Spectrum of β-Globin Gene Mutations and β-Thalassemia Haplotype Analysis among the Iranian Azeri Turkish Population
Received: 02-Sep-2015 / Accepted Date: 18-Dec-2015 / Published Date: 26-Dec-2015 DOI: 10.4172/2161-1165.1000210
Abstract
Background: A variety of mutations influencing gene transcription, translation, or mRNA processing have been identified in β-thalassemia. Several studies demonstrated a nonrandom linkage of particular RFLP haplotypes with specific β-thalassemia mutations. Linkage analysis using β-globin haplotypes is a valuable tool for indirect mutation detection. Our aim was to determine the spectrum and frequency of β-thalassemia mutations among Northwestern Iranians (Azeri Turkish population) along with determining the heterozygosity and polymorphism information content (PIC) value for seven β-globin markers. Method: We investigated spectrum of β-thalassemia mutations via ARMS-PCR technique followed by sequencing technique. Also β-globin gene cluster haplotypes was determined by PCR-RFLP technique on DNA samples of the patients and his (her) parents or siblings. Result: We detected 36 different mutations among 554 non-identical mutant chromosomes. The most frequent mutation was IVSII-1 (G>A) (23.6%), followed by IVSI-110 (G>A) (11.9%) and CD8 (-AA) (10.5%). Of the various β- globin gene cluster haplotypes, haplotypes I and IV were found to be most common among normal (21.36%) and mutant (15.76%) chromosomes, respectively. The highest observed heterozygosity (48%) was found for the HindIII Gγ and HindII 5'ψß polymorphic sites, whereas the highest expected heterozygosity (50%) was predicted for the HindIII Gγ and HindII sites, which also had the highest PIC value of 0.37. Conclusion: The current study implies mutational heterogeneity among investigated population. Haplotype study results (heterozygosity and PIC), clarifies β-globin markers usefulness for tracking mutant alleles as a complementary method to confirm the genotype in prenatal diagnosis (PND) among investigated population.
Keywords: β-thalassemia; Haplotypes; DNA polymorphism; Prenatal diagnosis
163972Introduction
β-Thalassemia, an inherited hemoglobin disorder, is characterized by the reduction or absence of β -globin chain synthesis [1]. Inheritance of a single defective gene in carriers causes microcytosis and mild anemia, while two β-globin gene mutations, one inherited from each parent, results in the clinically severe disease thalassemia major, also known as Cooley’s anemia [2]. The severe form necessitates life-long blood transfusions and other medical support along with iron-chelating agents for the treatment of iron overload [3]. The treatment is expensive, requiring huge payments by the available health care resources. The detection of ß-thalassemia mutation, genetic counseling, and prenatal diagnosis (PND) programs can pave the way for the prevention of the disease and ease this health burden [4,5].
β-thalassemia is the most frequent single gene disorder in Iran, with about 25,000 β -thalassemia major patients and more than two million carriers living in the country [6,7]. The prevalence of β-thalassemia is highest in the Northern and Southern provinces, with an approximate rate of 10% [8,9]. Pre-marriage and PND programs for the prevention of ß-thalassemia have been implemented in Iran since 1997 [10]. The Ministry of Health oversees the hemoglobinopathy control program.
At the present, more than 400 mutations in the ß-globin gene have been characterized all over the world [11]. β-globin gene mutations show allelic heterogeneity at the molecular level, with varied distribution among different ethnic groups and geographical locations [12-14].
When the direct method for the detection of mutations is unsuccessful, haplotype analysis is often helpful in the identification of a βthal chromosome, with accuracy as high as 90% in informative families [15,16]. Previous studies suggest that the association between mutations and restriction fragment length polymorphism (RFLP) haplotypes is not random, and that each haplotype is usually associated with one specific type of mutation [17]. RFLP analysis of the β-globin gene cluster and haplotype determination can prove advantageous for determining the origin and spread of ß-thalassemia and for defining the relationships between several human populations [17]. Given that the population of Iran includes various ethnic groups including Persian (51%), Azeri Turk (24%; approximately 15-20 million members reside in the northwestern part of Iran), Kurd (7%), Arab (3%) [18], and other minorities such as Armenians, the frequency of mutations and related haplotypes in hemoglobinopathies should be studied separately for each region or population. Accurate data on β-thalassemia mutations in a specific population offers valuable information for β-thalassemia prevention programs through heterozygote screening and PND [19,20].
In this study, we investigate the mutation spectrum and haplotype/mutation association among the Azeri Turkish population. We also describe the use of the ß-globin gene cluster haplotypes for prenatal diagnosis of β-thalassemia mutations in 45 pregnancies at risk for carrying fetuses homozygous for β-thalassemia.
