer S, Gannon G, Compagni A, Cotton M, et al. (2001) Mammalian Sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells. J Cell Biol 152: 1087-1098.
- Hanafusa H, Torii S, Yasunaga T, Nishida E (2002) Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signaling pathway. Nat Cell Biol 4: 850-858.
- Yusoff P, Lao DH, Ong SH, Wong ES, Lim J, et al. (2002) Sprouty2 inhibits the Ras/KAP kinase pathway by inhibiting the activation of Raf. J Biol Chem 277: 3195-3201.
- Sanui T, Tanaka U, Fukuda T, Toyoda K, Taketomi T, et al. (2015) Mutation of Spry2 induces proliferation and differentiation of osteoblasts but inhibits proliferation of gingival epithelial cells. J Cell Biochem 116: 628-639.
- Tanaka U, Sanui T, Fukuda T, Toyoda K, Taketomi T, et al. (2015) Sprouty2 inhibition promotes proliferation and migration of periodontal ligament cells. Oral Dis 21: 977-986.
- Atomura R, Sanui T, Fukuda T, Toyoda K, Tanaka U, et al. (2016) Inhibition of Sprouty2 polarizes macrophages toward an M2 phenotype by stimulation with interferon γ and Porphyromonas gingivalis lipopolysaccharide. Immun Inflamm Dis 4: 98-110.
Citation: Sanui T, Fukuda T, Tanaka U, Toyoda K, Yotsumoto K, et al. (2017) Sprouty2 Inhibition Resolves Inflammation in Periodontal Disease and Creates a Suitable Environment for Periodontal Tissue Regeneration. J Cell Biol Immunol 1: 101.
Copyright: © 2017 Sanui T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 6379
- [From(publication date): 0-2017 - Nov 25, 2024]
- Breakdown by view type
- HTML page views: 5414
- PDF downloads: 965