Research Article Open Access
Species Identification of Forensic Important Blowflies (Diptera, Calliphoridae) that occur in Jeddah, Kingdom of Saudi Arabia
Layla A.H. Al-Shareef*
King Abdulaziz University, Kingdom of Saudi Arabia
- *Corresponding Author:
- Layla A.H. Al-Shareef
Faculty of Science-Al Faisaliah, King Abdulaziz University
Ministry of Education, Kingdom of Saudi Arabia
Tel: + 966564469922
E-mail: Layladr@hotmail.com
Received date: May 11, 2016; Accepted date: July 14, 2016; Published date: July 21, 2016
Citation: Al-Shareef LAH (2016) Species Identification of Forensic Important Blowflies (Diptera, Calliphoridae) that occur in Jeddah, Kingdom of Saudi Arabia . Glob J Nurs Forensic Stud 1: 106. doi: 10.4172/2572-0899.1000106
Copyright: © 2016 Al-Shareef LAH. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Global Journal of Nursing & Forensic Studies
Abstract
An important goal in forensic entomology is the identification of necrophagous fly species that breed directly on carcasses. In kingdom of Saudi Arabia there are rarely studies on carrion breeding blowflies. Using rabbit carcasses as a model for study, a key for the identification of three species of blowflies (Diptera: Calliphoridae) collected from deferent habitat (desert, urban, agriculture and coastal) in Jeddah city, west region of the Kingdom of Saudi Arabia was prepared. The blowfly species which found were Chrysomya megacephala, Chrysomya marginalis and Chrysomya albiceps. This study was conducted to create database information on necrophagous flies in the Kingdom of Saudi Arabia geographical region, and it is essential to the progress of forensic entomology in the country.
Keywords
Chrysomya species; Blowflies; Kingdom of Saudi Arabia
Introduction
Forensic entomology applies insect evidence to legal problems. Compared to the traditional procedures in Forensic Medicine, insects are accuracy even in advanced stages of decomposition (after four to five days postmortem) when postmortem phenomena such as autolysis and putrefaction occur [1,2]. Flies from the family Calliphoridae are the first group that arrive to the cadaver [3,4], and attract by the odor produce during the early stages of decomposition. Since they lay eggs on a carrion within minutes after death, the estimation of the age of the oldest present eggs, larvae or pupae will provide a minimum postmortem interval (mPMI) [4-8]. Insect species associated with carrion and the period of life cycle vary according many factors, one of the most important being the geographic region or bio-geo climatic zone. The bio-geo climatic zone includes the habitat, vegetation, soil type and meteorological conditions of the area [9]. Therefore, knowledge of the insect fauna that are attracted to human cadavers in every geographical region is fundamental for the use of flies as forensic indicators, and databases should be developed [10,11]. This sort of information is obtained from field experiments on carrion decomposition, usually made with animal models [12]. However, in the Kingdom of Saudi Arabia only rarely studies have been conducted on this subject and the blowflies are poorly known, while using information from other bio-geographical areas, with different fauna and environmental conditions, may not provide a sufficient degree of accuracy for using flies in forensic cases [13]. For this purpose the current study aims to identify species and start to create a database for forensic important blowflies which present in the kingdom of Saudi Arabia particularly in Jeddah bio-geo climatic zone.
Materials and Methods
Specimens for this study were obtained from domestic rabbit carcasses located in four deferent habitat (desert, urban, agriculture and coastal), in Jeddah city in autumn, 2015. Jeddah city is located on the west coast of the Kingdom of Saudi Arabia (latitude 29.21 north & longitude 39.7 east), in the middle of the eastern shore of the Red Sea. The temperature and relative humidity at the time of collecting flies were varied from 26.83°C to 30.62°C and 15% to 89%, respectively. The flies were collected using entomological net and killed in 95% ethyl alcohol, then washed by water with small amount of detergent to remove dust. In some flies real body color was difficult to be observed and seems dull because of the excessively weathered, and we could to solve this problem by placing the specimens in xylene for two hours. The flies were identified with the aid of dissecting stereomicroscope from Leica Company (Leica M205 C stereomicroscope). Digital photographs were taken with Leica IC80 HD camera adapted to a Leica M205 C stereomicroscope. Measurement was given in millimeters. The taxonomy and terminology follows [14-18].
