Research Article Open Access
SPR Based Fiber Optic Sensor for the Detection of Vitellogenin: An Endocrine Disruption Biomarker in Aquatic Environments
Sachin K. Srivastava1*, Roli Verma2, Banshi D. Gupta3, Isam Khalaila4 and Ibrahim Abdulhalim1,5
1Department of Electro optic Engineering and Ilse Katz Institute for Nanoscale Science and Technology, Ben Gurion University, Israel
2School of Chemistry, Reymond and Beverly Sackler Faculty of Exact Sciences, Tel Aviv University, Israel
3Department of Physics, Indian Institute of Technology Delhi, India
4The Avram and Stella Goldstein-Goren Department of Biotechnology Engineering, Ben Gurion University, Israel
5School of Materials Science and Engineering, Nanyang Technological University, Singapore
- Corresponding Author:
- Sachin KS
Department of Electro optic Engineering and Ilse Katz Institute for Nanoscale Science and Technology
Ben Gurion University, Beer Sheva-84105, Israel
Tel: +972-587-120-784
E-mail: sachinchitransh@gmail.com
Received Date: January 25, 2015; Accepted Date: May 27, 2015; Published Date: May 29, 2015
Citation: Srivastava SK, Verma R, Gupta BD, Khalaila I, Abdulhalim I (2015) SPR Based Fiber Optic Sensor for the Detection of Vitellogenin: An Endocrine Disruption Biomarker in Aquatic Environments Biosens J. 4:114. doi:10.4172/2090-4967.1000114
Copyright: © 2015 Srivastava SK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biosensors Journal
Abstract
We have fabricated a fiber optic SPR biosensor for the detection of vitellogenin (Vg), an endocrine disruption biomarker in aquatic environments. The sensor was fabricated by immobilizing anti-Vg antibodies on the sensor surface. Control experiments performed on another similar protein, fetuin and on a sensing probe without the anti-Vg antibody receptor confirmed the specificity of the sensor. The limit of detection of the sensor was found to be as small as 1 ng/ml in our experimental window. The sensitivity of the sensor was 0.48 nm/(ng/ml). This sensor can be utilized for remote monitoring of the water bodies for any endocrine disruption phenomena.
to excite them this way due to the mismatch in the propagation constant (phase mismatch). However, the SPs get resonantly excited when the light incident somehow gains the propagation constant equal to the phase mismatch. Practically the phase matching is satisfied by employing a technique called Kretschmann configuration, where the base of a high index prism is coated with a thin layer of metal and the dielectric medium (with a refractive index lower than that of the prism) further surrounds it. The extra phase gained by the light is provided by the high index prism. In an optical fiber collaborated with SPR technology, the core of the optical fiber replaces the high index prism in the Kretschmann configuration. A small portion of the cladding from the optical fiber is removed and coated with a thin metal film, which comes in the contact of another dielectric layer. The light launched in the optical fiber gets guided through it due to the phenomenon of total internal reflection (TIR). The small part of the light guided through the optical fiber gains the required phase sufficient for the excitation of the SPs on the metal-dielectric interface. As a result of the SPR, a dip in the spectrum transmitted off the output end of the optical fiber is observed. The propagation constant and hence the SP resonance condition depends on the refractive indices of both the metal and the dielectric media. Any change in the refractive index of the dielectric medium leads to a change in the resonance wavelength. This property of SPs is generally utilized in sensing applications. A number of studies on chemical and bio sensors based on SPR based fiber optic probes have been reported in the literature [8-12]. The plasmons have also been utilized for the enhancement of the performance of various sensors [13-15]. Efforts have been made to enhance the performance of fiber optic plasmonic sensors as well [16].
