Review Article Open Access
Speciation via Hyphenation - Metal Speciation in Geological and Environmental Samples by CE-ICP-MS
Christina Hein, Jonas M Sander and Ralf Kautenburger*Saarland University, Inorganic Solid State Chemistry, Dudweiler, Germany
- *Corresponding Author:
- Ralf Kautenburger
Saarland University
Inorganic Solid State Chemistry
Dudweiler, Am Markt Zeile 5
66125 Saarbrücken, Germany
Tel: +49 681 302 2171
Fax: +49 681 302 70652
E-mail: r.kautenburger@mx.unisaarland.de
Received date: November 03, 2014; Accepted date: November 14, 2014; Published date: November 18, 2014
Citation: Hein C, Sander JM, Kautenburger R (2014) Speciation via Hyphenation - Metal Speciation in Geological and Environmental Samples by CE-ICP-MS. J Anal Bioanal Tech 5:225. doi: 10.4172/2155-9872.1000225
Copyright: © 2014 Hein C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
This review deals with different aspects of analytical speciation methods developed for metals and metal-containing biomolecules or metal complexes with natural organic and inorganic matter in geological and environmental samples. An overview of recent studies dealing with metal speciation in various geological and environmental sample matrices is presented. The focus of analytical speciation methods is limited to capillary electrophoresis (CE) hyphenated with Inductively Coupled Plasma (ICP) detection techniques. The main aspects discussed in this review are the separation and detection step for the metal species. Possible influences of the applied separation technique on the metal speciation have been considered. Prominent exampes are, the metal-NOM (natural organic matter) complex stability during the separation step by the use of CE or the problem of ion-exchange and adsorption processes in the case of silica tubes as CE-capillaries. Additionally, different matrix effects in ICP-MS analysis will be discussed. Furthermore, the major drawback of speciation analysis is the lack of suitable reference or standard materials for the purpose of quality assurance. Therefore, quantification and verification of separation efficiency and accuracy for both the separation and detection step of the species have been evaluated, too.
Introduction
In the last 20 years speciation analysis has become an important method for separation, identification and quantification of different species of one individual metal. Hitherto, the total concentration of an element in a sample matrix was the sole aspect being evaluated. Today it is known that the total concentration of an analyte is not a sufficient criterion to assess the true hazardousness. but that bioavailability, mobility and toxicity are dependent to a great extent on the form of the element, i.e. oxidation states and different bonding forms [1,2].
As a consequence, elemental speciation is becoming more important. In most cases, liquid chromatography (LC) or capillary electrophoresis (CE) are used to separate the different species in the samples. Standard detectors like a UV-detector show only a low sensitivity and in addition no metals can be detected without e.g. further derivatisation of the metals. Hyphenation with other instruments being able to detect and quantify metals are necessary. Therefore, mass spectrometry with inductively coupled plasma (ICP-MS) is often used as detector. The ICP-MS shows a very low limit of detection (LOD), has a wide linear range and can be used for multi-element and isotopic analysis [3].
In 1980 speciation was introduced by Florence and Batley [4] for the identification and quantification of different species. But not before the 90s the method has been accepted. Olesik [5] and Michalke [6] are the pioneers of the CE-ICP-MS hyphenation. Shortly after the publication of their work the number of new speciation methods increased but the application for natural samples was rare [4]. Whereas the number of publications with CE- ICP-MS slowly but constantly increased the papers with HPLC-ICP-MS increased rapidly [7].
Most research groups engaged with speciation analysis are particularly interested in the analysis of food, biological and medical samples like human serum, milk or urine [4]. Less common but not less important are environmental or geological applications. Especially important in this context are trace elements. They are essential for human health in low concentration but can become toxic in higher concentration. This is true for e.g. selenium, zinc, molybdenum and manganese [3]. Additionally, some metals are found alkylated in the environment due to anthropological input or after bio-methylation. Most interesting in this regard are the (semi) metals arsenic, bismuth, mercury, antimonium, lead and tin. A typical process is the biomethylation of metals on aqua-sediment-surfaces. The organic metal species are dissolved in the water and are accumulated in aquatic organisms for example methylmercury (MeHg(I)) in fish [8]. MeHg(I) is the most toxic species of mercury, can be accumulated through the food chain and is toxic for humans [9]. In some cases, it is possible that organometallic compounds interact with ligands present in the living organism. As a result, these toxic compounds are enabled to proceed along biochemical pathways normally only accessible for essential metals.