Materials and Methods
Subjects
One-thousand-two-hundred potential at-risk couples at the premarital or PND stages were referred to our center between 2011 and June 2014 by three health province centers (East Azerbaijan, West Azerbaijan, and Ardabil centers) based on hematological indices (MCV<80 fl, MCH<27 pg). Four hundred sixty eight couples with at least one partner with MCV<80 fl, MCH<27 pg, and HbA2>3.5% were considered potential carrier couples for β-thalassemia [21], and subjected to genetic studies for the detection of β-thalassemia mutations.
This study was approved by the Ethics Committee of Tabriz University of Medical Sciences. Written Informed consent was obtained from all the participants.
Haplotype analysis has been performed only for 122 couples (57 Unrelated and 65 consanguineous couples) who both were carrier of β-thalassemia.
We determined the β-haplotypes by family linkage study using PCR and restriction enzyme digestions. Haplotype analysis has been limited in 1330 chromosomes, including βthal carriers and their healthy non-carrier related family members. All participants were from Azeri Turkish background, residing in northwest of Iran.
Detection of mutations
ARMS-PCR technique was used for the detection of ß-thalassemia mutations, as described [22,23]. Seven mutations in the ß-globin gene, including IVSII-1 (G>A), IVSI-1 (G>T/A), CD 8/9 (+G), CD 8 (-AA), IVSI-5 (G>C), IVSI-110 (G>A), and IVSI-6 (T>C), were screened. When the mutation was not detectable by the ARMS-PCR method, the whole ß-globin gene was analyzed by sequencing of two PCR-amplified DNA fragments. The following primer pairs were used for the amplification of the 766 and 578 bp fragments, respectively: FA-Fwd (5’-CTGAGGGTTTGAAAGTCCAACTCC-3’) and FA-Rev(5’-GTGAAGCATCTCCTGGACTCA-3’);FBFwd(5’ATGTATCATGCCTCTTTGCACC3’)andFBRev(5’GCACTGACCTCCCACATTCC-3’) [24].
Haplotype analysis
Haplotype analysis has been carried out by PCR-RFLP on DNA samples of the carrier individual and his (her) parents or siblings in the form of family tree. Haplotypes were numbered according to Orkin et al. [25]. Seven restriction endonuclease sites within the β-globin gene cluster (5’ - HindII 5’ε, HindIII Gγ, HindIII Aγ, HindII 5’ψβ, HindII 3’ψβ, AvaII β, and HinfI β-3’) were used for haplotype construction [23,25].
Statistical analyses
Comparison of haplotype frequencies between wild-type and mutant chromosomes was carried out using either chi-square or Fisher’s exact test, as appropriate. Probability values of 0.05 or less were considered as statistically significant. The expected heterozygosities were calculated according to the Hardy-Weinberg equation. χ2 tests were used to inspect differences between observed and expected heterozygote frequencies. PIC was calculated according to Botstein et al. [26].
Results
Characterization of mutations
Six couples out of 468 potential b-thalassemia carriers were excluded from further study as no mutations were found in b-globin gene. In 260 out of 462 investigated families, only one of the partners was carrier of β-thalassemia while in 202 couples, both partners were found to be carriers of β-thalassemia. As 110 out of these 202 couples were consanguineous, totally 554 non-identical mutant chromosomes were identified.
The most prevalent mutation was IVSII-1 (G>A) with a frequency of 23.6%, followed by IVSI-110 (G>A) with a frequency of 11.9%, while CD8 (-AA) with 10.5% frequency, comprised the third common mutation among the 554 non-identical βthal examined chromosomes. Other mutations and their observed frequencies included CD8/9 (+G) at 7.6%, IVSI-1(G>A) at 7.4%, IVSI-6 (T>C) at 6.3%, IVSI-5 (G>C) at 5.2%, CD39(C>T) at 3.1%, both CD5 (-CT) and (-30) TATA box at 2.7% each and CD44 (-CT) at 2.3% (Table 1).