In the last few years, certain environmental contaminants that potentially affect the endocrine system have got lots of concerns due to the increase in the number of symptoms of new diseases. Numerous efforts have been made for the detection of endocrine disruption compounds (EDCs) or their markers [17-20]. The endocrine system is an ensemble of various glands which secrete different types of hormones directly into the circulatory system to maintain homeostasis, while the exocrine system secretes hormones via ducts. It regulates many vital processes such as metabolism, development, growth, reproduction, lactation and stress in a living organism. Any disturbance in the functioning of the endocrine system affects metabolism, birth rate, growth, etc., which lead to many health complexities, such as diabetes, infertility, indigestion, migraine, etc. Such compounds or factors which negatively affect the functions of the endocrine system are called endocrine disruptors. A few suspected EDCs can be named as heavy metals (such as Hg, As, Ni, Pb, Cd etc.), pesticides and herbicides (such as DDT, atrazine), personal care products (such as phthalates and benzaldehydes), pharmaceutical drugs (such as carticosteroids), synthetic and natural hormones (such as phytoestrogens), industrial chemicals (such as bisphenol A), etc. According to Kavlock et al. [17] “Endocrine disruptors are exogeneous agents that interfere with the production, release, transport, metabolism, binding, action or elimination of natural hormones in the body responsible for the maintenance of homeostasis and regulation of developmental processes” [21]. One of the main themes of work of the Organization for Economic Cooperation and Development (OECD) is the development of screening and testing programs for endocrine disrupting compounds in sea or other large fresh water bodies [22]. One of the potential endocrine disruption biomarker compounds is the egg yolk protein vitellogenin (Vg) of water vertebrate and invertebrate animals. It is a globular glycolipoprotein, which is produced as a yolk protein precursor in the liver of oviparous vertebrates. It is secreted by the hepatocytes when the estradiol receptors bind with 17 β-estradiol produced in the females during sexual maturation and is considered as a sign of the maturational state of the female [22]. About two decades ago, it was found that even male fish had surprisingly high Vg levels which might be caused by foreign chemicals or extrogeneous compounds [23,24]. During the reproductive cycle, the Vg expression changes primarily due to water contaminants such as synthetic estrogenic hormones, pesticides etc. present in sewages, streams, lakes, fish–foods and in sediments [25]. The increase of the levels of Vg is the consequence of increase in pollutants containing sex steroids [26]. Numerous studies have been performed on Vg as a marker for endocrine disruption in aquatic environments [20,23-25,27].
In the present report, we present our studies on the fabrication and characterization of a fiber optic SPR sensor for highly sensitive and specific detection of Vg. The SPR spectra for varying concentrations of Vg were recorded. Further, the control experiments were performed on a similar kind of protein fetuin and a fiber optic SPR probe without receptors to demonstrate the specificity of the sensor. The present sensor finds applications in remote monitoring of endocrine disruption in large water bodies.
Experimental Section
Reagents
Plastic clad silica (PCS) multimode optical fiber of 0.37 numerical aperture and 600 μm core diameter was purchased from Fiberguide Industries. Gold (Au) wire (99.99% pure) and chromium (Cr) (99.99% pure) were purchased from a local supplier. 4-Aminothiophenol (4-ATP), phosphate buffer saline (PBS), N-ethyl-N-(3- dimethylaminopropyl carbodimide) (EDC), N- hydroxysuccinimide (NHS), fetuin, bovine serum albumin (BSA) and glycine buffer were purchased from Sigma Aldrich. Ethanol (98.99% pure) was purchased from Bio Lab Ltd., Israel. All these chemicals were used without any further purification. Water used for making dilutions of buffers was taken from a Millipore® system.