Hyphenation of CE and ICP-MS
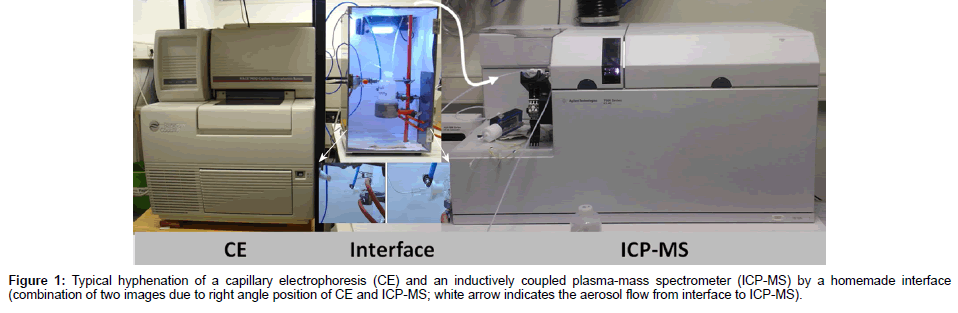
The hyphenation of CE and ICP-MS as shown e.g. in Figure 1 is used rarely compared to the hyphenation of HPLC and ICP-MS although the first hyphenation offers many advantages over the latter. For an analysis, only a small sample volume in the range of a few μL is required.
This is a great advantage for samples where only small volumes are available. Furthermore, the choice of the CE background electrolyte does not affect the plasma stability. Using smooth CE separation conditions, a separation of species with different charge and size in only one short analysis step is possible. High separation efficiency and low costs are further advantages too [10,11]. The requirement of a low sample volume leads to a LOD wich can be two orders of magnitude lower than in the case of hyphenation with HPLC.
As disadvantage, the applied separation conditions as well as the buffer- and electrolyte systems may influence the species distribution during the CE-separation thus falsifying the analytical outcome. Furthermore, due to the low sample flow rate from the CE instrument an additional make-up fluid can be necessary to guarantee a sufficient flow for constant nebulisation. Most of the time, nitric acid is used as make-up fluid due to its stabilising influence on the Ar-plasma. With other make-up liquids the ICP becomes imprecise and shows a worse sensitivity.
In the 90s the aims of the new CE-ICP-MS method were very elementary [6]. The total time of analysis was to be reduced and the LOD was to be lowered in order to enable the measurement of real samples taken from the environment. Today, the application of CEICP- MS for metal speciation in natural samples is still a problem.
In this review, only original research articles concerning speciation of geological or environmental samples by CE-ICP-MS are discussed. Further publications dealing with speciation analyses performed by CE-ICP-MS can be found in many other reviews over the last five years [12-27].
Geological Samples
The application of CE-ICP-MS for geological samples shows a high diversity of analytes, however, most of the studies analyse samples with spiked radionuclides as analytes. Objective of the research on the one hand is to optimate the separation method for different metal species, and on the other hand to evaluate complex stability constants of the spiked radionuclides with natural organic matter like fulvic or humic acids. An overview is given in Table 1.