| Mutation Name | βthal Chromosome number | Percentage% | Number | Related Haplotype/s | |
|---|---|---|---|---|---|
| 1 | IVSII-I(G>A) | 131 | 23.6 | 48 | A(4), I(3), II(4), VII(9), IV(26), IX(2) |
| 2 | IVSI-110(G>A) | 66 | 11.9 | 23 | A(3), VIII(6), V(3), I(8), II(3) |
| 3 | CD8(-AA) | 58 | 10.5 | 23 | IX(5), X(2), III(9), V(3), I(4) |
| 4 | CD 8/9(+G) | 42 | 7.6 | 10 | III(1), II(4), I(5) |
| 5 | IVSI-I (G>A) | 41 | 7.4 | 11 | III Atypic(3), I(2), VII(2), A3(1), VIII(2) |
| 6 | IVSI-6 (T>C) | 35 | 6.3 | 10 | A3(2), I Atypic (8) |
| 7 | IVSI-5 (G>C) | 29 | 5.2 | 10 | V(11) |
| 8 | CD39 (C>T) | 17 | 3.1 | 11 | A3(2), A(2), VII(2), I(1) |
| 9 | CD5(-CT) | 15 | 2.7 | 7 | A3(1), I Atypic(2), VII(1), IX(2) |
| 10 | (-30) TATA Box | 15 | 2.7 | 6 | (---++-+)(2), VI(1), V(1) |
| 11 | CD44(-CT) | 13 | 2.3 | 4 | A(1), VII(1), V(2) |
| 12 | CD36/37(-T) | 10 | 1.8 | 4 | VI(1) |
| 13 | CD15TGG>TGA | 9 | 1.6 | 2 | A3(3) |
| 14 | Sicillian | 8 | 1.4 | 1 | IX(1) |
| 15 | IVSI 3end25 del | 7 | 1.3 | 1 | I(1), I Atyipic(1) |
| 16 | IVSII-745 (C>G) | 6 | 1.1 | 1 | (++--+-+) (1) |
| 17 | CD22/23/24-7 bp (-AAGTTGG) | 6 | 1.1 | 3 | |
| 18 | (-28) TATA Box | 5 | 0.9 | 1 | |
| 19 | IVSI-128 (T>G) | 5 | 0.9 | 165 | |
| 20 | -87C>A | 4 | 0.7 | ||
| 21 | CAP+20 (G>A) | 4 | 0.7 | A3(1) | |
| 22 | CD82/83 (-G) | 4 | 0.7 | ||
| 23 | CD29 (GGC>GGT) | 3 | 0.7 | ||
| 24 | CD30(T30R) (G>C) | 4 | 0.5 | A3(3) | |
| 25 | CAP+22 (G>A) | 2 | 0.4 | (++--+-+) (1) | |
| 26 | IVSII-848 (G>A) | 2 | 0.4 | ||
| 27 | 1VSI-130 (G>C) | 2 | 0.4 | III Atypic(3), I(2), VII(2), A3(1), VIII(2) | |
| 28 | (-86) C>G /N | 2 | 0.4 | A3(2), I Atypic (8) | |
| 29 | CD16(-C) | 2 | 0.4 | V(11) | |
| 30 | CD41/42 (-CTTT) | 1 | 0.2 | A3(2), A(2), VII(2), I(1) | |
| 31 | c.*+96T>C | 1 | 0.2 | A3(1), I Atypic(2), VII(1), IX(2) | |
| 32 | TER CD +74 A>G | 1 | 0.2 | (---++-+)(2), VI(1), V(1) | |
| 33 | TER CD+93 (A>G) | 1 | 0.2 | A(1), VII(1), V(2) | |
| 34 | (-90) (C>T) | 1 | 0.2 | ||
| 35 | CD 25/26 (+T) | 1 | 0.2 | ||
| 36 | (-101) C>T | 1 | 0.2 | ||
| Total | 554 | 100% |
Table 1: Characterization of mutations.
The following mutations were observed at frequency below 2%: CD36/37(-T), CD15 (TGG>TGA), Sicilian, IVSI 3’ end25 end, IVSII-745(C>G), CD22/23/24-7 bp(-AAGTTGG), (-28) TATA Box, IVSI-128 (T>G), -87C>A, CAP+20 (G>A), CD82/83 (-G), CD30 (T30R) (G>C), CD29 (GGC>GGT), CAP+22 (G>A), IVSII-848 (G>A), 1VSI-130 (G>C), -86 (C>G), CD16(-C), CD41/42 (-CTTT), c.*+96T>C, TER CD +74 A>G, TER CD+93 (A>G), (-90) C>T, CD 25/26 (+T), (-101) C>T (Table 1).
Haplotype study
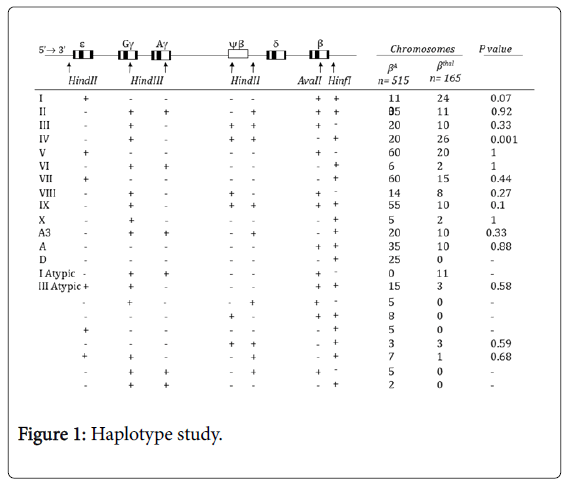
With considering heterozygosity, informativity and linkage phase of studied markers in investigated pedigrees, the haplotype could be unequivocally established in 515 normal and 165 mutant chromosomes. The results of the β-globin gene cluster haplotype analysis for the βA chromosomes are shown in Figure 1. Haplotypes I, V, IX, VII, II, and A together account for 68.9% of the βA chromosome haplotypes, with their individual frequencies being 21.36%, 11.65%, 10.68%, 11.6%, 6.8%, 6.8%, respectively (Figure 1). The following haplotypes were observed in low frequency in βA chromosomes:
D, IV, III, VIII, A3, III atypic, VI, X, (---+-++), (-+--++-), (---++-+), (++--+-+), (-++-++-), (+-----+) and (-++---+) (Figure 1).
