Spectrophotometric Determination of Nitrite and Nitrate in Some Selected Vegetables Cultivated in Adami Tulu Judo Kombolicha District Farms, Ethiopia
Received: 05-Sep-2018 / Accepted Date: 24-Sep-2018 / Published Date: 28-Sep-2018 DOI: 10.4172/2155-9872.1000411
Keywords: Vegetables; Nitrite; Nitrate; UV-visible spectrophotometer
Introduction
Vegetable is the designation given to that group of horticultural plants grown for human consumption either for their roots, tubers, shoots, stems, leaves, flower buds, flowers, fruit or seed (immature or mature). They are considered as a port of main foods for world population especially for the developed countries. About two-thirds of the world’s population relies largely on vegetarian diet [1]. It is widely accepted that vegetables help prevent a wide range of diseases [2,3]. Epidemiological data supported this protective effect against several types of cancers and cardiovascular diseases. The increasing scientific evidence that consumption of vegetables decreases the risk of several chronic diseases has created a firm basis for policy initiatives. Insufficient consumption of vegetables was among the risk factors recognized as contributing to the worldwide noncommunicable disease burden [2]. The World Health Organization (WHO) recommends consuming at least 400 g or five portions of fruits and vegetables (excluding tubers) a day [4]. However, these plant foods can also be a source of unfavorable contaminants like nitrates, nitrites, heavy metals and residues of pesticides [3].
While vegetables can impact health positively, their nitrate and nitrite levels should not be overlooked. Nitrate and nitrite are found naturally in the environment, and form part of the nitrogen cycle. They are chemicals used in fertilizers and can readily migrate from fertilized soil to groundwater [5]. In fact, a high nitrate concentration in vegetables is a worldwide problem. Nitrate contamination in vegetables occurs when crops absorb more than they require for their sustainable growth [6]. The amount of nitrate content is one of the most important factors of vegetable quality [1]. Due to the increased use of synthetic nitrogen fertilizers and livestock manure in intensive agriculture, vegetables and drinking water may contain higher concentrations of nitrate than in the past [7-9].
The nitrate ion has relatively low level of acute toxicity, but if transformed into nitrite, it may constitute a health problem. Reduction to nitrite may take place in the presence of bacteria or enzyme nitrate reductase, and in contact with metals [3]. Nitrites have been considered as a potentially hazardous compound for human health. Nitrites can easily react with secondary, tertiary amines and amides to form nitrosamines. Most nitrosamines are known to be highly carcinogenic and mutagenic [10]. It causes the formation of methemoglobin, for which its level in potable water and foods is restricted by regulations [11].
In order to control the nitrate and nitrite intake by consumers in general and on babies in particular who are the most vulnerable to the adverse effects of these two compounds, a maximum acceptable limit of these compounds were suggested. European Union food commission (1992) states the daily acceptable intake of nitrate and nitrite as 0-3.65 and 0-0.07 mg/kg, respectively. Similarly, FAO and WHO food commission report the average daily nitrate and nitrite intake of a 60 kg person as 220-240 mg and 16-32 mg, respectively [1].
In view of the importance of vegetables to health and fact that many people have now resulted to eating vegetables for the well-being of their health, the aim of this study is therefore to determine nitrate and nitrite concentrations in vegetables. This was carried out by analyzing spectrophotometrically the levels of nitrate and nitrite in samples of three different vegetables [(cabbage (Brassica oleracea), tomato (Lycopersicon esculentum) and onion (Allium cepa)] collected from vegetable farms of Bochessa, Edo kontol and Dodich which supply most of the vegetables consumed in Adami Tulu judo Kombolicha district.
A variety of analytical methods, including spectrophotometry, high performance liquid chromatography (HPLC), ion chromatography (IC), gas chromatography (GC), polarography, and capillary electrophoresis (CE) for the determination of nitrate and nitrite in food have been developed [12]. The present study is based on Spectrophotometric determination of nitrite and nitrate in vegetables by forming colored compounds [13].