| Elements/ Isotopes | Method | Species | Buffer | Separation conditions |
LOD | Ref. |
|---|---|---|---|---|---|---|
| Lanthanides, Cs-133,134, 135, 137, Ba-134,135, 136,138 |
CE-ICP-QMS/ CE-ICP-SFMS | different isotopes and elements | 15 mM Phosphate pH 2.5 for Cs / 0.8 mM picolinic acid, 10 mM HIBA, 25 mM formic acid pH 4.7, adjusted with Tris |
hydrodynamic injection, separation at 30 kV | QMS: 6 µgCs L-1 / 8 µgLn L-1/ SFMS: 9 ng Cs L-1 / 7 ngLn L-1 |
[28] |
| Eu-153, Gd-158 |
CE-ICP-MS | Eu3+, Eu-HA, Gd3+, Gd-HA | 100 mM acetic acid + 10 mM Na-acetate, pH 3.7 |
hydrodynamic injection, separation at 30kV + 3psi | 100 ng L-1 | [35,36] |
| Nd-146 | CE-ICP-MS | Nd-FA / Nd-EDTA | 500 pmol L-1 | [33] | ||
| Np-237 | CE-ICP-MS | NP(V), NP(IV) | 1 M acetic acid, pH 2.4 |
hydrodynamic injection, separation from 15 to 30kV | NP(IV) 1 nmol L-1 Np(V) 0.5 nmol L-1 | [30] |
| Pu-239, Np-237 |
CE-ICP-SFMS | NpO2+; NpO2-carbonates; PuO2+; PuO2-carbonates |
100 mM Na2CO3, 50 mM Good buffer, 2 mM tetradecyl-trimethyl-ammonium bromide pH 5.3 -11.5 |
10 kV / -10 kV in dependency of pH | 1 pmol L-1 | [31] |
| Pu-239, Np-238 |
CE-ICP-MS | NpO2+; NpO2-clorides, sulfates; PuO2+; PuO2-chlorides, sulfates |
0-0.15 M Na2SO4; 0-1 M NaCl; pH 6 |
hydrodynamic injection; separation from 5 to 10 kV at 0.8 psi | 1 pmol L-1 | [32] |
| Np-237, U-238, La; Th |
CE-ICP-MS | NpO2+; UO22+; La3+, Th4+ | 1 M acetic acid, pH 2.4 |
50 µg L-1 | [29] | |
| U-238 | CE-ICP-MS | UO22+, UO2-HA |
100 mM acetic acid + 10 mM Na-acetate, pH 3.8 |
hydrodynamic injection, separation at 30 kV + 2psi | 50 ng L-1 | [37] |
| Pa-233 | CE-ICP-MS | PaO(C2O4)+; PaO(C2O4)2-; PaO(C2O4)32- | 0.5 M oxalic acid | hydrodynamic injection, separation at -3kV and 0.8psi | 1 pmol L-1 | [40] |
Table 1: Application of CE-ICP-MS for metal speciation in spiked geological samples
CE-ICP-MS is used in the context of the safety analysis for nuclear waste repositories. Pitois et al. [28] have determined fission products for safety analysis in geological disposals for radioactive wastes and analysed leak water of MOX fuel rods. The single ICP-MS method could not be used due to the isobaric interferences at which the isotopes of fission products interfere with the natural isotopes of other elements. A preceding chemical separation is necessary. In this case the partition of Ba2+ and Cs+ with a phosphate buffer was conducted. The barium was complexed by phosphate und therefore migrates slower than the cesium ion. The advantage of the speciation is that all isotopes can be analysed by ICP-MS. In comparison to gamma spectroscopy where only gamma emitters like Eu-155 can be determined.
The research group of Reich and Trautmann [29,30] are dealing with a similar problem. In a nuclear disposal in a deep geological formation the elements neptunium and plutonium are primarily responsible for the amount of radiation present after 1000 years. The separation of Np and Pu species allows an assessment of the behaviour of these metals in clay as natural host rock or in the pore water of the clay. As a result, the Np(V) species is the stable species over a wide pH range, is well soluble in water and shows no relevant tendency for sorption onto the host rock. Under redox conditions, e. g. in presence of Fe2+, Np(V) is reduced to Np(IV) which shows a high sorption tendency. The redox speciation of Np(V) and Np(IV) can be conducted under natural conditions in clay pore water. A LOD of 10-9 mol L-1 when using a Mira Mist CE nebuliser has been achieved.