Procurement of the Vitellogenin (Vg) protein
The female crustacean hemolymph was collected as previously described elsewhere [28] and was further subjected to a two-step density gradient centrifugation as mentioned in ref. [29]. Briefly, the lipoproteins were isolated by adjusting the hemolymph to a density of 1.35 g/ml with NaBr and ultracentrifuging it at 100,000 × g, at 4°C, for 24 hours. The lipoproteins were taken from the top of the centrifuge tube and re-adjusted to a density of 1.35 g/ml. Then the lipoproteins were placed at the bottom of an ultracentrifuge tube. The fractions of PBS adjusted with NaBr were prepared with densities: 1.25, 1.2, 1.15, 1.1, and 1.05 g/ml and then layered on the top of the lipoprotein fraction. After a second ultracentrifugation (100,000 × g, 4 °C, 24 h) was performed, the Vg-containing layer was collected, dialyzed against PBS containing 150 mM NaCl and stored at -70 °C until use. Polyclonal antibodies were raised against Vg in rabbits by immunizing them, as previously described elsewhere [30]. Briefly, a quantity of 1 mg of the purified Vg mixed with freund's complete adjuvant (Sigma, Israel) was injected subcutaneously into a rabbit. Three additional immunization of 1 mg Vg with incomplete freund's adjuvant were given once every 4 weeks. A total of 50 ml of blood was withdrawn from the rabbit ear veins at the end of the immunization period. The blood was allowed to clot at 4°C overnight. The clotted blood was centrifuged at 16,000 g for 10 min, and the serum was aliquoted and stored at -20°C until use.
Fiber optic SPR probe fabrication
Several pieces of PCS optical fiber of 600 μm core diameter and 0.37 NA were taken and a length of 1 cm of them was uncladded from the middle. Both the protective plastic jacket and the polymer cladding were removed with the help of a sharp razor blade. The uncladded portion of the fiber was cleaned with water, ethanol and acetone and blow dried with air. The uncladed fibers were then kept in the vacuum chamber of a coating unit utilizing thermal evaporation. The fibers were further cleaned for 5 minutes with high tension (HT) air plasma generated in the vacuum chamber by letting a small amount of air flow in the vacuum chamber. The plasma cleaning process removes any dirt particles on the fibers. The pressure of the chamber was kept at 5×10-2 mbar while the plasma cleaning. After the plasma cleaning, the air flow in the chamber was stopped and the high vacuum valve was opened to achieve a pressure of 5 × 10-6 mbar. The coating of the unclad core was performed at this vacuum. At such a small pressure, the mean free path of the metal flux becomes greater than the height of the vacuum chamber and negligibly small amount of air molecules remain in the chamber; which lead to negligible scattering of the evaporated flux and hence produce a very smooth coating. After a stable vacuum condition was reached, the low tension (LT) current was slowly increased until the Cr/Au in the metallic boat in the vacuum chamber starts to melt. The shutter was then opened to let the evaporated metal plume coat on the optical fiber. The fibers were rotated at an angle of 1200 and the coating was performed in 3 steps to achieve a uniform film. The films of 2 nm of Cr and 50 nm of Au were coated using the above technique. Cr acts as an adhesive layer between the optical fiber core and Au [8]. The thickness of the metal film and the rate of deposition were measured online with the help of a digital thickness monitor inside the vacuum chamber. It is basically a quartz crystal microbalance. The details of its working can be found elsewhere [31]. The fibers were kept in the vacuum chamber, until further use.