Materials and Methods
Description of the study area
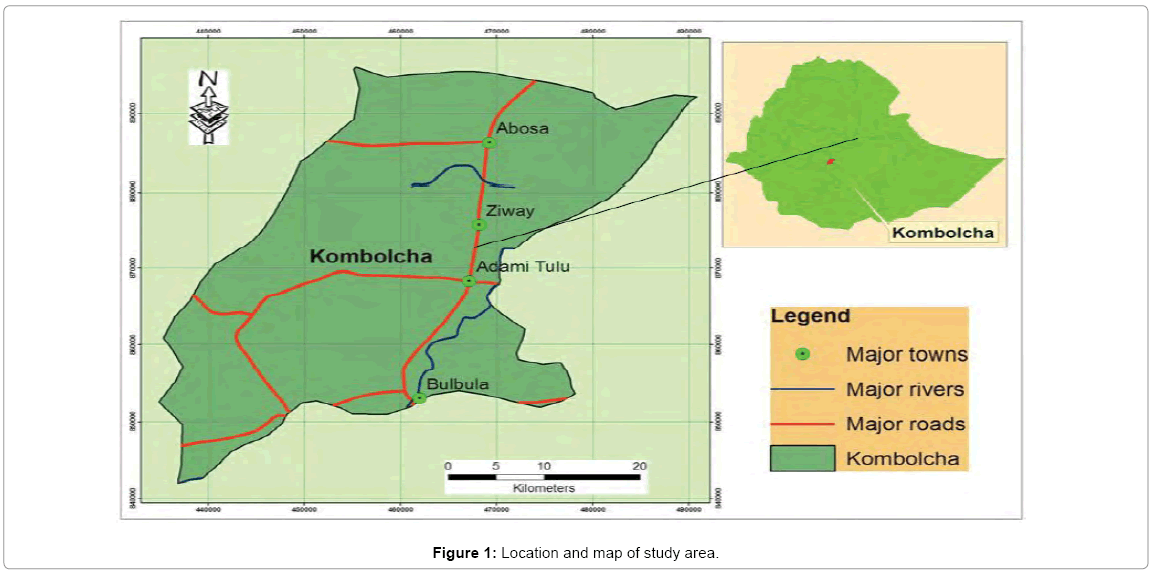
The study was conducted in Adami Tulu-Jido Kombolcha woreda (Figure 1), which is part of the East Showa Zone of the Oromia Regional State. Three kebeles viz. Dodich, Bechesa and Edo Kontol were selected to conduct the research [14,15]. They were chosen because the area is known for producing large amount of vegetable all around Zeway Lake and also it is located in the vicinity of industrial zone. Geographically the area is located between 38°20’ and 38.5°5’ E and 7°35’and 8°05’ N. The District covers an area of 1403.3 km2. Zeway (Battu) town is the administrative center of the District [16].
Chemicals, reagents and standard solutions
All chemicals used were of analytical reagent grade, and distilled water was used in the preparation of all solutions in the experiments. Nitrite solution (1000 mg/L) was prepared by dissolving 0.1500 g sodium nitrite (which was dried in drying oven at 105°C for 24 h) in water and diluting to 100 mL. The solution was treated with one pellet of sodium hydroxide to prevent liberation of nitrous acid and 1 mL of chloroform to preserve solution [17]. Nitrate solution (1000 mgL-1) was prepared by dissolving 0.1631 g potassium nitrate (which was dried in drying oven at 105°C for 24 h) in water and diluting to 100 mL. Working standard solutions were prepared by appropriate dilution of stock solution daily. P-nitroaniline (PNA, 0.05% in 2.5M HCl) was prepared by dissolving 0.05 g of PNA (BDH) in 21.5 mL concentrated HCl and diluting to 100 mL with distilled water and 1-naphthol solution (0.03%) was prepared by dissolving 0.03 g of 1-naphthol (LOBA CHEMIE) in 4 mL of 2M KOH and diluting to 100 mL with distilled water. 98% of H2SO4 acid was used for nitration of phenol [18]. 8% phenol solution was prepared by dissolving 8 g of phenol in 100 mL of distilled water and heated on boiling water bath for a couple of minutes. The following reagents were prepared by dissolving appropriate amounts of reagents in distilled water: 2M HCl, 2M KOH and 2M NaOH.
Collection of samples
Vegetable samples were collected in February 2013. Fresh vegetable samples were collected from farmlands of three areas in Adami tulu judo kombolicha particularly Bochessa, Dodich and Edo Kontol. These sampling sites were selected based on their large scale production of vegetables in woreda so that the sample represents the whole vegetable cultivated in woreda. To draw the representative sample from each sampling site, three sub samples (500 g each) were randomly chosen from the three triangular corners of the area which are roughly 500 meters away from each other. During each collection, samples were randomly collected from different plots and homogenized into three composite samples in the three sampling areas [19].