A total of 16 haplotypes were found to be associated with the βthal chromosomes (Figure 1). The most frequent β-globin gene cluster haplotypes observed among the βthal chromosomes were IV, I and V with frequencies of 15.76%, 14.55%, 12.1%. Haplotype VII was observed with frequency of 9.1%. The observed frequency of haplotypes, II, III, I Atypic, IX, A3 and A was around 6% in each. Haplotype VIII showed frequency of 4.8%. The frequency of other identified haplotypes were less than 2% (Figure 1).
The following β-thalassemia mutations were included in the haplotype study: IVSII-1 (G>A) (48 chromosomes), IVSI-110 (G>A) and CD8 (-AA) (23 chromosomes each), IVSI-5(G>C) (10 chromosomes), IVI-I, CD8/9 (+G) and IVSI-6 (T>C) (10 chromosomes each), CD39(C>T) (7 chromosomes), CD5(-CT) (6 chromosomes), CD44(-CT) and (-30) TATA Box (5 chromosomes each), CD15(TGG>TGA) (3 chromosomes), IVSI-3’end25del (2 chromosomes) and Sicilian, CD36/37(-T), CAP+22 (G>A), IVSII-745(C>G) (1 chromosome each) (Table 1).
The allele frequencies of all the markers are shown in Table 2, with (+) and (-) denoting digestible and indigestible alleles, respectively.
| Marker | Allele | Chromosomes | Frequency | H(Obs.) | H (Exp.) | PICa | P value |
|---|---|---|---|---|---|---|---|
| HindII e | + | 640/1330 | 0.48 | 0.44 | 0.5 | 0.37 | 0.856 |
| - | 690/1330 | 0.52 | |||||
| HindIII Gg | + | 630/1330 | 0.47 | 0.48 | 0.5 | 0.37 | 0.95 |
| - | 700/1330 | 0.53 | |||||
| HindIII Ag | + | 245/1330 | 0.18 | 0.3 | 0.3 | 0.26 | 1 |
| - | 1085/1330 | 0.82 | |||||
| HindII 5'yβ | + | 430/1330 | 0.32 | 0.47 | 0.44 | 0.33 | 0.9 |
| - | 900/1330 | 0.68 | |||||
| HindII 3'yβ | + | 490/1330 | 0.37 | 0.45 | 0.48 | 0.34 | 0.91 |
| - | 840/1330 | 0.63 | |||||
| HinfIβ | + | 835/1330 | 0.63 | 0.34 | 0.46 | 0.34 | 0.14 |
| - | 495/1330 | 0.37 | |||||
| AvaIIβ | + | 935/1330 | 0.7 | 0.4 | 0.42 | 0.32 | 0.94 |
| - | 395/1330 | 0.3 |
Table 2: The allele frequencies of all the markers.
The highest heterozygosity was observed for HindIII Gγand HindII 5'Ψβ polymorphic markers with a value of 48%, while the other markers HindII 3'ψβ, HindII ε, AvaII β, HinfI β3’, and HindIII Aγshowed lower heterozygosity values of 0.45%, 0.44%, 0.40%, 0.34%, and 0.30%, respectively. The expected heterozygosity did not exhibit a similar trend, as shown in Table 2; however, the differences between observed and expected heterozygosity rates for all the seven markers were found to be statistically insignificant.
Overall, the gene frequencies of each marker revealed the population to be in Hardy-Weinberg equilibrium.
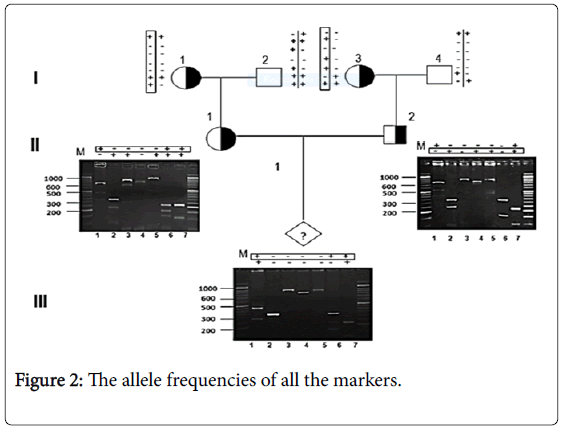
However, the PIC value of the HindIII Gγand HindII εmarkers (0.37) was the highest among the seven polymorphic sites, while the markers HindII 3'Ψβ and HinfI β had PIC values of 0.34, and the HindII 5'Ψβ, AvaII β, and HindIII Aγmarkers had lower PIC values of 0.33, 0.32, and 0.26, respectively. We also successfully applied RFLP linkage analysis for 45 out of 53 PND cases of β-thalassemia (Figure 2).