Functionalization of the sensor surface
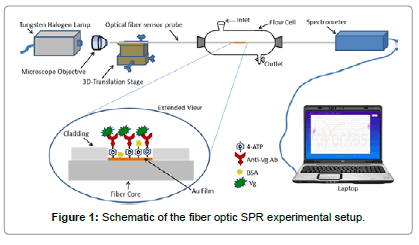
The fiber optic SPR probes were incubated in 1% 4-ATP solution in ethanol (W/W) for overnight. The 4-ATP molecules get tightly bound to the Au surface because of thiol bonding. The overnight incubation of Au coated region of the optical fiber in 4-ATP solution provided a monolayer of 4-ATP molecules adsorbed on the Au surface. The incubated optical fibers were rigorously washed in ethanol and Millipore® water to remove any unbound 4-ATP molecules. The –NH2 group of 4-ATP provides an opportunity for the linkage with other molecules. Anti-vitellogenin (Anti-Vg) antibody was immobilized by covalent bonding on the –NH2 terminal of 4-ATP, by incubating the 4-ATP functionalized fiber probes in the antibody solution. Before immersing the optical fibers in the antibody solution, the anti-Vg antibody was conjugated by EDC-NHS Chemistry to increase the coupling efficiency. A rigorous discussion of the process can be found elsewhere [32]. The conjugation of the antibody with EDC-NHS allowed the direct immobilization of the antibody on the 4-ATP functionalized fiber optic SPR probes. Briefly, 0.2 mM solution of EDC and 0.05 mM solution of NHS were prepared in 50 mM PBS solution. Equal volumes of EDC and NHS mixtures were then mixed with the antibody serum to react for two hours at 4°C. The 4-ATP functionalized optical fibers were then incubated in the EDC-NHS modified antibody serum for overnight at 4°C. This incubation provided the immobilization of the antibodies on the –NH2 terminals of ATP layer. Afterwards, the optical fibers were taken out of the antibody solution, washed rigorously with Millipore® water, dried in nitrogen gas and incubated in BSA solution of 1 mg/ml concentration for one hour at 4°C. The incubation of antibody immobilized fiber optic SPR probe in BSA solution was performed to make the sensor surface anti-fouling. That is to say, BSA binds on any sites of the sensor surface which can offer any kind of non-specific binding. This step makes the sensor highly specific to Vg only. The sensors probes were rigorously washed with PBS and Millipore® water and blow dried with nitrogen after all the incubations and kept into a refrigerator at 4°C before further use. A schematic of the functionalized sensor surface is shown in Figure 1 in the extended view of the sensor surface.
Experimental setup
The sample solutions of varying concentrations ranging from 0 ng/ ml to 25 ng/ml of Vg and fetuin were prepared in 50 mM PBS solution by making appropriate dilutions. The fiber optic sensor was fixed in a flow cell as shown in Figure 1. The flow cell has the provisions for input and output of liquid samples. Before fixing the fiber probe into the flow cell, both the ends of it were cleaved with a tungsten carbide cutter. After fixing the fiber into the flow cell, both the ends were wiped with acetone soaked optical tissue. The polychromatic light from a power stabilized tungsten-halogen light source was coupled to the input end of the optical fiber sensor probe with the help of a microscope objective of 0.65 NA. The light transmitted off the output end of the optical fiber sensor was coupled to a spectrometer interfaced with a laptop to record the SPR spectra. The spectrometer recorded the optical power transmitted off the output end of optical fiber as a function of wavelength. The SPR spectra for the varying concentrations of Vg and fetuin were recorded. The sensor surface was incubated in the Vg sample for 2 minutes before recording the SPR spectrum for each concentration. After recording of SPR spectrum for each sample solution, the sensing region was regenerated for the use of the sensor for the next time. In the regeneration process, the sensing region was firstly washed with running PBS buffer and then regenerated with running glycine buffer (0.2 M of pH 2.4) for 2 minutes. After emptying the glycine buffer from the flow cell, the sensing surface was washed by Millipore® water for removing all the flavor of glycine buffer. Moreover, PBS was introduced in the flow cell to further wash the sensing region. After removing the PBS solution from the flow cell, another sample solution of Vg/fetuin was introduced into the flow cell. The same process was followed in between the recording of SPR spectra for any two sample solutions of Vg or fetuin.