Topin et al. [31,32] are interested in neptunium and plutonium speciation, too. The research group investigated the complexation of these metals with sulfate, chloride and carbonate and found only small complex stability constants. At higher concentrations the Pu(V) disproportionates into Pu(IV) and Pu(VI). Therefore speciation studies are only possible with very small concentrations (<10-8 mol L-1). Apart from Pu(V) Np(V) is the most stable actinide and shows a similar behaviour as Pu. The influence of small, environmentally relevant, inorganic ligands on these metals is very important due to a possible mobilisation of the metals in a future disposal. In the speciation analysis experiments conducted using CE-ICP-MS the complex ligands have a concentration of 1 M at maximum. Higher concentrations produce Joule heat and are clogging the nebulizer. The Np and Pu species show the same migration time. The values determined for the sulfate (log β(PuO2SO4 -) = 1.30 ± 0.11; log β(NpO2SO4 -) = 1.34 ± 0.12) and chloride complexes of Np and Pu (log β(PuO2Cl) = -(0.40 ± 0.07); log β(NpO2Cl) = -(0.40 ± 0.07) are in the same order of magnitude. An analogous behaviour of Pu and Np is verified.
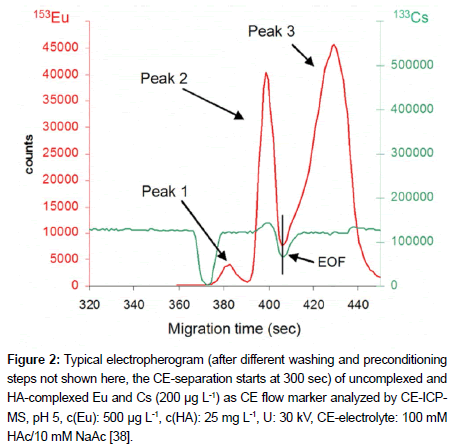
Sonke and Salters [33,34] as well as Kautenburger et al. [35-39] investigate the complexation behaviour of different humic substances like fulvic (FA) and humic acid (HA) with REEs as chemical homologues of the actinides. These experiments are important to assess the behaviour of actinides in a nuclear disposal in presence of organic substances. Some of the actinides show high sorption behaviour onto the potential host rock Opalinus clay. In presence of organic substances like FA and HA the species distribution changes and partly the metal mobility increases. The stability constants log β are important parameters in order to understand the above described process and to design subsequent experiments. Both groups apply different methods for the determination of their log β values. To avoid an adsorption of the high valent metal ions onto the capillary wall the addition of Ca2+ is one possibility. But the added metal can influence the equilibrium between analysed metal and humic/fulvic acid. The modification of the capillary surface charge with a surface modifier inhibits the sorption of metal ions onto the capillary wall. However, the employed organic complex ligands can sorb very good on the modified wall surface. As an alternative, EDTA was added to the sample. This method, the so called disequilibrium method, was chosen by Sonke and Salters [33,34]. The lanthanides dissociate partly out of the HA/FA complex and are complexed by the present EDTA. The inert metal-EDTA and metal- HA/FA species (chelate complexes) are separated by CE. Complexes of REE with EDTA are well known and the log β values can be found in the literature. The log β values of HA and FA with REE can be calculated from the known log β values of EDTA-REE complexes via the distribution of the different metal-organic species. For this calculation Sonke and Salters assumed a monodentate carboxyl-bonding process. The total ligand concentration was calculated out of the carboxyl group density and the dissolved HA/FA concentration. The determined log β values are clearly higher than log β values determined by other methods. Additionally with the disequilibrium method weak metal organic complexes are only analysed if kinetically slow metals are used which are stable during the separation. Kautenburger et al. [35- 39] used the applied voltage to influence the equilibrium during the CE-separation. During the measurement the applied voltage of 30 kV pulls the weakly bound metal out of the HA complex. As result, three species were separated, the free, HA uncomplexed metal (peak 1), the weakly complexed metal (peak 2) and the strongly bound metal (peak 3) as shown in Figure 2. With this method, the influence of competing cations on the complex stability can be determined [35,38] and log β values can be calculated too (log β (EuHA) = 6.43 ± 0.15; log β (UO2HA) = 4.51 ± 0.17) [36,37]. The determination of weak complexes is not possible because of the applied high voltage which destroys the complexes during the CE separation. Additionally, a modification of the HA is necessary for successful detection of the HA in the ICP-MS. In this case, an iodination reaction was chosen to introduce iodine as ICP-MS marker into the HA.
Figure 2: Typical electropherogram (after different washing and preconditioning steps not shown here, the CE-separation starts at 300 sec) of uncomplexed and HA-complexed Eu and Cs (200 μg L-1) as CE flow marker analyzed by CE-ICPMS, pH 5, c(Eu): 500 μg L-1, c(HA): 25 mg L-1, U: 30 kV, CE-electrolyte: 100 mM HAc/10 mM NaAc [38].
