Case Report Open Access
Spinal Analgesic Drug Delivery for Ehlers-Danlos Hypermobility Type Chronic Pain Treatment: A Case Report
| Van Tilburg CWJ* | |
| Consultant Anesthesiologist and Pain Specialist, Department of Anesthesiology, Bravis hospital, The Netherlands | |
| *Corresponding Author : | Cornelis W. J. van Tilburg MD, FIPP, Consultant Anesthesiologist and Pain Specialist Multidisciplinary pain center, Department of Anesthesiology Bravis hospital, Boerhaaveplein 1, 4624 VT Bergen op Zoom, The Netherlands Tel: 0031-887067697 Fax: 0031 887067699 E-mail: vtilburg@ziggo.nl |
| Received: January 17, 2016; Accepted: January 17, 2016; Published: February 26, 2016 | |
| Citation: Van Tilburg CWJ (2016) Spinal Analgesic Drug Delivery for Ehlers-Danlos Hypermobility Type Chronic Pain Treatment: A Case Report. J Pain Relief 5:235. doi:10.4172/2167-0846.1000235 | |
| Copyright: © 2016 Van Tilburg CWJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pain & Relief
Abstract
Study background: Chronic pain in patients with Ehlers-Danlos hypermobility type can be a severe, debilitating disorder. Scientific literature on pain treatment in these patients is scarce. We present a case report on spinal analgesic drug delivery for diminishing pain. Methods: Multidisciplinary consultation in adult female patient with Ehlers-Danlos, hypermobility type. Implanting Synchromed® II pump (Medtronic, Minneapolis, MN, United States of America) after successful trial period. One year follow-up. Results: During the trial period, verbal Numerical Rating Scale decreased from 8 to 3 with 0.72 mg of spinal morphine (2 mg/mL, 0.36 mL/day) and no oral or transdermal opioids. The spinal morphine/hydromorphone infusion schemes and verbal NRS for pain after implantation are presented. Baseline and follow-up results at one year from the other questionnaires are presented. Wound healing was successful. Conclusion: Spinal analgesic drug delivery can be an option to treat chronic, widespread pain in patients with Ehlers-Danlos syndrome, hypermobility type. Multidisciplinary consultation is necessary to deal with the wide variety of problems in these patients
| Keywords |
| Ehlers-Danlos; Hypermobility type; Spinal analgesic drug delivery; Joint hypermobility syndrome; Spinal analgesia; Intrathecal analgesic drug delivery |
| Abbreviations |
| 4DSQ: Four Dimensional Symptom Questionnaire; CPAQ: Chronic Pain Acceptance Questionnaire; EDS: Ehlers-Danlos syndrome; HADS: Hospital Anxiety and Depression Scale; NRS: Numerical Rating Scale for pain; ODI: Oswestry Disability Index; PCI: Pain Coping Inventory; RAND-36: RAND 36 item health survey; TENS: Transcutaneous Electric Nerve Stimulation; TSK: Tampa Scale for Kinesiophobia |
| Introduction |
| A young, female patient (22 years, Caucasian, Body Mass Index 18.7) was referred by one of the neurologists to the pain center because of chronic, refractory lumboradicular pain, having had nucleoplasty two times at L4-L5 and one time at L5-S1, lumbar epidural infiltration, radiofrequent disc denervation at L4-L5 and transcutaneous electric nerve stimulation (TENS) in another hospital. Consultation of a psychologist as well as rehabilitation specialist had already been established, and treatment with anti-nociceptive (Paracetamol, Tramadol) medication had commenced. Genetic counselling, recommended by the rehabilitation specialist had revealed Ehlers- Danlos syndrome (EDS), hypermobility type. No further (genetic) investigations were performed at that moment. |