Results and Discussion
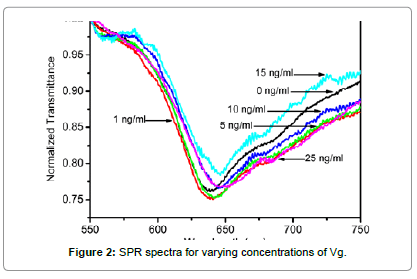
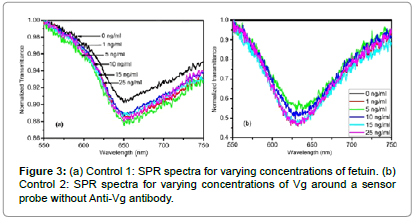
SPR spectra for varying concentrations of Vg are shown in Figure 2. It is observed that a dip in output transmitted power is achieved due to the excitation of surface plasmons on the interface of the metal and sensing medium (Vg sample solution) for all the samples of different concentrations of Vg. The wavelength corresponding to the minimum transmission is termed as resonance wavelength, and is a characteristic of the Vg sample of a particular concentration. A red shift in the SPR spectra is observed with an increase in the Vg concentration around the sensing region. To demonstrate the specificity of our sensor, we performed two control experiments: one on the same sensor probe with another similar protein called fetuin, which we named as Control 1; while another on a fiber optic SPR sensing probe without the attachment of the receptor, i.e. the anti-Vg antibody, named as Control 2. In the Control 1 experiment, the SPR spectra were recorded for varying concentrations of fetuin around the sensor probe with the anti- Vg antibody receptor. The SPR spectra for varying concentrations of fetuin are plotted in Figure 3a. It is observed that similar to Vg, a dip in the transmission curve is obtained for all the sample solutions. This dip is attributed to the SPR phenomena due to the liquid medium around the sensor probe. However, no significant shift in the SPR curves is observed with an increase in the fetuin concentration around the sensor probe. In the Control 2 experiment, a fiber optic SPR probe with the same fabrication parameters was fixed in the same flow cell and the SPR spectra for varying concentration of Vg around this probe were recorded. The sensor surface in the present case was not functionalized with the receptor to prove the fact that it is the binding of the Vg with anti-Vg antibody which leads to a change in the resonance wavelength and not the change in the refractive index of the sample solution with an increase in the Vg concentration. The refractive indices of the sample solutions for such a small concentration variation are generally the same up to the third decimal place of the refractive index. Even then it might be claimed that the shift in the resonance wavelength is due to small changes in the refractive index around the probe. The SPR spectra for varying concentrations of Vg, for Control 2 experiment have been plotted in Figure 3b. It is observed that no shift in the SPR spectrum is observed with an increase in the Vg concentration in the sample solutions around the probe. Hence, it is proved that the shift in the SPR curves, shown in Figure 2, is totally due to the binding of the Vg molecule to anti-Vg antibody. To access a quantitative response of the sensor
We have plotted the change in resonance wavelength referenced to that of 0 ng/ml concentration (λSample–λ0) for both the proteins with respect to the change in concentration in Figure 4. A close observation of the SPR spectra for all the above mentioned cases reveals that the SPR resonance wavelengths are found in different ranges of the electromagnetic spectrum even for the sample solution of 0 ng/ml concentration, which should have been the same in all the aforementioned cases. Such a response is obtained because of the light launching conditions in the optical fiber. Since the dimensions of the fiber core are very small, it is practically impossible to control the excitation of desired number of modes in a highly multimode optical fiber. Also, off-axis excitation leads to the guidance of skew rays, which affect the performance of the sensor [33,34]. Since the excitation of the same modes is not possible in different optical fiber sensing probes, characterized separately, the reference resonance wavelengths differ for sample of 0 ng/ml concentration. However, the difference in the resonance wavelengths for the sample solutions in the considered range of operation was found to be the same. Therefore, in Figure 4, we have plotted the shift in resonance wavelength from the reference (resonance wavelength corresponding to 0 ng/ml). The response curves for Control 1 and Control 2 experiments have been plotted alongside to that of Vg to make a quantitative comparison. The symbols represent the experimental data pints extracted from Figures 2 and 3, while the curves passing through them are the best linear fits. The X and Y-error bars correspond to the maximum probable errors, which were calculated by considering the errors which might have occurred due to inherent errors of the micro-pipettes, measuring cylinders, and the spectrometer used in our study. It is not possible to see the error bars due to very small values of error. Therefore, we have presented a zoomed in section of the response curves in the inset to visualize the error bars. It is observed that with an increase in the Vg concentration from 0 to 25 ng/ml, a total shift of 12.03 nm occurs in the resonance wavelength. However, almost no net shift