Results
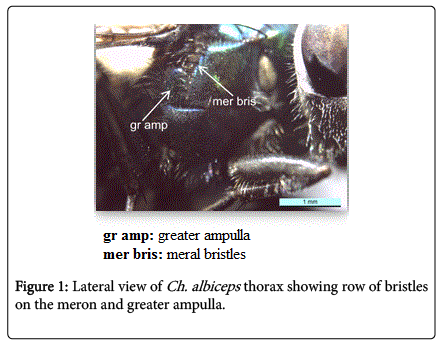
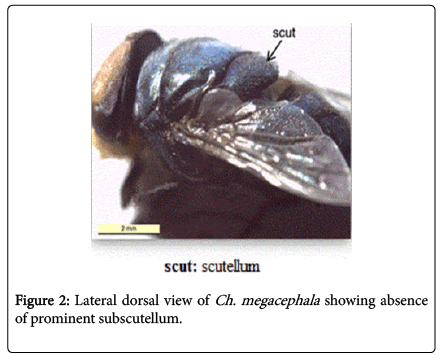
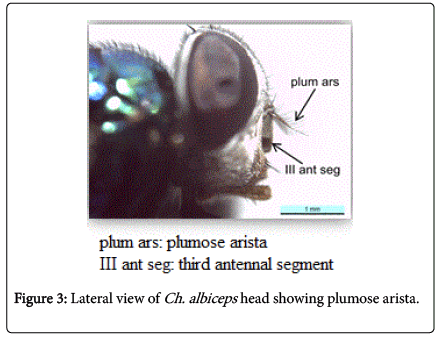
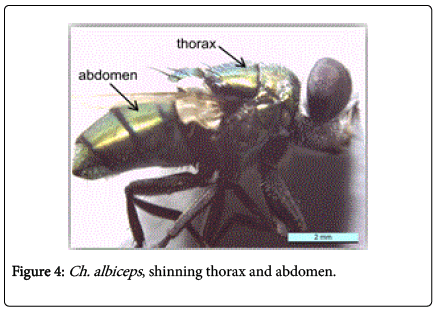
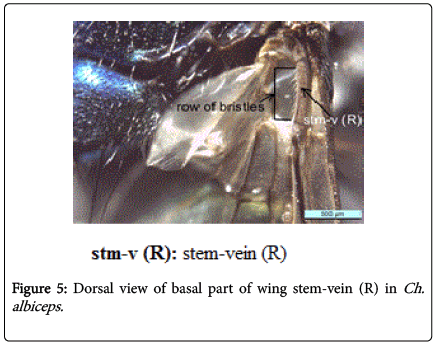
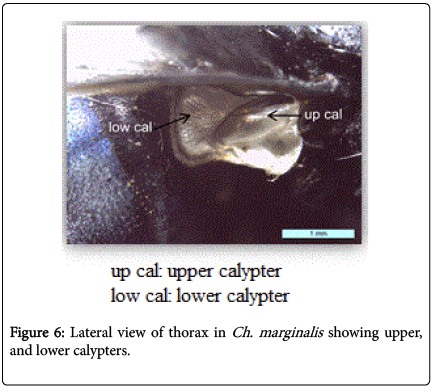
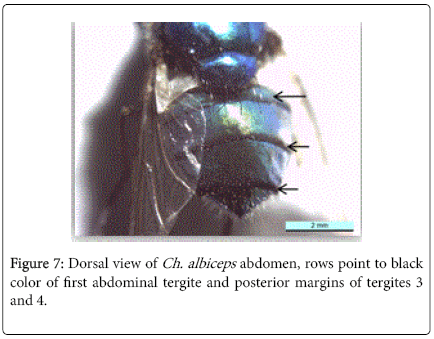
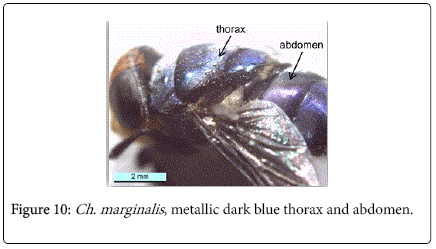
During this study adult fly specimens were obtained from domestic rabbit carcasses located in four deferent habitats (desert, urban, agriculture and coastal) in Jeddah city, kingdom of Saudi Arabia. These flies were observed among the initial colonizers of the corpse, arriving in short time after death and laying their eggs into the natural orifices. All collected flies were belong to family Calliphoridae (blowflies), which identified by presence row of bristles on the meron (meral bristles) (Figure 1), absence of prominent subscutellum (Figure 2), third antennal segment has plumose arista (Figure 3), they were medium to large flies (4-16 mm in length) with metallic blue or green thorax and abdomen (Figure 4). All these blowflies from Subfamily; Chrysomyinae which easily recognized by being base of stem-vein (R) with row of bristles on the dorsal surface (Figure 5). The flies belong to genus Chrysomya Robineau-Desvoidy which characterized by reniform greater ampulla with stiff erect hairs (Figure 1), dorsal surface of lower calypter with dense hairs (Figure 6), dorsum of first abdominal tergite (syntergite 1+2) and posterior margins of tergites 3 and 4 black (Figure 7), genal dilation (cheek) whitish or yellowish (Figures 8- 10).