Discussion
The purpose of this study was to obtain data on the prevalence of β-thalassemia mutations and to compare the haplotypes associated with ßthal and ßA chromosomes in northwestern Iran. Iran is a multi-ethnic country, and each region of the country has its own distinct set of β-globin mutations. To our knowledge, there isn’t any previous study associating particular β-globin gene cluster haplotypes and mutations in northwestern Iran with the Azeri Turkish population. Although more than 400 β-thalassemia alleles have been characterized worldwide [11], few mutations are common among any given population in the areas in which β-thalassemia is prevalent [27]. Population studies demonstrate that 40 mutations account for 90% or more of the β-thalassemia mutations worldwide [27]. In the countries of the Middle East, IVSI-5 (G>C), IVSI-110 (G>A), IVSI-1(G>A), codon 39 (C>T), IVSI-6 (T>C), IVSII-1 (G>A), and codon 5 (-CT) together account for>90% of the β-thalassemia mutations [28]. Previous studies in Iran, which has a multi-ethnic population, indicate that each province has its distinctive mutation spectrum, with IVSII-1 (G>A), IVSI-5 (G>C), CD8/9 (+G), and IVSI-110 (G>A) constituting the most prevalent mutations [5,29].
In the current study, a total of 554 non-identical mutant chromosomes were examined, among which 36 different mutations were observed. The most frequently observed mutation was IVSII-1 (G>A) (23.6%), followed by IVSI-110 (G>A) (11.9%). The IVSII-1 (G>A) mutation, known as the Mediterranean type, has been previously reported as the most frequently occurring mutation in most of the Arab countries [30], as well as in the Kurdestan and fars provinces of Iran, which are located west and south, respectively [31,32,33]. The seven most common mutations, which include IVSII-1 (G>A), IVSI-110 (G>A), CD8 (-AA), CD8/9 (+G), IVSI-I, IVSI-6 (T>C), and IVSI-5 (G>C), together accounted for 72.6% of the mutant alleles.
Orkin et al. have shown that there is strong linkage disequilibrium between the β-globin gene cluster haplotypes and β-thalassemia mutations within regional populations. However, a high level of heterogeneity likely exists in these associations both within and between populations [34-36].
An investigation of the association of specific mutations with different haplotypes showed that IVSII-1 (G>A), the most common mutation, was associated with six different haplotypes, with haplotype IV being predominant (54%). This haplotype was not associated with other mutations. Haplotype VII was the second most common haplotype linked with the IVSII-1 (G>A) mutation. In contrast, previous studies show that the IVSII-1 (G>A) mutation was associated with haplotypes III and V in the Turkish population [35] and with haplotypes I and III, respectively, in the Palestinian [37] and Lebanese [38] populations.
Of the 23 mutant chromosomes with IVSI-110 (G>A) that were analyzed, eight Mediterranean haplotype I chromosomes were found. In addition, haplotypes VIII (6 chromosomes), II (3 chromosomes), V and A (3 chromosomes each) were the other haplotypes associated with the IVSI-110 (G>A) mutation in our study. The association of the IVSI-110 (G>A) mutation with haplotypes I, II, IV, VII, and IX have been reported in world populations [35-39].
The CD8 (-AA) mutation was first identified in Turkish patients and subsequently detected in the Middle East region, and is reported to occur at very low frequencies among the Mediterranean populations [40,13]. In the current study, the association of the CD8 (-AA) mutant chromosomes with haplotypes I, III, V, X and IX was revealed. This mutation has been previously reported to show linkage with haplotypes IV in Moroccan population [40], IX in Algerian [13], and IV and VII in Turkish populations [35].
The IVSI-5 (G>C) mutation has been reported to be linked with haplotypes I and V in Brazilian β-thalassemia patients [41]. In association India, on the other hand, the IVSI-5 (G>C) is the most common mutation, and showed with eight different haplotypes, haplotype V being predominant (54%) [42]. Assessment of haplotypes linked with the IVSI-5 (G>C) mutation in the current study revealed association only with haplotype V.
The prevalence of the haplotypes varied among patients from different geographical areas. For instance, among Mediterranean patients, haplotype I was found to be the most prevalent haplotype (47%) associated with βthal chromosomes, followed by haplotypes II (17%) and V (12%) [34]. Among the Corsican population, strong association between CD39 (C>T) as the most common mutation (88.40%) with haplotype II was revealed [43]. CD39 (C>T) and the IVS-I-110 (G>A) mutations as two most frequent mutations in Northern Ireland exclusively linked with haplotype I [44].