in the resonance wavelength is observed for fetuin in Control 1 and Vg in Control 2 experiments respectively for the considered sample concentration range. This confirms the specificity of our sensor. Slight fluctuations in resonance wavelength are observed in both the control experiments, which lie well within the accuracy and resolution of the spectrometer used. The refractive indices of all the sample solutions of Vg are the same up to the third decimal place. Even then, a red shift in resonance wavelength is observed with an increase in Vg concentration. The mechanism of red shift in SPR curves and hence in resonance wavelength with an increase in the Vg concentration can be understood by following mechanism. When the Vg molecules come into the vicinity of the sensor surface, immobilized with the anti-Vg antibody, they get attached to it, which leads to a change in the local refractive index near the sensor surface. This small change in the local refractive index is further translated to the shift in the SPR curve. Successive binding of higher concentrations of Vg leads to greater increase in the local refractive index and hence a red shift in the resonance wavelength is observed. While, no shift in resonance wavelength is observed for fetuin, because the fetuin molecules do not bind to the sensor surface and hence no change in the local refractive index occurs, which translates into a static SPR curve with respect to concentration. The response of the sensor is linear in our experimental window. The sensitivity can be defined as the shift in the resonance wavelength for unit change in concentration of the analyte medium. The sensitivity of the sensor calculated from the slope of the response curve is 0.48 nm/(ng/ml), which is not as high as that of SERS based sensors [27]. However, for aquatic environments, the sensitivity of the sensor is fairly enough to detect any endocrine disruption, as the elevated levels of Vg reach to the concentrations as high as few μg/ml [25]. The resolution of the sensor is limited by the resolution of the spectrometer. In our case, the resolution of the spectrometer was 1 nm. This implies that resolution of our sensor with the present spectrometer integrated with it was about 2 ng/ml. For a spectrometer with improved spectral resolution, the resolution of the sensor can further be improved. The same sensor was used for the characterization of all the sample solutions. The repeatability of the response was confirmed for three times at different days with different fiber optic sensing probes. Hence, the present sensor has the capabilities of reusability and repeatability. Further, the sensor presents additional advantages of online measurements and remote sensing, as the optical fibers can be used for carrying information without much loss to large distances. The sensor utilizing evanescent wave approach of excitation of SPR in the present case makes its performance unaffected by any kind of impurities/scatterers/opacities in the sample, which might lead to additional scattering and hence a change in response of the SERS based sensors. Hence, the present sensing mechanism might be used for the sensing of even non-transparent media such as blood, etc.
Conclusion
We have fabricated and characterized a SPR based fiber sensor for the detection of endocrine disruption biomarker vitellogenin in aquatic environments. The control experiments on fetuin established the specificity of our sensor for Vg. The response of the sensor was found to be linear in our experimental window from 0 to 25 ng/ml with a sensitivity equals to 0.48 nm/(ng/ml) while the resolution was 2 ng/ml. Such a sensor with high sensitivity and capability of ng concentrations detection has proven to be advantageous over state of the art endocrine disruption techniques. The sensor presents added advantages of capabilities of online monitoring and remote sensing in large aquatic environments.
Acknowledgements
This Research was conducted by NTU-HUJ-BGU Nanomaterials for Energy and Water Management Programme under the Campus for Research Excellence and Technological Enterprise (CREATE), that is supported by the National Research Foundation, Prime Minister’s Office, Singapore. The support of the Council for Scientific and Industrial Research (CSIR), India is also appreciated. Sachin K. Srivastava and Roli Verma thank to the Council for Higher Education of the Government of the State of Israel for PBC post-doctoral fellowships.
Author Contribution
Sachin K. Srivastava performed all the surface functionalizations and characterization experiments. He analyzed the results and wrote the manuscript. Roli Verma fabricated the SPR based optical fiber probe. Isam Khalaila extracted and procured the Vg and anti-Vg antibodies. Banshi D. Gupta and Ibrahim Abdulhalim supervised the project, helped in analysis of experimental results and wrote parts of the manuscript.
References
- Jorgenson RC, Yee SS (1993) A fiber-optic chemical sensor based on surface plasmon resonance. Sensors and Actuators B: Chemical 1: 213-220.