Key for the identification of the species of Calliphoridae with forensic importance in Jeddah city, Kingdom of Saudi Arabia
Blowflies from family Calliphoridae , subfamily Chrysominae , genus Chrysomya - 1
1. Anterior spiracle dark- brownish (Figure 11) - Chrysomya megacephala
1’. Anterior spiracle white (Figure 12) - 2
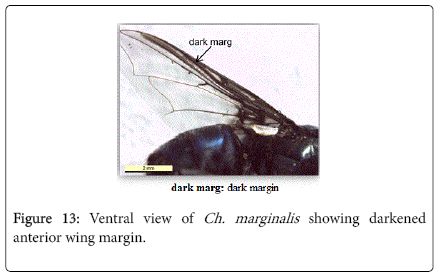
2. Anterior wing margin strongly darkened (Figure 13) - Chrysomya marginalis
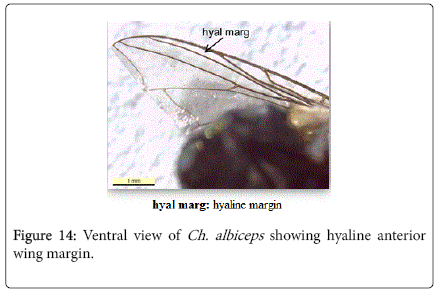
2’. Anterior wing margin hyaline (Figure 14) - Chrysomya albiceps
Notes on species
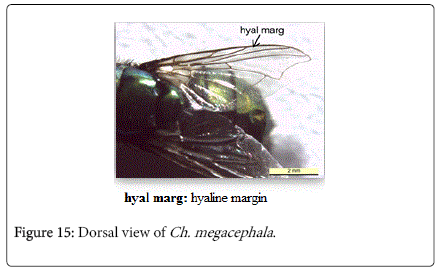
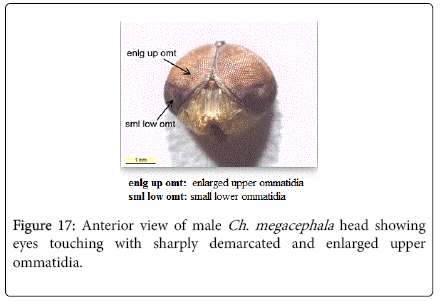
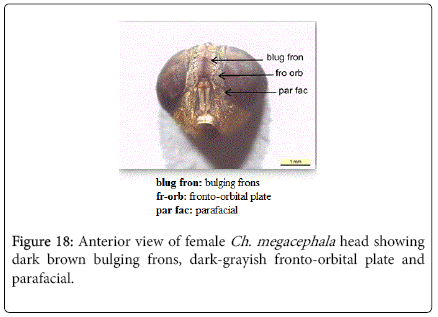
Chrysomya megacephala (Fabricius, 1794): Diagnosis- Flies with medium size 6-9 mm in length. Surface of genal dilation and post-gena orange with orange to yellow hair, anterior spiracle brownish (Figure 9). Wings entirely hyaline (Figure 15). Lower calypter yellowish to dirty gray (Figure 16). Male frons very narrow, eyes touching with ommatidia in upper two thirds enlarged and sharply demarcated from small ones in lower third (Figure 17). Frons of female bulging in the middle, not paralled-sided, with dark brown to black color, the frontoorbital plate and parafacial dark grayish (Figure 18).
Distribution: Chrysomya megacephala was first recorded in the late 1970’s [19-21], then spread quickly and now present in most continents [22]. This species has been recorded in continental Europe [23,24], Mediterranean region[24,25], Palaearctic region (Middle East, Canary Is.) [26,27], presently covering the Siberian sub-region, Spain, Iran, Afghanistan, China, Japan, [28], United States [29-34], West Indies, and Brazil [35]. It is also recorded in southern Africa [36], Egypt, Kuwait, Oman, Pakistan, United Arab Emirates, Kingdom of Saudi Arabia [37].