Mendes et al. [40] have set their focus on protactinium whose isotopes Pa-233 and Pa-231 originate from thorium reactors. The behaviour of Pa in combination with naturally occurring organic complexation substances, for example oxalate, and the obtained structural and thermodynamic data are particularly important for the radioactive waste management.
The stable oxidation state of Pa is +5 and it has a strong tendency for hydrolysis which allows a sorption onto solid surfaces. This relation is not yet fully explored and at lower concentrations even less results can be obtained because of an irreversible polymerisation in the presence of higher concentraded HCl or H2SO4. As CE make-up fluid a 2% HNO3 with 10% ethanol was used. Bismuth was added as internal standard and 0.5 mol/l oxalic acid was used as electrolyte. To facilitate the interpretation of charge and structure of the protactinium oxalate complex the results are compared with the oxalate complexes of uranium(VI) and neptunium(V). The uranium oxalate migrates slower than the neptunium oxalate. This can be explained with the formation of a fourfold negatively charged uranium complex while the other complexes are only threefold negatively charged. The Pa oxalate complex is smaller than the Np oxalate which results in a shorter migration time. The Pa forms only one complex with oxalate. The CE-ICP-MS hyphenation method is not able to analyse this complex. The structure was resolved with XAS methods and quantum chemical calculations in which the PaO(Ox)33- complex was examined.
Environmental Samples
At the end of the 20th century the dumping of Pt into the environment increased strongly due to the use of catalytic converters in automobiles. Pt can be found in soil sediments near the streets and the prevalent species is of interest. Lustig et al. [41,42] determined the different species in dust and soil. At this time, the experiments with CE-ICP-MS were in the early stages. Therefore, it was important to double-check the behaviour of the CE- electrode which is made of Pt too. The authors confirm the assumption that all of the Pt species originate from the sample and no alteration of the electrode was detected. Additionally, they found out that the species distribution of Pt changes once in contact with soil very fast. To a great degree, this observation is dependent on the starting species.
Since the 21st century researchers in the field of speciation analysis are dealing with other topics. An overview of the applications of CEICP- MS for environmental samples is given in Table 2.
| Elements/ Isotopes | Method | Species | Buffer | Sample | Separation conditions | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| Se-78,80 | octopole reaction cell-CE-ICP-MS | Se(IV), Se(VI), selenocysteine (SeCys2), selenomethionine (SeMet) | 20 mM NaH2PO4 + 10 mM Na2B4O7 + 0.2 mM cationic surfactant (CTAB) pH 8.6 | rice seaweed | -16 kV | 0.1-0.9 µg L-1 | [46] |
| As | CE-ICP-MS | As(III), As(V), monomethyl-arsonic acid (MMA), dimethylarsinic acid (DMA) | 20 mM NaH2PO4 + 5 mM Na2B4O7, pH 6.5 | shrimps clams | 12 kV | 0.03-0.043 µg L-1 | [53] |
| As | CE-ICP-SFMS | As(III), As(V), MMA, DMA, arsenobetaine (AsB), arsenocholine (AsC) | 16 mM H3BO3, pH 3 | soil solution, soil water extracts | 30 kV | 5-12 ng L-1 | [58,59] |
| As | CE-ICP-MS | As(III), As(VI), MMA, DMA, AsB, AsC | 15 mM Tris + 15 mM sodium dodecyl sulfate (SDS), pH 9 | fish, oyster | hydrodynamic injection, separation at 22 kV | 0.3-0.5 µg L-1 | [54] |
| As | CE-ICP-MS / CE-ESI-TOF-MS | As(III), As(V), AsB, DMA | 25 mM borate buffer with 10% methanol, pH 9.2 | fish | 30 kV | ICP-MS: 62-95 nmol L-1 ESI-TOF-MS: 0.1-1 µmol L-1 | [52] |
| As | CE-ICP-MS | As(III), As(V), AsC, AsB, MMA DMA | 20 mM borate buffer with 6% methanol, pH 9.2 | fish | 30 kV | ICP-MS: 0.10-1.08 µg L-1 | [60] |
| As | CE-ICP-MS | As (III), As (V), DMA, MMA, AsB, AsC, 3-NHPAA, 4-NPAA,o-ASA (o-arsanilic acid) and p-UPAA | 12 mM NaH2PO4 + 8 mM H3BO3, pH 9.2 | lobster, fish protein, plants | 30 kV | ICP-MS: 0.9-3.0 ng g-1 | [61] |