Vegetable sample preparation for nitrite and nitrate analysis
Vegetable samples were thoroughly washed with tap water and thereafter rinsed with distilled water so as to remove surface contaminants like soil, dust and spray residues. Then sliced into nearly uniform sizes to facilitate drying at the same rate. The sliced samples were then dried in an oven at 70°C for 24 hours until they become brittle and crisp. The dried samples were mechanically ground into fine particles using clean mortar and pestle and sieved to obtain <2 mm fractions [20]. A portion (2 g) of each of the sieved samples were taken separately in 125 mL Erlenmeyer flask, 20 mL of hot distilled water were added and allowed to stand in a boiling water bath for 30 min. The contents were cooled to room temperature by immersing the flask in cool water bath. Then, the solution was filtered through Whatman No. 41 filter paper to remove the residues and the filtrates were kept at 4°C in refrigerator until analysis [21].
Determination of nitrite and nitrate in vegetable samples
To the extracted and filtered sample solutions, 1 mL of 0.05% of p-nitroaniline and 1 mL of 2.5M HCl were added and the solution was shaken thoroughly to allow the diazotization reaction to go to completion. Then, 2 mL of 0.03% 1-naphthol and 2 M KOH (2 mL) were added to form an azo dye and the contents were diluted to the mark with distilled water and left for five minutes in order to give time for violet color development [22]. Absorbance was measured at wavelength of maximum absorption (570 nm) against the corresponding reagent blank using single beam UV-Visible spectrophotometer. The amount of nitrite present in the unknown solution was computed from the calibration graph. While for the determination of nitrate, to the filtrated sample solutions, 1 mL of 5% Ag2SO4 solution was added followed by subsequent addition of 7 mL of 98% H2SO4 and 0.1 mL of 8% phenol solution. The solution was allowed to stand for 5 min while shaking occasionally. Then, 10 mL of 2M NaOH was added to form yellow sodium nitrophenoxide derivative. Absorbance was measured at wavelength of maximum absorption (400 nm) against the corresponding reagent blank [23].
Results and Discussion
Determination of maximum absorption wavelengths of azo dye and nitrophenoxide
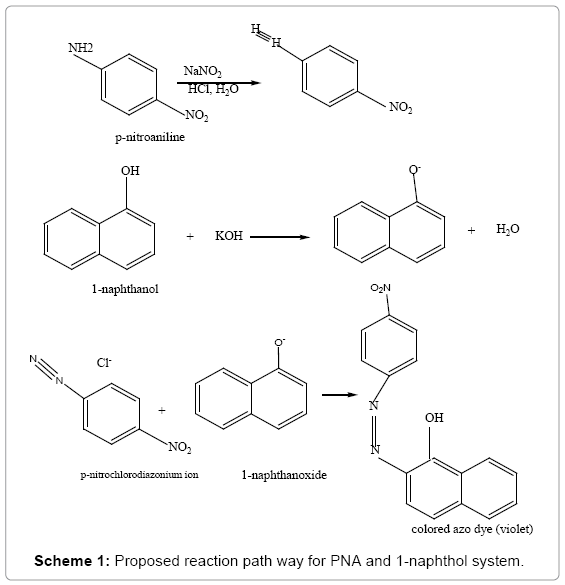
The method was based on diazo-coupling reaction between nitrite with amino group of PNA in acidic medium with the coupling agent 1-naphthol, in alkaline medium to produce violet colored azo dye with maximum absorption at 570 nm in alkaline medium (Scheme 1).
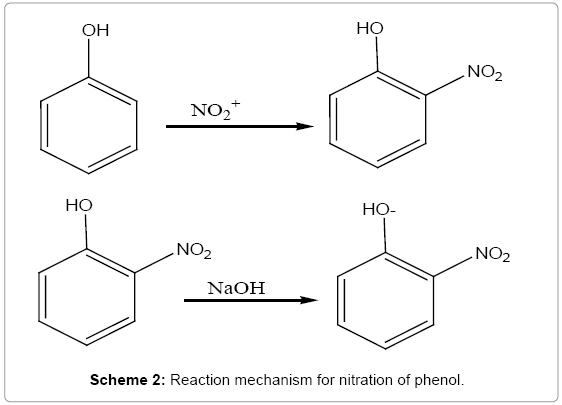
In the determination of maximum wavelength for yellow sodium nitrophenoxide derivative, the method was based on the nitration of phenol in the presence of sulphuric acid and the formation of the corresponding yellow sodium nitrophenoxide derivative in alkaline medium with maximum absorption at 400 nm was obtained. The reaction mechanism involved based on literature is presented as shown in Scheme 2.