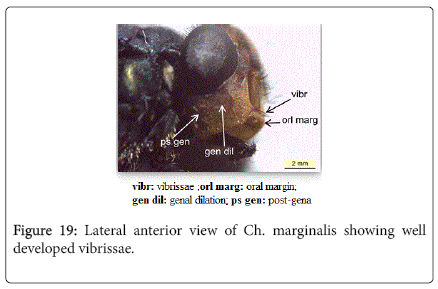
Chrysomya marginalis (Wiedemann, 1819): Diagnosis - Large species, usually 8-12 mm in length. The body is metallic dark blue (Figure 10). Surface of genal dilation and post-gena orange with orange to yellow hairs, vibrissae well above the oral margin (Figure 19). It is ch aracteristic by deeply darkened of anterior margin wings (Figure 11).
Distribution: This species generally common in sub-Saharan Africa [38]. It was recorded in Burkina Faso, Central African Republic, Egypt, Congo, Kenya, Madagascar, Mali, Namibia, Nigeria, Rwanda, South Africa, Senegal, Somalia, Sudan, Tanzania, Zimbabwe [38-46]. Also occurs in kingdom of Saudi Arabia, Yemen, Oman, United Arab Emirates, Iran, Pakistan [37].
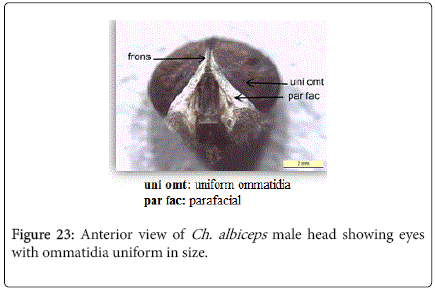
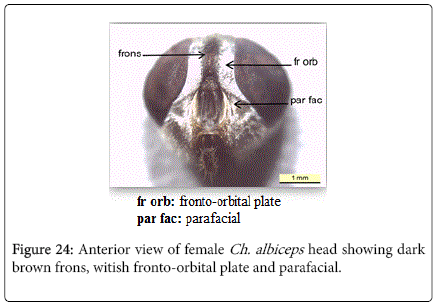
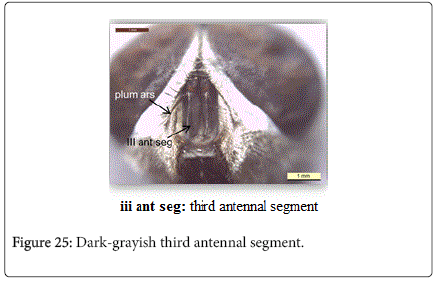
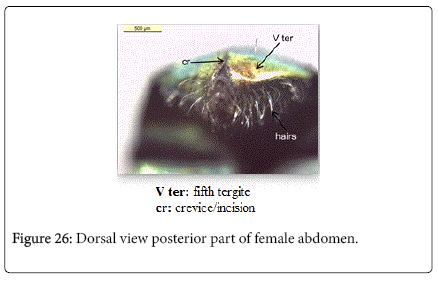
Chrysomya albiceps: Diagnosis - Flies with medium size (6-9 mm in length). Thorax and abdomen metallic blue to green (Figure 4), wings entirely hyaline (Figure 12). Anterior spiracle whitish, proepisternal seta (blew anterior spiracle) absent (Figure 20). katepisternal setae 1+1 (Figure 21). Male and female vertix with well-developed outer vertical setae (Figure 22). Surface of genal dilation and post-gena whitish with white hairs (Figure 8). Male frons very narrow, eyes very close to each other with ommatidia uniform in size (Figure 23). Frons of female with dark brown to black color, the fronto-orbital plate and parafacial whitish (Figure 24). Third antennal segment wholly dark-grayish (Figure 25). At least some hairs on lateral surfaces of tergite V white, posterior edge of tergite V of female with crevice/incision (Figure 26).
Distribution: Chrysomya albiceps is very common species in the Mediterranean regions, and has been reported in Egypt, Iran, Iraq, Kuwait, Lebanon, Libya, Oman, Pakistan, Kingdom of Saudi Arabia, Syria, United Arab Emirates and Turkey [37]. It is recently shown expansion towards central Europe [47-49]. This species is already established in Portugal [50] and other European countries [51], recently having reached Poland [49]. It was also recorded in America [16], West Indies.