Haplotype I was also found to be the most prevalent haplotype among β-thalassemia patients and healthy individuals (35.7% and 43.3%, respectively) in the western region of Iran (with Kurdish ethnic population) [31,45], as also among the β-thalassemia minor individuals or normal controls (46.2% and 43.3%, respectively) from Southern Iran [15]. However, the second most prevalent haplotypes for β-thalassemia patients and controls were haplotypes III (28.6%) and V (14.3%), respectively, in western Iran, and haplotypes V (15.4%) and III (15.4%), respectively, in southern Iran [15,31,45]. The current study revealed that haplotypes I (27.1%) and IV (18.1%) were the most prevalent haplotypes among βA and βthal chromosomes, respectively. The second most prevalent haplotype included haplotype I among βthal chromosomes (15.1%) and haplotype V (10.67%) among βA chromosomes. These differences in haplotype frequencies could be attributable to the diversity in ethnic backgrounds that exist in different regions of Iran.
In the current study, haplotype IV was found to be specifically associated with the most common mutation, IVSII-I, with a frequency of 54.1%; therefore, it can be concluded that the high frequency of the IVSII-I mutation resulted in the high prevalence of haplotype IV in our study. On the other hand, a comparison of the frequencies of different haplotypes associated with βthal and βA chromosomes did not reveal any significant differences, with the exception of haplotype IV, which was significantly higher in β thal chromosomes (p value<0.05; Figure 1).
Four of the 5’ haplotypes (5’ - HindII 5’ε, HindIII Gγ, HindIII Aγ, HindII 5’ψβ, HindII 3’ψβ, -3’) accounted for 86% and 81.8% of the βA and βthal chromosomes, respectively. The frequencies of these haplotypes, namely (+----), (-+-++), (-----), and (-++-+), were 45.6%, 18.4%, 11%, and 11% for βA chromosomes, and 35%, 27.8%, 6%, and 13% for βthal chromosomes. Haplotype (+----) was the most frequent 5’ haplotype among the Azeri Turkish population, followed by the haplotype (-+-++).
Among Mexican population, two of three most frequent mutations, the CD39 (C>T) and IVS-I-1 mutations, were observed with five 5' haplotypes with haplotypeIng the most prevalent. Whereas the IVS-I-110 was only found in association with haplotype I [46].
PND helps prevent the birth of offsprings with thalassemia among high-risk couples (with both partners being carriers), and can be performed in the first trimester of pregnancy by direct and indirect analyses of DNA extracted from amniocytes and CVS. Haplotype analysis can be used for the verification of the direct method or independently as an indirect method, and is also helpful to rule out the possibility of fetal sample contamination with maternal blood or tissue. In the current study, haplotype analysis proved useful for diagnosis in 45 out of 53 at-risk couples under investigation.
Conclusion
The identification of 36 different mutations implies high rates of mutational heterogeneity, and offers useful information about the types and geographic or ethnic distribution of β-thalassemia mutations in northwest Iran, thereby allowing rapid detection of mutations.
In addition, the occurrence of 16 different haplotypes on βthal chromosomes and the recognition of dissimilar haplotypes as most prevalent on βthal and βA chromosomes provide evidence that β-thalassemia mutations might have originated as a result of gene flow due to population migration along with new mutation events in a common chromosomal background in the population.
Our results show that (+----), (-+-++), (-----), and (-++-+) are the dominant 5’ haplotypes, together accounting for 86% and 81.8% of the βA and βthal chromosomes, respectively.
Among the investigated polymorphic sites, the highest heterozygosity of 48% was observed for the HindIII Gγand HindII 5'Ψβ markers, whereas the highest heterozygosity of 50% was expected for HindIII Gγand HindII εmarkers, which have the highest PIC value of 0.37. The current study presents the levels of informativity of the seven RFLP markers investigated in the β-globin locus, and show that haplotype analysis using these polymorphic markers can prove useful for carrier detection, particularly in cases where unknown mutations are encountered and confirming PND reliability in the target population.
Acknowledgements
This paper was funded by Hematology Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
References
- Karimi M, Giti R, Haghpanah S, Azarkeivan A, Hoofar H, et al. (2009) Malignancies in patients with beta-thalassemia major and beta-thalassemia intermedia: a multicenter study in Iran. Pediatr Blood Cancer 53: 1064-1067.
- Divoky V, Mrug M, Thornley-Brown D, Divoka M, Prchal JT (2001) Non-anemic homozygous beta(o) thalassemia in an African-American family: association of high fetal hemoglobin levels with beta thalassemia alleles.Am J Hematol 68: 43-50.