NO3- + H2SO4 → HSO4- + HNO3
HNO3 + H2SO4 → NO2+ + -OSO3 + H2O
Optimization of reagent concentration for azo dye formation
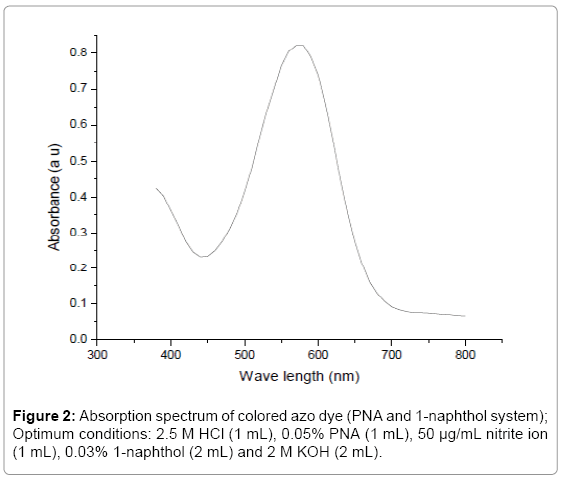
Effect of acid concentration on diazotization: The effect of acidity on diazotization reaction was studied in the range 1-4.5 M HCl acids and, the increase absorbance was observed in the range 1-2 M and reaches maximum at 2.5 M. Above this concentration of HCl a decrease in the absorbance was observed as shown in Figure 2. The optimum acidity for the diazotization was fixed at 2.5 M for complete diazotization [24].
The effect of the p-nitro aniline concentration: The effect of the PNA concentration on the color intensity was studied using the proposed procedure in the range 0.01-0.1%. The result showed that a 0.05% PNA solution was sufficient for the complete color development (Figure 3). Higher concentration did not enhance the absorbance further, and lower concentration did not give good results.
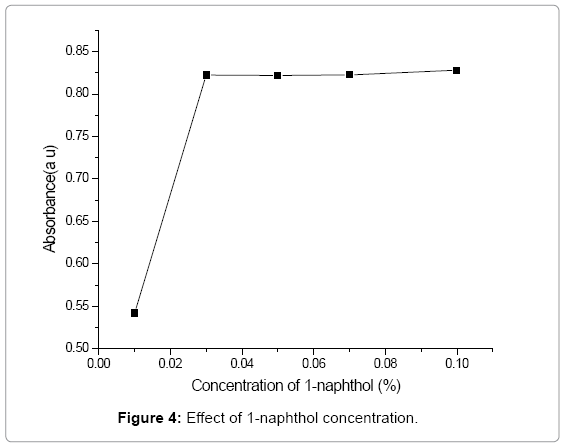
Effect of 1-naphthol concentration: The effect of 1-naphthol on coupling reaction was studied in the range 0.01-0.1%. It was observed that maximum absorption and stable color was formed with 2 mL of 0.03% of 1-naphthol. Further increases in 1-naphthol concentration do not increase the absorbance significantly as indicated in Figure 4. Therefore, the optimum concentration of 1-naphthol for the coupling reaction was fixed at 0.03%.
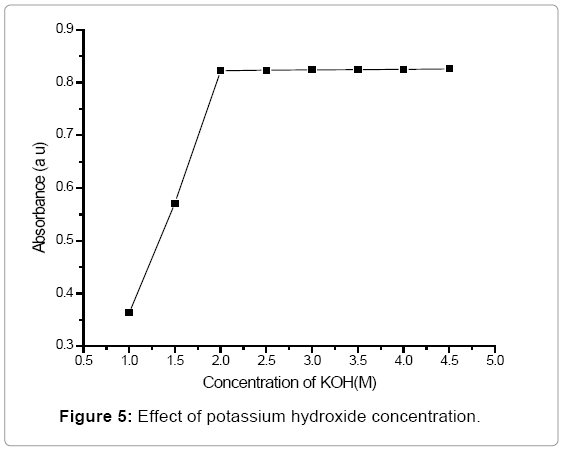
Effect of potassium hydroxide: The effect of KOH on the deprotonation of 1-naphthol was studied in the range 1-4.5 M. The absorbance increased instantaneously at additions of 1-2 M of potassium hydroxide and maximum and constant absorbance obtained at additions of 2-4.5 M of potassium hydroxide (Figure 5) [25]. 2 M KOH solutions were chosen. Other alkaline solutions were investigated, but best results were obtained by using potassium hydroxide.