Discussion
Blowflies occur in all biogeography regions and contain around 1500 species [52]. The fauna of blowflies has been studied all over the world; in South America [53], Argentina [54], North America [16,18], Portugal [55], East and South Asia [56], Africa [57]. It was also studied in European and Mediterranean regions [58], Afrotropical region [17]. Blowfly species in the Middle East region were studied by Akbarzadeh et al. [37] and a key was accomplished. However, the fauna of blowflies of forensic important was not studied enough in the Arabian Gulf region, and there is not any key to identify species in the Kingdom of Saudi Arabia. This situation stops broad application of insects for medico-legal purposes due to a lack of knowledge proper tools for species identification of the local fauna. According to [53], the definition of the carrion flies faces two major problems: the lack of taxonomists and the lack of keys. Therefore, recent study is a part of large ongoing effort to identify carrion-feeding entomofauna and to start to build a checklist of necrophagous species present in this geographical area; the Kingdom of Saudi Arabia.
The recent study proved that there are three species of blowflies attracted to the rabbit carcasses in Jeddah city, all of them were belong to genus Chrysomya Robineau-Desvoidy. Until now, Chrysomya Robineau-Desvoidy flies were confined to the Old World tropics and subtropics regions, where they were among the most abundant and economically important blowflies [59].
In this study, Chrysomya megacephala (Fabricius) was observed among the first wave of flies visiting rabbit carcasses. This species known as the ‘oriental latrine fly’ due to its favorite place of ovipositing in latrines [60], and it is may be origins in New Guinea [61]. Larvae are commonly breed in feces and carrion [30,62]. Due to its close association with humans, it was considered to be a potential mechanical vector of faecal pathogens [63]. Chrysomya megacephala was recorded in Kuwait by Al-Mesbah et al. [64]. It was used in forensic entomology cases, for postmortem interval determination [65-67]. In the recent study, the blowfly Chrysomya marginalis (Wiedemann) was attracted to the rabbit carcasses. This species is normally feces and carrion breeder and one of the primary flies associated with decomposing corpses in areas in which the species is present [53]. It is rarely enters houses, but are common around butcher shops, and also feed from sores and wounds of cattle [62]. Braack [68] found large numbers of Ch. marginalis on freshly death impala in Kruger Park. In the Arabian Gulf region Chrysomya marginalis was reported in the Kingdom of Saudi Arabia on rabbit carcasses in the mountains of Al-Baha Province at southwestern area [69]. It was also recorded in United Arab Emirates, Oman and Yemen [37]. One of the most abundant blowflies species associated with rabbit carcasses in this study was Chrysomya albiceps . It is strong fliers, and the maximum dispersal rates for this species were estimated as 16 km/12 days [70]. Chrysomya albiceps larvae are predatory on other larvae present in carrion [62]. They are typically found in carrion but are also able to consume decomposing flesh on wounds of living animals, and considered a primary myiasis fly [26]. Adults prefer high temperatures and humidity, and can be attracted in large numbers by meat [62]. Buttiker et al. [71] proved that Chrysomya albiceps is endophilic in Saudi Arabia and a nuisance in homes and markets there.
Conclusion
It was recorded in the Kingdom of Saudi Arabia by [69] in Al-Baha Province at southwestern region, and in Jeddah city at the west region by [72], it was also reported in Qatar [73] and in Kuwait [64]. Al- Ghamdi et al. [74] collected Chrysomya megacephala and Chrysomya albiceps from different locations of Jeddah city, and they mentioned some morphological characters for these two species which were not enough to understand taxonomical features, also they didn’t prepare any taxonomical key.
References
- Benecke M (2004) Forensic Entomology: Arthropods and Corpses. Forensic Pathology Reviews, Humana Press, Totowa, New Jersey, pp.207-240.
- Saukko PJ, Knight B (2004) Knight’s Forensic Pathology (3rdedn) Arnold, Oxford University Press, London, UK.
- Nuorteva P (1977) Sarcosaprophagous Insects as forensic indicators, In: Eckert CG, Tedeschi LG (eds.) Forensic Medicine: A Study in Trauma and Environmental Hazards, Trauma. Saunders and Co, Pennsylvania, pp. 1637.
- Goff ML (1993) Estimation of postmortem interval using arthropod development and succession patterns. Forensic Science Review 5: 81-94.
- Catts EP (1990) Analyzing entomological data. Entomology & Death: A Procedural Guide. In: Catts EP, Haskell NH (eds.) Joyce’s Print Shop, Clemson, South Carolina, pp. 124-137.
- Catts EP, Goff ML (1992) Forensic entomology in criminal investigations. Annu Rev Entomol 37: 253-272.
- VanLaerhoven SL, Anderson GS (1999) Insect succession on buried carrion in two biogeoclimatic zones of British Columbia. J Forensic Sci 44: 32-43.