- Nirschl M, Reuter F,Vörös J (2011) Review of transducer principles for label-free biomolecular interaction analysis. Biosensors (Basel) 1: 70-92.
- Srivastava SK,Hamo HB, Kushmaro A, Marks RS, Grüner C, et al. (2015) Highly sensitive and specific detection of E. coli by a SERS nanobiosensor chip utilizing metallic nanosculptured thin films. Analyst 140: 3201-3209.
- Ebbesen TW, Lezec HJ, Ghaemi HF, Thio T, Wolff PA (1998)Extraordinary optical transmission through sub-wavelength hole arrays. Nature 39: 667-669.
- Abdulhalim I, Karabchevsky A, Patzig C, Rauschenbach B, Fuhrmann B et al. (2009) Surface-enhanced fluorescence from metal sculptured thin films with application to biosensing in water. Applied Physics Letters 94: 063106-063106-063103.
- Xiao N, Wang C, Yu C (2013) A Self-Referencing Detection of Microorganisms Using Surface Enhanced Raman Scattering Nanoprobes in a Test-in-a-Tube Platform. Biosensors (Basel) 3: 312-326.
- Osawa M (2006) Surface-enhanced infrared absorption spectroscopy. In Handbook of vibrational spectroscopy, John Wiley & Sons, Ltd: 2006.
- Srivastava SK, Gupta BD (2013)Fiber optic plasmonic sensors: Past, present and future. The Open Optics Journal Bentham.
- Srivastava SK, Verma R, Gupta BD (2012) In Surface plasmon resonance based fiber optic glucose biosensor, Proceedings of SPIE 83511Z-83516.
- Verma R, Gupta BD (2013) Optical fiber sensor for the detection of tetracycline using surface plasmon resonance and molecular imprinting. Analyst 138: 7254-7263.
- Verma R, Gupta BD (2014) A novel approach for simultaneous sensing of urea and glucose by SPR based optical fibermultianalyte sensor. Analyst 139: 1449-1455.
- Verma R, Gupta BD (2015) Detection of heavy metal ions in contaminated water by surface plasmon resonance based optical fibre sensor using conducting polymer and chitosan. Food Chemistry 166: 568-575.
- Poghossian A, Weil M, Cherstvy AG, Schöning MJ (2013) Electrical monitoring of polyelectrolyte multilayer formation by means of capacitive field-effect devices. Anal BioanalChem 405: 6425-6436.
- Cherstvy AG (2014) Electrostatics and charge regulation in polyelectrolyte multilayered assembly. The Journal of Physical Chemistry B 118: 4552-4560.
- Abouzar MH, Poghossian A, Cherstvy AG, Pedraza AM, Ingebrandt S et al. (2012) Label-free electrical detection of DNA by means of field-effect nanoplate capacitors: Experiments and modeling. physica status solidi 209: 925-934.
- Srivastava SK, Gupta BD (2011) A multitaperedfiber-optic spr sensor with enhanced sensitivity. Photonics Technology Letters, IEEE 23: 923-925.
- Dostálek J,Pribyl J, Homola J, Skládal P (2007) Multichannel SPR biosensor for detection of endocrine-disrupting compounds. Anal BioanalChem 389: 1841-1847.
- Gobi KV, Kataoka C, Miura N (2005) Surface plasmon resonance detection of endocrine disruptors using immunoprobes based on self-assembled monolayers. Sensors and Actuators B: Chemical 108: 784-790.
- Karabchevsky A,Tsapovsky L, Marks RS,Abdulhalim I (2013) Study of Immobilization Procedure on Silver Nanolayers and Detection of Estrone with Diverged Beam Surface Plasmon Resonance (SPR) Imaging. Biosensors (Basel) 3: 157-170.
- Kim N, Kim DK, Cho YJ, Moon DK, Kim WY (2008) Carp vitellogenin detection by an optical waveguide lightmode spectroscopy biosensor. BiosensBioelectron 24: 391-396.