- Joulaei H, Shahbazi M, Nazemzadegan B, Rastgar M, Hadibarhaghtalab M, et al. (2014) The diminishing trend of β-thalassemia in Southern Iran from 1997 to 2011: the impact of preventive strategies.Hemoglobin 38: 19-23.
- Najmabadi H, Ghamari A, Sahebjam F, Kariminejad R, Hadavi V, et al. (2006) Fourteen-year experience of prenatal diagnosis of thalassemia in Iran.Community Genet 9: 93-97.
- Abolghasemi H, Amid A, Zeinali S, Radfar MH, Eshghi P, et al. (2007) Thalassemia in Iran: epidemiology, prevention, and management.J Pediatr Hematol Oncol 29: 233-238.
- Najmabadi H, Teimourian SH, Khatibi T (2001) Amplification refractory mutation system (ARMS) and reverse hybridization in the detection of beta thalassemia mutations. Arch Irn Med 4: 165-170.
- Hadavi V, Taromchi AH, Malekpour M, Gholami B, Law HY, et al. (2007) Elucidating the spectrum of alpha-thalassemia mutations in Iran.Haematologica 92: 992-993.
- Karimi M, Alavian G, Kadivar MR (2007) Regional mapping of the gene frequency of ß-thalassemia in Fars province, Iran during 1997-1998. Irn J Med Sci 25: 134-137.
- Samavat A, Modell B (2004) Iranian national thalassaemia screening programme.BMJ 329: 1134-1137.
- Hardison RC, Chui DH, Giardine B, Riemer C, Patrinos GP, et al. (2002) HbVar: A relational database of human hemoglobin variants and thalassemia mutations at the globin gene server.Hum Mutat 19: 225-233.
- Akhavan-Niaki H, Derakhshandeh-Peykar P, Banihashemi A, Mostafazadeh A, Asghari B,et al. (2011) A comprehensive molecular characterization of beta thalassemia in a highly heterogeneous population. Blood Cells Mol Dis 47:29-32.
- Boudrahem-Addour N, Zidani N, Carion N, Labie D, Belhani M, et al. (2009) Molecular heterogeneity of beta-thalassemia in Algeria: how to face up to a major health problem.Hemoglobin 33: 24-36.
- Thein SL (2013) The molecular basis of β-thalassemia.Cold Spring Harb Perspect Med 3: a011700.
- Rahimi Z, Merat A, Akhzari M, Haghshenass M, Ronald L, et al. (2005) ß-Globin Gene Cluster Haplotypes in Iranian Patients with ß-Thalassemia. IJHOBMT 2:30-34.
- Kazazian HH Jr, Antonarakis SE, Cheng T, Boehm CD, Waber PG (1983) Use of haplotype analysis in the beta-globin gene cluster to discover beta-thalassemia mutations.Prog Clin Biol Res 134: 91-98.
- Kazazian HH Jr, Orkin SH, Markham AF, Chapman CR, Youssoufian H, et al. (1984) Quantification of the close association between DNA haplotypes and specific beta-thalassaemia mutations in Mediterraneans.Nature 310: 152-154.
- Bonyadi M, Omrani O, Rafeey M, Bilan N (2011) Spectrum of CFTR gene mutations in Iranian Azeri Turkish patients with cystic fibrosis.Genet Test Mol Biomarkers 15: 89-92.
- Patrinos GP, Kollia P, Papadakis MN (2005) Molecular diagnosis of inherited disorders: lessons from hemoglobinopathies.Hum Mutat 26: 399-412.
- Xu XM, Zhou YQ, Luo GX, Liao C, Zhou M, et al. (2004) The prevalence and spectrum of alpha and beta thalassaemia in Guangdong Province: implications for the future health burden and population screening.J Clin Pathol 57: 517-522.
- Zeinalian M, Nobari RF, Moafi A, Salehi M, Hashemzadeh-Chaleshtori M (2013) Two decades of pre-marital screening for beta-thalassemia in central Iran.J Community Genet 4: 517-522.
- Weatherall DJ, Clegg JB (2001) The Thalasseamia syndromes, 4th edn. Oxford, Blackwell science. pp: 711-716.
- Old J, Henderson S (2010) Molecular diagnostics for haemoglobinopathies.Expert Opin Med Diagn 4: 225-240.
- Arab A, Karimipoor M, Rajabi A, Hamid M, Arjmandi S, et al. (2011) Molecular characterization of β-thalassemia intermedia: a report from Iran.Mol Biol Rep 38: 4321-4326.
- Orkin SH, Kazazian HH Jr, Antonarakis SE, Goff SC, Boehm CD, et al. (1982) Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster.Nature 296: 627-631.
- Botstein D, White RL, Skolnick M, Davis RW (1980) Construction of a genetic linkage map in man using restriction fragment length polymorphisms.Am J Hum Genet 32: 314-331.