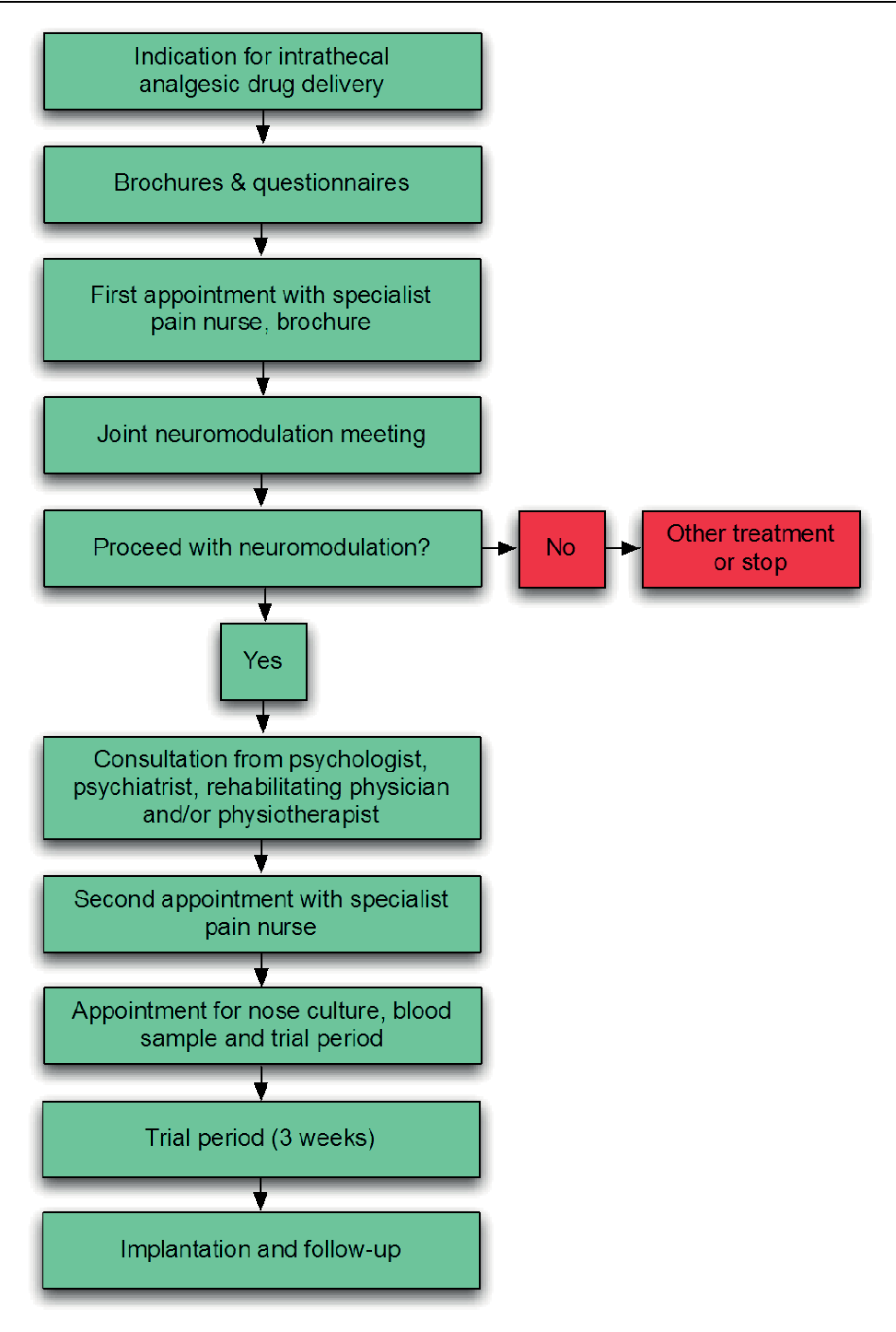
| During medical history she complained of wide spread pain (back pain above all), refractory to all offered treatment so far, as well as fatigue, wrenching and a decreased effect of local anaesthesia revealed at the age of 10. We decided to initiate oral (Gabapentine, ceased because of side effects) and transdermal (Buprenorphine, slightly effective but with side effects) medication, changing the regimen to oxycodon (moderately effective but with side effects) and fentanyl (moderately effective but with side effects). After extensive follow-up we decided to initiate the flowchart for spinal analgesic drug delivery (Figure 1), for which she gave informed consent. Special attention was paid to the issue of wound healing. |
| Materials and Methods |
| After multidisciplinary consultation and pre-operative screening (Figure 1), a trial period with spinal analgesic drug delivery was initiated, using an Ascenda® spinal catheter (model 8781, Medtronic, 710 Medtronic Parkway, Minneapolis, MN, United States of America), a port-a-cath system (Porthales® 4000M, Medtronic, 710 Medtronic Parkway, Minneapolis, MN, United States of America) and a Crono five ambulatory infusion pump (Intrapump® infusion systems, 920 Minters Chapel Rd, suite 200, Grapevine, TX, United States of America). Placement of the port-a-cath occurred at the site of pump implantation. An infusion regimen with morphine (Table 1) was started and oral medication was gradually decreased and stopped. After a successful three-week trial period the port-a-cath system was replaced by a Synchromed® II programmable infusion pump (model 8637-40, Medtronic, 710 Medtronic Parkway, Minneapolis, MN, United States of America), using the same flow and concentration found during the trial period. During the implantation procedures special attention was paid to wound healing (e.g. approximation of the wound edges and using Steri-strip® surgical skin closure (3M, 3M Center, St. Paul, Maplewood, Minnesota, United States of America). |
| Results |
| During the trial period, verbal Numerical Rating Scale (NRS) for pain decreased from 8 to 3 with 0.72 mg of spinal morphine (2 mg/mL, 0.36 mL/day) and no oral or transdermal opioids. The spinal morphine - and hydromorphone infusion schemes and verbal NRS for pain after implantation are presented in Table 2. Baseline and follow-up results from other questionnaires are presented in Table 3. The Hospital Anxiety and Depression Scale (HADS), RAND 36 item health survey (RAND- 36), Pain Catastrophizing Scale (PCS), Four Dimensional Symptom Questionnaire (4DSQ) and Pain Coping Inventory (PCI) revealed improvement in parameters like general health, pain acceptance, anxiety and the catastrophizing pain condition; other questionnaires (Chronic Pain Acceptance Questionnaire (CPAQ), Oswestry Disability Index (ODI) and Tampa Scale for Kinesiophobia (TSK)) could not demonstrate an improvement. Wound healing was successful. |
| Discussion |
| This case report demonstrates that spinal analgesic drug delivery can be an option to treat chronic, widespread pain in patients with EDS, hypermobility type. Multidisciplinary consultation is necessary to deal with the wide variety of problems in these patients. Randomized controlled trials investigating the efficacy of spinal analgesia in patients with EDS (or other groups of patients with chronic pain) are needed; hopefully this case report can act as a stepping-stone towards conducting such a trial. |