- Utsumi T, Yoshimura Y(2011) Applicability of lectinhistochemistry in a test system with in ovo treatment for detecting androgenic and antiandrogenic effects of chemicals in japanese quail (coturnix japonica). Poultry Science 90: 168-174.
- Nilsen BM, Berg K, Eidem JK, Kristiansen SI, Brion F, et al. (2004) Development of quantitative vitellogenin-ELISAs for fish test species used in endocrine disruptor screening. Anal BioanalChem 378: 621-633.
- Sumpter JP,Jobling S (1995) Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect 103 Suppl 7: 173-178.
- Harries JE, Sheahan DA, Jobling S, Matthiessen P, Neall P et al. (1997) Estrogenic activity in five united kingdom rivers detected by measurement of vitellogenesis in caged male trout. Environmental Toxicology and Chemistry 16: 534-542.
- Rodríguez EM,Medesani DA, Fingerman M (2007) Endocrine disruption in crustaceans due to pollutants: a review. Comp BiochemPhysiol A MolIntegrPhysiol 146: 661-671.
- Scott AP, Katsiadaki I, Kirby MF, ThainJ (2006) Relationship between sex steroid and vitellogenin concentrations in flounder (platichthysflesus) sampled from an estuary contaminated with estrogenic endocrine-disrupting compounds. Environmental Health Perspectives 114: 27-31.
- Srivastava SK, Shalabney A, Khalaila I, Grüner C, Rauschenbach B, et al. (2014) SERS biosensor using metallic nano-sculptured thin films for the detection of endocrine disrupting compound biomarker vitellogenin. Small 10: 3579-3587.
- Okuno A, Yang WJ, Jayasankar V, Saido-Sakanaka H, Huong DTT et al. (2002) Deduced primary structure of vitellogenin in the giant freshwater prawn, macrobrachiumrosenbergii, and yolk processing during ovarian maturation. Journal of Experimental Zoology 29: 417-429.
- Roth Z,Parnes S, Wiel S, Sagi A, Zmora N, et al. (2010) N-glycan moieties of the crustacean egg yolk protein and their glycosylation sites. Glycoconj J 27: 159-169.
- Sagi A, Khalaila I, Abdu U, Shoukrun R, Weil S (1999) A newly established elisa showing the effect of the androgenic gland on secondary-vitellogenic-specific protein in the hemolymph of the crayfish cheraxquadricarinatus. General and Comparative Endocrinology 115: 37-45
- Gupta BD,Srivastava SK, Verma R (2015)Fiber optic sensors based on plasmonics. World Scientific Publishing Co: Singapore.
- Verma R,Srivastava SK, Gupta BD (2012) Surface-plasmon-resonance-based fiber-optic sensor for the detection of low-density lipoprotein. Sensors Journal, IEEE 1: 3460-3466.
- Dwivedi YS, Sharma AK, Gupta BD (2007) Influence of skew rays on the sensitivity and signal-to-noise ratio of a fiber-optic surface-plasmon-resonance sensor: a theoretical study. Appl Opt 46: 4563-4569.
- Srivastava SK, Verma RK, Gupta BD (2009) Theoretical modeling of a localized surface plasmon resonance based intensity modulated fiber optic refractive index sensor. Appl. Opt. 48: 3796-3802.
Relevant Topics
- Amperometric Biosensors
- Biomedical Sensor
- Bioreceptors
- Biosensors Application
- Biosensors Companies and Market Analysis
- Biotransducer
- Chemical Sensors
- Colorimetric Biosensors
- DNA Biosensors
- Electrochemical Biosensors
- Glucose Biosensors
- Graphene Biosensors
- Imaging Sensors
- Microbial Biosensors
- Nucleic Acid Interactions
- Optical Biosensor
- Piezo Electric Sensor
- Potentiometric Biosensors
- Surface Attachment of the Biological Elements
- Surface Plasmon Resonance
- Transducers
Recommended Journals
Article Tools
Article Usage
- Total views: 15853
- [From(publication date):
June-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11053
- PDF downloads : 4800