- Thein SL (2005) Genetic modifiers of beta-thalassemia.Haematologica 90: 649-660.
- Najmabadi H, Karimi-Nejad R, Sahebjam S, Pourfarzad F, Teimourian S, et al. (2001) The beta-thalassemia mutation spectrum in the Iranian population.Hemoglobin 25: 285-296.
- Cao A, Gossens M, Pirastu M (1989) Beta thalassaemia mutations in Mediterranean populations.Br J Haematol 71: 309-312.
- Zahed L (2001) The Spectrum of beta-Thalassemia Mutations in the Arab Populations.J Biomed Biotechnol 1: 129-132.
- Rahimi Z, Muniz A, Akramipour R, Tofieghzadeh F, Mozafari H, et al. (2009) Haplotype analysis of beta thalassemia patients in Western Iran.Blood Cells Mol Dis 42: 140-143.
- Yousefi MH (1979) Beta Thalassemia trait in Sanandaj.Scientific Journal of Kurdestan University of Medical Sciences 4:11-13.
- Modjtahed Zadeh F (1999) Beta Thalassemia gene mutations in Thalassemic patients referred to Boo Ali Sina Hospital of Sari the year 1373. Journal of Mazanderan University of Medical Science 23-22: 37-42.
- Antonarakis SE, Kazazian HH Jr, Orkin SH (1985) DNA polymorphism and molecular pathology of the human globin gene clusters.Hum Genet 69: 1-14.
- Diaz-Chico JC, Yang KG, Stoming TA, Efremov DG, Kutlar A, et al. (1988) Mild and severe beta-thalassemia among homozygotes from Turkey: identification of the types by hybridization of amplified DNA with synthetic probes. Blood 71:248-251.
- Hockham C, Piel FB, Gupta S, Penman BS (2015) Understanding the contrasting spatial haplotype patterns of malaria-protective β-globin polymorphisms.Infect Genet Evol 36: 174-183.
- El-Latif MA, Filon D, Rund D, Oppenheim A, Kanaan M (2002) The beta+-IVS-I-6 (T-->C) mutation accounts for half of the thalassemia chromosomes in the Palestinian populations of the mountain regions.Hemoglobin 26: 33-40.
- Makhoul NJ, Wells RS, Kaspar H, Shbaklo H, Taher A, et al. (2005) Genetic heterogeneity of Beta thalassemia in Lebanon reflects historic and recent population migration.Ann Hum Genet 69: 55-66.
- Orkin SH, Goff SC (1981) Nonsense and frameshift mutations in beta 0-thalassemia detected in cloned beta-globin genes.J Biol Chem 256: 9782-9784.
- Agouti I, Badens C, Abouyoub A, Khattab M, Sayah F, et al. (2007) Genotypic correlation between six common beta-thalassemia mutations and the XmnI polymorphism in the Moroccan population.Hemoglobin 31: 141-149.
- Martins JT, Bordin S, de Albuquerque DM, Saad ST, Costa FF (2005) DNAase I hypersensitive site 3' to the beta-globin gene cluster containing two TAA insertions and a G-->A polymorphism is predominantly associated with the beta+-thalassemia IVS-I-6 (T-->C) mutation.Hemoglobin 29: 85-89.
- Gupta A, Sarwai S, Pathak N, Agarwal S (2008) Beta-Globin Gene Mutations in India and Their Linkage to beta-Haplotypes. Int J Hum Genet 8:237.
- Falchi A, Giovannoni L, Vacca L, Latini V, Vona G, et al. (2005) beta-globin gene cluster haplotypes associated with beta-thalassemia on Corsica island.Am J Hematol 78: 27-32.
- Knott M, Ramadan KM, Savage G, Jones FG, El-Agnaf M, et al. (2006) Novel and Mediterranean beta thalassemia mutations in the indigenous Northern Ireland population.Blood Cells Mol Dis 36: 265-268.
- Rahimi Z, Muniz A, Mozafari H (2010) Abnormal hemoglobins among Kurdish population of Western Iran: hematological and molecular features.Mol Biol Rep 37: 51-57.
- Morales KR, Magaña MT, Ibarra B, Perea FJ (2009) Diversity of the 5' beta-globin haplotype of four beta-thalassemia mutations in the Mexican population.Hemoglobin 33: 66-71.
Citation: Derakhshan SM, Khorrami A, Feizi AHP, Khaniani MS (2015) Spectrum of β-Globin Gene Mutations and β-Thalassemia HaplotypeAnalysis among the Iranian Azeri Turkish Population. Epidemiology (sunnyvale) 5:210. DOI: 10.4172/2161-1165.1000210
Copyright: © 2015 Derakhshan SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11670
- [From(publication date): 12-2015 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 10754
- PDF downloads: 916