- Byrd JH, Castner JL (2009) Insects of forensic importance. In: Byrd JH, Castner JL (eds.) Forensic Entomology: The Utility of Arthropods in Legal Investigations. CRC Press, Boca Raton, pp. 43–79.
- Anderson GS (2010) Factors that influence insect succession on carrion. Forensic Entomology: The utility of arthropods in legal investigations, CRC Press, Boca Raton, Florida, pp. 201-250.
- Anderson GS (1995) The use of insects in death investigations: an analysis of cases in British Columbia over a five year period. Can Soc Forensic Sci J 28: 277-292.
- Anderson GS, VanLaerhoven SL (1996) Initial studies on insect succession on carrion in southwestern British Columbia. J Forensic Sci 41: 617- 625.
- Wells JD, LaMotte LR (2010) Estimating the Postmortem Interval. Forensic Entomology: The Utility of Arthropods in Legal Investigations, CRC Press, Boca Raton, Florida, pp. 367-388.
- Arnaldos MI, Romera E, Presa JJ, Luna A, García MD (2004) Studies onseasonal arthropod succession on carrion in the southeastern Iberian Peninsula. Int J Legal Med 118: 197-205.
- James MT (1947) The flies that cause muaisis in man. Misc. Publication 631, US Department of Agriculture.
- Spradbery JP (2002) A manual for the diagnosis of screw-worm fly. Agriculture, Fisheries and Forestry, Australia.
- Marshall SA, Whiteworth T, Roscde L (2011) Blow flies (Diptera: Calliphoridae) of eastern Canada with a key to Calliphoridae subfamilies and genera of eastern North America, and a key to the eastern Canadian species of Calliphorinae, Lucilinae and Chrysomyiinae. Can J Arthropod Identification No. 11.
- Irish S, Lindsay T, Wyatt N (2014) Key to adults of Afrotropical species of the genus ChrysomyaRobineau-Desvoidy (Diptera: Calliphoridae). AfrEntomol 22: 297–306.
- Whitworth T (2006) Keys to the genera and species of blow flies (Diptera: Calliphoridae) of America north of Mexico. ProcEntomolSoc Wash 108: 689–725.
- Kurahashi H (1978) The oriental latrine fly: Chrysomyamegacephala (Fabricus) newly recorded from Ghana and Senegal, West Africa.
- Prins AJ (1979) Discovery of the oriental latrine fly Chrysomyamegacephala (Fabricius) along the south-western coast of South Africa. So AfrMus Ann 78: 39-47.
- Williams KA, Villet MH (2014) Morphological identification of Luciliasericata, Luciliacuprina and their hybrids (Diptera, Calliphoridae). ZooKeys 420: 69–85.
- Verves YG (2003) A preliminary list of species of Calliphoridae and Sarcophagidae (Diptera) of the Republic of Seychelles. Phelsuma 11: 1–16.
- Vel´asquez Y, Martinez-Sanchez A, Rojo S (2008) Autumn colonization of pig carrion by blowflies (Diptera: Calliphoridae) in a Mediterranean urban area (SE Spain). Proceedings of the Sixth Meeting of the European Association for Forensic Entomology, Kolymbari, Crete.
- Prado e Castro C, Garcia MD (2009) First record of Chrysomyamegacephala (Fabricius, 1794) (Diptera, Calliphoridae) from Portugal.
- Ebejer MJ (2007) The occurrence of Chrysomyamegacephala (Fabricius) (Diptera, Brachycera) in Malta and records of other Calliphoridae from the Maltese Islands. Entomologist’s Monthly Magazine 143: 165–170.
- Zumpt F (1965) Myiasis in man and animals in the old world. Butterworths. London, pp. 267.
- Smith KGV (1986) A Manual of Forensic Entomology. The Trustees of the British Museum (Natural History) London, pp. 205.
- Martinez-Sanchez A, Marcos-Garca MA, Rojo S (2001) First collection of Chrysomyamegacephala (Fabr.) in Europe (Diptera: Calliphoridae). Pan- Pacific Entomologist 77: 240-243.
- Greenberg B (1988) Chrysomyamegacephala (F.) (Diptera: Calliphoridae) collected in North America and notes on Chrysomya species present in the New World. J Med Entomol 25: 199-200.
- Wells JD (1991) Chrysomyamegacephala (Diptera: Calliphoridae) has reached the continental United States: Review of its biology, pest status, and spread around the world. J Med Entomol 28: 471-473.