Optimization of reagents concentration for sodium nitrophenoxide derivative formation
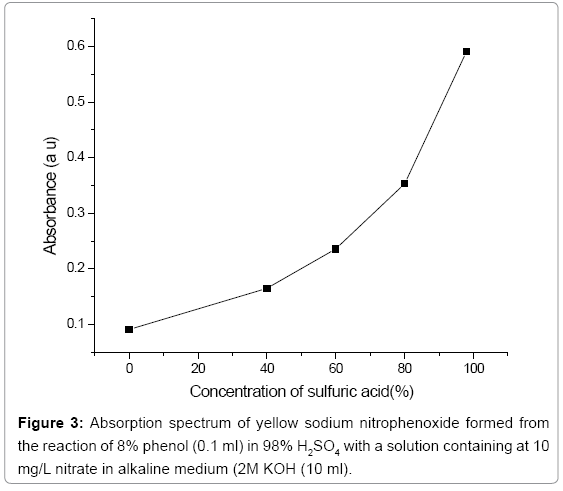
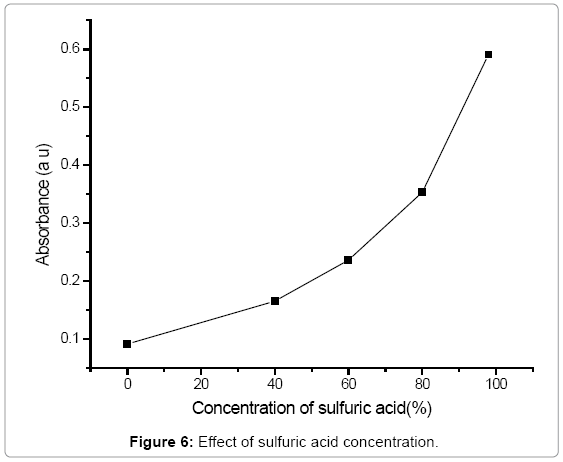
Effect of sulphuric acid: The investigations were studied in the range of 0-98% of sulphuric acid. Absorbance increased as the concentration of the acid is increased as shown in Figure 6. The use of 98% sulphuric acid was used for nitration of phenol.
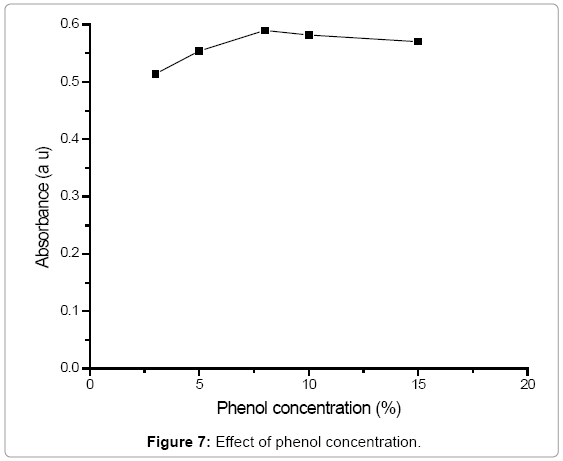
Effect of phenol solution: The effect of phenol solution was studied in the range of 3-15%. Nearly equal amounts of nitrate were determined successfully in the aliquots of standard nitrate solutions at a level of 10 mg/L by using different phenol concentrations (Figure 7). In the proposed procedure 8% phenol solution was used in the determination of nitrate.
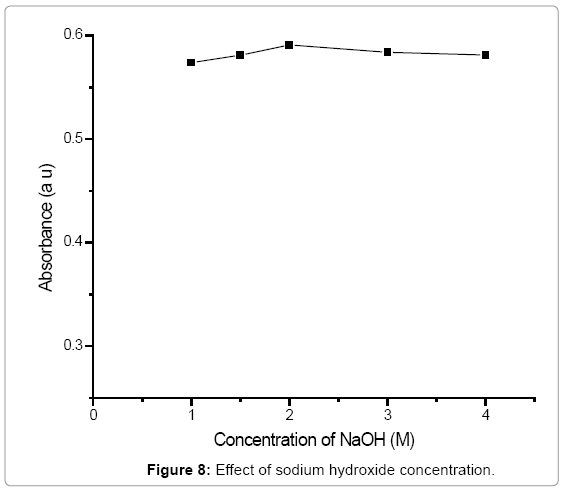
Effect of sodium hydroxide: The effect of sodium hydroxide on the formation of yellow sodium nitrophenoxide derivative was studied in the range 1-4 M. There was no significant difference in the amount of nitrate determined with NaOH concentrations as shown in Figure 8. Therefore, in the procedure 2 M sodium hydroxide was recommended [26].