| EDS is a group of inherited collagen disorders, affecting more than 1.5 million people around the world and producing a wide range of symptoms and severity, three of them being chronic, debilitating pain, fatigue and poor wound healing. Men and women of all racial and ethnic backgrounds are affected. The hypermobility type (former type III) is most common [1-3]. Chronic pain is treated with opioids, heat therapy, splints or braces and surgical interventions [4]. The Ehlers- Danlos National Foundation has published multi-modal management programs for diminishing pain in patients with EDS, ranging from single (or a combination of) analgesics to surgical pain interventions such as implantation of spinal pumps [5]. |
| In the patient presented in this case report, her chronic pain, measured by means of the verbal NRS for pain, decreased from 8 to 2 using continuous spinal analgesic drug delivery. Other questionnaires also revealed improvement in parameters like general health, pain acceptance, anxiety and the catastrophizing pain condition, but not all questionnaires demonstrated such a rise (Table 3). Because of an increase in weight with 15 kg, a change was made from morphine to hydromorphone and consultation from an internist was sought after, who found no explanation. After this change, weight decreased with 5 kg, but not to the level as before the use of spinal analgesic drug therapy. Changing the analgesic regimen also resulted in a temporary increase in NRS for pain, gradually declining from 7 to 2 after several months; reasons for this to occur could be the possibility that the dose and/or flow rate were insufficient, as compared to the morphine dose and flow rate. Before implantation of the spinal pump the patient complained of voiding problems as well as sexual (orgasm) problems; hormone levels were normal. The patient received therapy from a specialist pelvic physiotherapist. Voiding problems ceased to exist, the sexual problems remained unaltered. |
| Acknowledgements |
| The author wishes to acknowledge Mrs. N. Kroonen, RN, who is involved in data collection and J. G. Groeneweg, PT, PhD, who is involved in proof reading. |
| References |
References
- Sacheti A, Szemere J, Bernstein B, Tafas T, Schechter N, et al. (1997) Chronic pain is a manifestation of the Ehlers-Danlos syndrome. J Pain Symptom Manage 14: 88-93.
- Voermans NC, Knoop H, Bleijenberg G, van Engelen BZ (2010) Pain in Ehlers-Danlos syndrome is common, severe, and associated with functional impairment. J Pain Symptom Manage 40: 370-378.
- Castori M, Morlino S, Celletti C, Celli M, Morrone A, et al. (2012) Management of pain and fatigue in the joint hypermobility syndrome (a.k.a. Ehlers-Danlos syndrome, hypermobility type): principles and proposal for a multidisciplinary approach. Am J Med Genet A 158A: 2055-2070.
- Arthur K, Caldwell K, Forehand S, Davis K (2015) Pain control methods in use and perceived effectiveness by patients with Ehlers-Danlos syndrome: adescriptive study. Disabil Rehabil 24:1-12.
- Ehlers-Danlos National Foundation (2013) Pain management medical resource guide.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 11956
- [From(publication date):
March-2016 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 11102
- PDF downloads : 854