- Baumgartner DL (1993) Review of Chrysomyarufifacies (Diptera: Calliphoridae). J Med Entomol 30: 338-352.
- De Jong GD (1995) Report of Chrysomyamegacephala (Diptera: Calliphoridae) in northern New Mexico. Entomological News 106: 192.
- Wells JD (2000) Introduced Chrysomya (Diptera: Calliphoridae) flies in northcentral Alabama. J EntomolSci 35: 91-92.
- Tomberlin JK, Reeves WK, Seppard DC (2001) First record of Chrysomyamegacephala (Diptera: Calliphoridae) in Georgia, USA Florida Entomologist 84: 300-301.
- Guimaraes JH, Prado AP, Linhares AX, (1978) Three newly introduced blowfly species in southern Brazil (Diptera: Calliphoridae). RevistaBrasileira de Entomologia 22: 53-60.
- Baumgartner DL, Greenberg B (1984) The genus Chrysomya (Diptera: Calliphoridae) in the new world. J Med Entomol 21: 105-113.
- Akbarzadeh K, Wallman JF, Sulakova H, Szpila K (2015) Species identification of Middle Eastern blowflies (Diptera: Calliphoridae) of forensic importance. Parasitol Res 114: 1463-1472.
- Zumpt F (1956) Calliphoridae (DipteraCyclorrhapha) Part I: Calliphorini and Chrysomyiini. Exploration du Parc National Albert Mission G F De Witte (1933–1935), 87: 1–201.
- Rickenbach A (1967) Calliphoridae de RepubliqueCentrafricaine avec la description d’une nouvelle espèce: Isomyiayvorei n. sp. Bulletin de la SociétéEntomologique de France 72: 45–52.
- Rickenbach A, Hamon J, Ovazza M (1962) Calliphoridae de HauteVolta et de Côte d’Ivoire. Bulletin de la SociétéEntomologique de France 67: 132–141.
- Pont AC (1980) Family Calliphoridae, In: Catalogue of the Diptera of the Afrotropical Region. British Museum (Natural History), London, UK, pp. 779–800.
- Prins AJ (1982) Morphological and biological notes on six South African blowflies (Diptera, Calliphoridae) and their immature stages. Ann S Afr Museum 90: 201–217.
- Braack LEO (1987) Community dynamics of carrion-attendant arthropods in tropical African woodland. Oecologia 72: 402-409.
- Rognes K (2002) Blowflies (Diptera: Calliphoridae) of Israel and adjacent areas, including a new species from Tunisia. Insect SystEvol Supplements 59: 1-148.
- Verves YG (2005) A catalogue of Oriental Calliphoridae (Diptera). Dipterological Research 16: 233-310.
- Kurahashi H, Kirk-Spriggs A (2006) The Calliphoridae of Namibia (Diptera: Oestroidea). Zootaxa, 1322: 1–131.
- Povoln´y D (2002) Chrysomyaalbiceps (Wiedemann, 1819): the first forensic case in Central Europe involving this blowfly (Diptera, Calliphoridae). ActaUniversitatisAgriculturae et SilviculturaeMendelianaeBrunensis L 3: 105–112.
- Verves YG (2004) Records of Chrysomyaalbiceps in the Ukraine. Med Vet Entomol 18: 308-310.
- Szpila K, Pape T, Rusinek A (2008) Morphology of the first instar larva of Calliphoravicina, Phormiaregina and Luciliaillustris (Diptera, Calliphoridae). Med Vet Entomol 22: 16–25.
- Prado e Castro C (2011) Seasonal carrion Diptera and Coleoptera communities from Lisbon (Portugal) and the utility of Forensic Entomology in Legal Medicine. PhD Thesis, University of Lisbon, Portugal.
- Rognes K (2004) Fauna Europaea: Calliphoridae. In: Pape T (ed.) Fauna Europaea: Diptera. Fauna Europaean version.
- Thompson FC (2006) Nomenclator Status Statistics. Retrieved January, 10, 2007, from The Diptera site. The BioSystematic Database of World Diptera.
- Carvalho CJB, de Mello-Patiu CA (2008) Key to the adults of the most common forensic species of Dipterain South America RevistaBrasileira de Entomologia 52: 390-406.
- Mariluis JC (1982) Contribución al conocimiento de lasCalliphoridae de la Argentina. Opera Lilloana 33: 3-59.
- Prado e Castro C, Serrano A, Martins Da Silva P, García MD (2012) Carrion flies of forensic interest: a study of seasonal community composition and succession in Lisbon, Portugal. Med Vet Entomol 26: 417-431.