Determination of anions in vegetable samples
The concentrations (mg kg-1) of anions in the samples were calculated using the following equation:
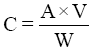
Where, C=total anion concentration (mg/kg); A=mg/L of anion in a sample; V=total volume of the sample solution (ml); W=weight of extracted sample (gm).
Nitrite: Concentrations of nitrite found in vegetables were summarized in Table 1. From the results obtained, the three samples, i.e., cabbage, tomato and onion of three sampling areas all contain nitrite. Nitrite which is toxic and hazardous to man was found to be within the range of 0.46-1.44 mg/kg. Nitrite had its lowest concentration in tomato and highest concentration in cabbage. The relatively high concentration of nitrite in cabbage suggests that leafy vegetables are accessible for the accumulation of this anion than fruits and bulbs. The variation in the level of nitrite may be because of the different capacity of the accumulation of nitrite in vegetables. Vegetable samples of Edo Kontol contain highest concentrations of nitrite followed by Dodich and Bochessa.
| Vegetables | Study sites | Mean (NO2-) mg/kg ± SD | Mean (NO3-) mg/kg ± SD |
|---|---|---|---|
| Cabbage | Bochessa | 1.25 ± 0.027 | 186.58 ± 18.84 |
| Dodich | 1.38 ± 0.038 | 211.5 ± 19.34 | |
| Edo kontol | 1.44 ± 0.029 | 230.5 ± 12.99 | |
| Onion | Bochessa | 0.86 ± 0.087 | 78.35 ± 4.36 |
| Dodich | 0.93 ± 0.06 | 87.47 ± 9.37 | |
| Edo kontol | 1.04 ± 0.038 | 95.36 ± 5.46 | |
| Tomato | Bochessa | 0.48 ± 0.025 | 28.74 ± 3.35 |
| Dodich | 0.46 ± 0.014 | 32.77 ± 2.81 | |
| Edo kontol | 0.49 ± 0.014 | 34.02 ± 1.18 |
Values are means ± SD and ranges (minimum and maximum). Each type of vegetable was analyzed in triplicate.
Table 1: Average concentrations (mean ± SD, n=3, mg/kg) of nitrite and nitrate in vegetable samples.
There is no significant difference in the levels of nitrite at 95% (P>0.05) confidence level among the three onion, tomato and cabbage samples obtained from Bochessa, Dodich and Edo Kontol. This insignificant difference may be due to similar environmental conditions (i.e., length of exposure to sunlight, cultivation methods and seeding time) of the sampling areas. Among vegetables (cabbage, onion and tomato) of different sampling sites, the mean concentrations of nitrite showed significantly different at 95% (P<0.05). This difference may be due to the different capacity of the accumulation of this anion among different vegetables [26].
As indicated in Table 1, the concentrations of nitrate in all vegetable samples were found lower than the limit set by WHO (100 mg/kg). The nitrite levels generally were below the allowable range set by Korean environmental protection agency. The nitrite content results in this survey were lower than 1 mg/Kg except in cabbage samples which were higher than 1 mg/Kg. However, it is not possible to decide whether they are harmful or not during the consumption of these vegetables in these areas. Since the Korean climatic condition, cultivation, soil type, variety of vegetables and harvesting time may differ from Ethiopia [5].
Nitrate: From Table 1, nitrate was detected in all vegetable samples. Nitrate occurred in all the samples investigated in varying concentrations, with the highest concentrations of 186.58-230.5 mg/kg of cabbage. While the lowest concentration of 28.74-34.02 mg/kg was detected in tomato. The highest concentrations of nitrate come from vegetable samples of Edo Kontol followed by Dodich and Bochessa.
The concentrations of nitrate were not significantly different in all same vegetable samples at the different farms. Absence of significant difference of nitrate mean concentration in same vegetable samples of farms indicates that since these areas may be under the same geographical location, same soil type and share common climatic conditions and being irrigated with same water source [10]. Among vegetables (cabbage, onion and tomato) of different sampling sites, the mean concentrations of nitrate showed significantly different at 95% (P<0.05). These results showed the differences in absorption, reduction and transport of nitrate among different vegetables and that some vegetables were better adopted to conditions of high nitrate fertilization.
The concentrations of nitrate in vegetables were lower than the pollution level (NO3-N>325 mg/kg) set by Chinese environmental protection agency. However, it is too difficult to fix on the harmless effect of eating these vegetables. Since, the Chinese climatic condition, cultivation, eating style, usage of fertilizers and type of soil may differ from Ethiopia. In addition, levels of nitrate content were compared with WHO permissible limit (300 mg/kg) and all values were found below this allowable limit.