|
As-750, Se-82, |
CZE-ICP-MS | AS(III), As(V), MMA, DMA/ Se(IV), Se(VI), SeCys2, SeMet, Sb(V), Te(IV), Te(VI) | sodium chromate (0.5 mmol), sodium trimethyltetradecylammonium bromide (0.5 mmol), pH 11.2 | soil | -20 kV | 6-58 µg L-1 | [47] |
| P-31 | CE-ICP-MS with collective sample introduction technique | organo-phosporus pesticides: dimethoat, trichlorphon, glyphosate | 150 mM Na2B4O7 + 50 mM H3BO3 + 20 mM SDS, pH 8.5 | spiked, vegetable sample (cabbage) | eletromigration injection, separation at 15 kV | 0.05-0.07 mg L-1 | [45] |
| P-31 | CE-ICP-MS | organo-phosporus pesticides (OPP): glyphosate, glufonisate, aminomethyl-phosphonic acid (AMPA) | 40 mM NH4 acetate, pH 9 | natural river water | 20 kV | 0.11-0.19 mg L-1 | [44] |
| Hg | CZE-ICP-MS | Hg(II), MeHg, EtHg | 50 mM H3BO3 + 12.5 mM Na2B4O7, pH 9.2 | fish, natural water | 18 kV | 0.021-0.032 µg L-1 | [50] |
| Hg | CE-volatile species generation (VSG)-ICP-MS | MeHg(I)Cys-, EtHg(I)Cys-, Hg(II)Cys2- | 20 mM Na2B4O7, pH 9.3 | certified biological reference material (DOLT-2) | hydrodynamic injection, separation at 25 kV | 1-30 µg L-1 | [51] |
| Hg | short column CE-ICP-MS | Hg(II), MeHg | 30 mM H3BO3 + 5% methanol, pH 8.6 | spiked, river water | hydrodynamic injection, separation at 21 kV | 9.7-12 µg L-1 | [48] |
| V-51, Fe-56 | CE-bandpass reaction cell-ICP-MS | VO2-EDTA3-, VO2-Fe-EDTA-, Fe-phen2+ | 15 mM tris(hydroxymethyl) aminomethan (Tris)+ 0.5 mM EDTA or 0.5 mM o-phenatroline (phen), pH 8.75 | waste water | 22 kV | V: 0.1-0.5 µg L-1; Fe: 1.2-1.7 µg L-1 | [59] |
| Cr-52 | CE-bandpass reaction cell-ICP-MS | CrO42-, Ce-DTPA | 10mM ammonium citrate + 0.5mM diethylentriamine pentaacetic acid (DTPA) + 0.01% polybrene | waste water | -22 kV | 0.4-1.3 µg L-1 | [59] |
| Pt-195 | CE-ICP-MS | PtCl62-, PtCl42-, Pt-HA | 50 mM NaH2PO4 + 50 mM N2HPO4, pH 6 | Pt treated soil, tunnel dust | - | - | [41,42] |
| Cd-111,114, Cu-63, Zn-64 |
LVSS-CE-ICP-QMS | metallothioneine | 70 mM Tris + 5% methanol, pH 7.4 | eel | hydrodynamic injection, separation at 20 kV | 6-454 µg L-1 (Cd) | [56] |
| Cd-111,114, | CE–VSG–ICP–(Q)MS | metallothioneine | 70 mM Tris + 5% methanol, pH 7.4 | rabbit | hydrodynamic injection, separation at 20 kV | 0.1-4.33 mg L-1 | [57] |
| Pb | CE-ICP-MS | Pb(II), trimethyl lead (TML), triethyl lead (TEL) | 15 mM Tris + 10 mM SDS + 0.2 mM EDTA, 2% methanol, pH 8.25 | fish | hydrodynamic injection, separation at 26 kV | 0.2-0.6 µg L-1 | [55] |
| Pb | CE-ICP-MS | Pb(II), trimethyl lead (TML), triethyl lead (TEL) | 70 mmol/L H3BO3, 17.5 mmol/L Na2B4O7, 0.4 mmol/L CTAB, 2% methanol pH 8.85 | clam oyster | electromigration injection −12.5 kV | 0.012-0.084 µg L-1 | [62] |
Table 2: Overview of the applications of CE-ICP-MS for metal speciation in environmental samples
The application of pesticides is inevitable to guarantee the nutrition of the increasing human population. However, the use of pesticides has disadvantages, too, since the uptake of pesticides can bear harmful consequences for the human being especially for the nervous system. Usually, pesticides are incorporated together with the food plant upon its consumption. This increases the health risk for the consumer. In order to minimize the risk, for some pesticides a maximum concentration inside the plants was regulated. Owing to the close chemical relationship between different pesticides their differentiation poses an analytical problem. Wuilloud et al. [43] hold the opinion that CE is a good method for separation of different pesticides even if the differences in their structures are small. Nevertheless, UV detection shows a low sensitivity when detecting pesticides. Again, this problem’s solution is coupling of CE with ICP-MS. The next critical aspect lies in the element to be quantified. Some pesticides, namely the organo phosphorus pesticides, contain phosphate. Though, the P-31 has a high ionization energy and polyatoms like 15N16O+ show the same mass-to-charge-ratio. As a consequence, the measurement of this isotope results in a false high reading. With the developed octopole reaction or collision cell the mentioned polyatomic interferences are excluded thereby offering a better detection of P-31. In addition, with this hyphenation LODs being ten to twenty times lower than with photometric flame analysis are possible.