- Yang ST, Kurahashi H, Shiao SF1 (2014) Keys to the blow flies of Taiwan, with a checklist of recorded species and the description of a new species of Paradichosia Senior-White (Diptera, Calliphoridae). Zookeys 434: 57-109.
- Youmessi FFD, De Coninck E, Bilong BCF, Hubrecht F, Djiéto-Lordon C, et al. (2012) First records on five species of Calliphoridae (Diptera) reared from maggot collected on rat carrions corpse during a forensic entomology experiment in the campus of the University of Yaounde I-Cameroon. Int J Biosciences 2: 75-80.
- Szpila K (2010) Key for identification of European and Mediterranean blowflies (Diptera, Calliphoridae) of forensic importance Adult flies. Nicolaus Copernicus University Institute of Ecology and Environmental Protection Department of Animal Ecology.
- Guimarães, JH, Papavero N (1999) Myiasis in man and animals in the Neotropical region. Bibliographic database, São Paulo.
- Laurence BR (1988) The tropical African latrine blowfly, Chrysomyaputoria (Wiedemann). Med Vet Entomol 2: 285-291.
- Kurahashi H (1982) Probable origin of a synanthropic fly Chrysomyamegacephala, in New Guinea (Diptera: Calliphoridae). MonographiaeBiologicae 42: 689–698.
- Greenberg B (1971) Flies and Disease. Ecology, Classification, and Biotic Associations. Princeton University Press, Princeton, New Jersey, USA.
- Bohart GE, Gressitt JL (1951) Filth-Inhabiting Flies of Guam. Hawai'i.
- Al-Mesbah H, Al-Osaimi Z, El-Azazy OME (2011) Forensic entomology in Kuwait: The first case report. Forensic SciInt 206: 1-218.
- Goff ML, Odom CB (1987) Forensic entomology in the Hawaiian Islands. Three case studies. Am J Forensic Med Pathol 8: 45-50.
- Goff ML, Omori AI, Gunatilake K (1988) Estimation of postmortem interval by arthropod succession. Three case studies from the Hawaiian Islands. Am J Forensic Med Pathol 9: 220-225.
- Goff ML (1992) Problems in estimation of postmortem interval resulting from wrapping of the corpse: a case study from Hawaii. J AgrEntomol 9: 237-243.
- Braack LEO (1986) Arthropods associated with (Diptera: Calliphoridae) collected in North America and notes on Chrysomya species present in the New World. J Med Entomol 25: 199–200.
- Abouzied EM (2014) Insect Colonization and Succession on Rabbit Carcasses in Southwestern Mountains of the Kingdom of Saudi Arabia. J Med Entomol 51: 1168-1174.
- Greenberg B (1973) Flies and disease. Princeton University Press, New Jersey, pp. 2: 447
- Buttiker W, Attiah MD, Pont AC (1979) Diptera: synanthropic flies, In: Wittmer W, W Buttiker (eds.) Fauna of Saudi Arabia. Ciba-Geigy Ltd, Switzerland, pp. 352-367.
- Al-Shareef LAH, Al-Qurashi SID (2016) Study of some biological aspects of the blowfly Chrysomyaalbiceps (Wiedemann 1819) (Diptera: Calliphoridae) in Jeddah, Saudi Arabia. Egyptian J Forensic Sci 6: 11–16.
- Abdu RM, Shaumar NF (1985) A preliminary list of the insect fauna of Qatar. Qatar UnivSci Bull 5: 215-232.
- Al-Ghamdi KM, Alikhan M, Mahyoub JA, Alanazi NA, Al-Najada AR, et al. (2015) Characterization of Forensically Important Necrophagus Flies (Diptera) of Jeddah, Saudi Arabia. Adv Environ Biol 9: 58-71.
Relevant Topics
- Adult Protective Care Unit
- Correctional Nursing
- Critical Care Nursing
- Emergency and Acute Care Setting
- Forensic and Victimology
- Forensic Mental disorder
- Forensic Mental Health Nursing
- Forensic Mental Illness
- Forensic Nursing
- Forensic Nursing Care
- Forensic Nursing Clinical Practice
- Forensic Nursing Science
- Healthcare Management
- Interpersonal Violence
- Intimate Partner Violence
- Nursing research
- Post Exposure Prophylaxis
- Psychosocial Intervention
- Trauma Nursing
Recommended Journals
Article Tools
Article Usage
- Total views: 15975
- [From(publication date):
September-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 14740
- PDF downloads : 1235