Comparison of the results of the present study with the reported data
In general, from a comparison of the results reported in the study it can be seen that the mean concentrations of anions were more or less comparable with the reported literature values as shown in Tables 2 and 3. However, higher values of nitrate in onion were observed in this
| Nitrite content in the present work | Reported nitrite content in other studies | |||
|---|---|---|---|---|
| Vegetables | Nitrite content | Nitrite content | Country | Reference |
| 16.9 ± 0.68 | Nigeria | [9] | ||
| 0.18 ± 0.3 | Syria | [26] | ||
| Tomato | 0.46 - 0.49 | 0.31 ± 0.07 | Romania | [22] |
| 0.15 | Palestine | [24] | ||
| 0.44 | Great Britain | [14] | ||
| 14.69 ± 0.50 | Nigeria | [9] | ||
| Onion | 0.86-1.04 | 0.5 | China | [15] |
| 4.5 | Iran | [1] | ||
| Cabbage | 1.25-1.44 | 10.36 ± 1.94 | Nigeria | [9] |
| 0.625 | Palestine | [24] | ||
| 0.5 | Great Britain | [14] | ||
| 2.255 | Turkey | [18] | ||
| 3.2 | Iran | [1] | ||
Table 2: Comparison of nitrite content (mg/kg) in the vegetables studied in Adami Tulu Judo Kombolicha district, and the nitrite content reported in other studies.
| Vegetables | Nitrate content | Nitrate content | Country | References |
|---|---|---|---|---|
| Tomato | 28.74-34.02 | 39.99 ± 8.87 | Nigeria | [9] |
| 10.89 ± 13.96 | Syria | [26] | ||
| 104.7 ± 15.99 | Romania | [22] | ||
| 16.955 | Palestine | [24] | ||
| 35 | China | [17] | ||
| Onion | 78.35-95.36 | 29.93 ± 2.14 | Nigeria | [9] |
| 49.835 | Palestine | [24] | ||
| 23 | Korea | [16] | ||
| 13 | China | [15] | ||
| Cabbage | 186.58-230.5 | 174.63 ± 17 | Nigeria | [9] |
| 141.845 | Palestine | [24] | ||
| 510 | Turkey | [18] | ||
| 1200 | China | [15] | ||
| 161 | Iran | [26] |
Table 3: Comparison of nitrate content (mg/kg) in the vegetables studied in Adami Tulu Judo Kombolicha district, and the nitrate content reported in other studies.
study than other reported literature value.
Conclusion and Recommendation
In this research simultaneous determination of nitrate and nitrite were conducted with single beam UV-Visible spectrophotometer for the analysis of vegetables (cabbage, tomato and onion) and supportive soil In Adami Tulu Judo Kombolicha district.
From the results obtained, it is seen that the study area is an inhabitant of anions, like nitrite and nitrate. The results of the study showed that nitrite content in vegetables ranged from 0.46-1.44 mg/ kg. Nitrate content also ranged from 28-230.5 mg/kg. The highest value of nitrite and nitrate comes from cabbage followed by onion and tomato. As much as these anions are good in maintaining a balance health, condition their tendencies of being toxic have given rise to this research. This research is meant to stand as a guide to other researchers, nutritionists, and agriculturists who seek information on the level of anions in crops grown in this study area and also give anyone who has the opportunity of laying hands on this research the knowledge of the effects associated with the intake of these vegetables.
In this study the concentrations of nitrate and nitrite in vegetable samples were within the safe limit of WHO. Therefore, the consumption of these vegetables has no health effect related to the toxicity of nitrite and nitrate.
Acknowledgments
We are thankful to Dilla University for providing financial assistance to carry out the study. We are also grateful for Dr. Ahmed Hussen and Dr. Getachew Adam for advising to achieve this study.
References
- Ahmed S, Naser A, Farideh S (2007) Evaluation of Nitrate and Nitrite of Southern Iran (Ahwaz) Vegetables during winter and spring of 2006. Asian Journal of Plant Sciences 6: 1197-1203.
- Antonio A (2004) Measuring intake vegetables and fruits. Unit of Epidemiology, Catalan Institute of Oncology (ICO), Spain.
- Olga P, Vicent Y, Pilar V, José A (2009) Monitoring programme on nitrate in vegetables and vegetable-based baby foods marketed in the Region of Valencia: levels and estimated daily intake. Journal of Food Additives and Contaminants, pp: 1-26.
- WHO (2003) Nitrate and potential endogenous formation of N-nitroso compounds). In: Safety evaluation of certain food additives. Food Additives Series 50.