Yang et al. [44] examined a similar problem. In the case of real food samples a high dilution is inescapable due to sample preparation and dilution with the CE buffer. Hence and owing to the high LOD, only a qualitative analysis is possible with the online hyphenation of CE and ICP-MS. The only approach to a proper quantification of the different pesticides in food is a sample collection after the separation step and subsequent CE quantification. The LODs of this offline method are quite better than those of the online method. A further drawback is the inability to differ pesticides with differences of less than 20 seconds in their migration times. Yang et al. show that synthetically prepared samples that mimic natural samples can be quantified well both with and without a reaction cell.
Another important element for speciation analysis is selenium. As trace element, Se is an essential element for humans. It acts as anticancer agent and is very important to the immune system of both humans and animals. A selenium deficiency e. g. triggers many diseases and can lead to deformity of the unborn child. The uptake of Se through the human body is dependent on the available species.
First separation of different Se species was performed by Michalke [6,45] in 1997 and 1998. With the separation of inorganic Se species such as Se(IV) and Se(VI) the CE method was optimized. The LOD was decreased and the time per analysis was reduced. Additionally, no suction flow was observed and no system peaks were found. This optimisation makes the method interesting for real, natural samples. Besides of the inorganic selenium Se(IV) and Se(VI), organic selenium compounds like cystamine, glutathione, methionine (SeMet) and cysteine can be separated by CE. However, the concentration of selenium in natural samples is very small and therefore lies below the LOD in most of the cases. For such samples a preconcentration step is necessary which provokes further problems. Zhao et al. [46] identified Se species in rice samples and pointed out the unfavourable ionisation behaviour of Se in the ICP-MS plasma. An addition of methanol to the CE electrolyte increases the amount of ionized Se in the plasma significantly and lead to a good sensitivity. Additionally, a modification of the capillary surface with CTAB is necessary for a successful separation of the different Se species due to their negative charge. In rice samples only the SeMet species was found. Quantification of the Se species shows that the concentration for SeMet is identical to the total concentration of Se.
Besides of Se, Cassiot et al. [47] have determined the elements arsenic, antimony and tellurium. Once released to the environment, all of these elements form metalloid species. In experiment, they can be very well separated by CE. Once more, the UV detector is not suitable for the complex matrix of natural samples necessitating a coupling of CE to ICP-MS. In this regard, Cassiot et al. describe issues with the interface and the placement of the capillary in the nebuliser. The inlet and outlet capillary ending should be on the same height to reduce siphonic effects. Otherwise, instability of the separation voltage and a reverse flow of make-up fluid towards the beginning of the capillary can be the result. Moreover, the addition of an electro osmotic flow modifier to the background electrolyte is crucial in order to reverse the electro osmotic flow thus increasing the migration velocity of the Se species towards the detector.