- Thomson B (2004) Nitrates and nitrites dietary exposure and risk assessment: prepared as part of a New Zealand Food Safety Authority contract for scientific services. Institute of Environmental Science & Research Limited. Christchurch Science Centre.
- Özdestan O, Üren A (2011) Effects of boiling parameters on the levels of nitrate, nitrite and color values of wild Radish (Raphanus raphanistrum). GIDA 36: 193-200.
- Santamaria P, Elia A, Serio F, Todaro E (1999) A survey of nitrate and oxalate content in fresh vegetables. Journal of the Science of Food and Agriculture 79: 1882-1888.
- Santamaria P (2006) Nitrate in vegetables: toxicity, content, intake and EC regulation. Journal of the Science of Food and Agriculture 86: 10-17.
- Uwah J, Abah J, Ndahi N, Ogugbuaja V (2009) Concentration levels of nitrate and nitrite in soils and some leafy vegetables obtained in Maiduguri, Nigeria. Journal of Applied Sciences in Environmental Sanitation 4: 233-244.
- Pandey A, Nigam P, Soccol VT (2000) Biotechnological potential of agro-industrial residues I: sugarcane bagasse. Bioresource Technology 74: 69-80.
- Aydin A, Ercan A, Tascloglu S (2005) A novel method for spectrophotometric determination of nitrite in water. Talanta 66: 1181-1186.
- Chou S, Chung J, Hwang D (2003) A high performance liquid chromatography method for determining nitrate and nitrite levels in vegetables. Journal of Food and Drug Analysis 11: 233-238.
- Badiadka N, Kenchaiah S (2009) Spectrophotometric method for the determination of nitrite and nitrate. Eurasian J Anal Chem 4: 204-214.
- CECSCCF (Commission of the European Communities Scientific Committee for Food) (1997) Report of Scientific committee for food on nitrate and nitrite. 38th Series.
- Center for Food Safety (CFS) (2010) Nitrate and Nitrite in Vegetables Available in Hong Kong. Risk Assessment Studies, Report No 40.
- Chung SY (2003) Survey of nitrate and nitrite contents of vegetables grown in Korea. Food Additives and Contaminants 20: 621-628.
- Feng J (2006) Assessment of nitrate exposure in Beijing residents via consumption of vegetables. Chinese Journal of Food Hygiene 18: 514-516.
- Fredvis M, Fatma S, Neslihen E (2010) Nitrate and nitrite contents in some vegetables consumed in South province of Turkey. Agricultural Journal 5: 142-145.
- Gaya U, Alimi S (2006) Spectrophotometric determination of nitrate in vegetables using phenol. J Appl Sci Environ Mgt 10: 79-82.
- Gouda A, Shehab W, Sheikh R, Shohaib S (2008) Sensitive spectrophotometric determination of carbaryl in its formulations and environmental samples. Third Environment Conference, pp: 21-34.
- Nidal Z, Maher A, Abdullah F (1999) Spectrophotometric determination of nitrite and nitrate using phosphomolybdenum blue complex. Talanta 50: 819-826.
- Simion V, Câmpean G, Vasile G, Artimon M, Catana L, et al. (2008) Nitrate and nitrite accumulation in tomatoes and derived products. Roumanian Society of Biological Sciences 13: 3785-3790.
- Terje T, Mari R, Kadrin J, Toomas T, Alida K (2005) Nitrates and nitrites in vegetables and vegetable-based products and their intakes by Estonian population. Journal of Food Additives and Contaminants 23: 1-25.
- Feng J (2006) Assessment of nitrate exposure in Beijing residunts via consumption of vegetables. Chinese Journal of Food Hygiene 18: 514-516.
- WHO (1995) Evaluation of certain food additives and contaminants. 44th Report of the Joint FAO/WHO Expert Committee on Food Additives. Technical Report Series No. 859. Geneva, WHO, pp: 29-35.
- Zamrik M (2013) Determination of Nitrate and Nitrite Contents in Tomato and Processed Tomato Products in Syrian Market. Int J Pharm Sci Rev Res 19: 1-5.
Citation: Ajebe EG, Bahiru TB (2018) Spectrophotometric Determination of Nitrite and Nitrate in Some Selected Vegetables Cultivated in Adami Tulu Judo Kombolicha District Farms, Ethiopia. J Anal Bioanal Tech 9: 410. DOI: 10.4172/2155-9872.1000411
Copyright: © 2018 Ajebe EG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 6986
- [From(publication date): 0-2018 - Apr 28, 2025]
- Breakdown by view type
- HTML page views: 5923
- PDF downloads: 1063