Typical natural samples are different types of fish and seafood. Since both serve as foodstuff stringent quality control checks are obligatory. (Semi) metals such as lead, arsenic or mercury have toxic effects on humans and often critical quantities thereof are present in fish and seafood.
Mercury is one of the most toxic metals and is on the third place of the ATSDR most hazardous substances priority list. Of course, the chemical form of mercury influences its bioavailability, the mobility in the environment and the lifetime. A severe problem with mercury is the bioaccumulation in fish and seafood. Through accumulation a fish can contain hundred thousand times more mercury than the water which surrounds it. Beyond, 95% of this Hg is methyl-Hg which is formed by bio organisms. Therefore, in the 70s mercury containing chemicals were forbidden as fertilisers. The major sources of release to the environment are volcanic eruptions, decomposition of rocks and minerals and the man-made pollution as a result of production processes connected with electric instruments, paper, fungicides, colours and pharmaceuticals.
Li, Zhao et al. and Silva da Rocha et al. [48-51] have determined mercury species in environmental samples. Typical CE capillaries have a separation time of three to five minutes. During this time the ICPMS is idle. This dead time could be increased with a higher separation voltage but this option is limited in the CE system due to Joule heat. An alternative solution is the use of a shorter CE column. That is the concept of Li and coworkers who employ a short CE column resulting in a better efficiency compared to normal capillaries [9,49]. Fortunately, the optimised method can be applied for natural waters, too.
Zhao et al. [50] describe the separation of Hg(II), Me-Hg and Et-Hg as difficult due to the undissociated Hg species in solution. As remedy an ionic substance with functional thiol groups e. g. cysteine or MMA can be added. The thiol groups can form coordination complexes with Hg thus enabling a separation of different Hg species with CE.
Silva da Rocha et al. [51] points out difficulties with the intake of the nebuliser. The capillary creates a laminar flow which brings along negative effects on the separation of species. Contrarily, in the case of an online generation of volatile species with addition of NaBH4 no such sucking-in effect was observed.
The bioaccumulation in seafood and fish is observed for arsenic, too. The toxicity of As is strongly dependent on the availability of organic or inorganic species. Arsenite and arsenate are very toxic and the methylated arsenic acid is know to be a cancer promoter. Meermann et al. [52] applies CE-ICP-MS for quantification of positively and negatively charged arsenic species. Supplementary, a hyphenation of CE and ESI-TOF-MS for qualification of unknown samples was developed.
Yang et al. [53] extracted arsenic species from dried shrimps and soft-shell clams. The extraction process has a key position because the species may not altered during the extraction step. With CE-ICP-MS four different As species (As(III), As(V), dimethyl-arsinic acid (DMA), monomethyl-arsonic acid (MMA)) were separated. In all samples As(III) is found, in the clams additionally DMA and in shrimps MMA is present. The sum of the different species is equal to the total concentration of As.
Yeh and Jiang [54] determined As species in fish and oyster after a microwave assisted extraction of dried samples. The chosen extraction step involves problems with the As(III) species in oyster samples. The As(III) is either too volatile or remains in the matrix during the centrifugation step. An identification of all different As species is not possible due to the high number of different species present in fish and oyster. Therefore a hyphenation of CE and ICP-MS is necessary.
Lee and Jiang [55] used the CE-ICP-MS for separation of different Pb species in fish. Their results differ from the results of other research groups. After a microwave assisted extraction they found Pb(II) as main component. The Pb(II) concentration is equal to the total concentration. Alvarez-Llamas et al. [56] introduced the large volume sample stacking (LVSS) for CE measurements. This online preconcentration system facilitates low detection limits in real samples. In the case of Cd the detection limit is decreased by one magnitude with the LVSS. For Cu and Zn the detection limits decrease too. All of these metals are present in fish liver proteins. Additionally, Alvarez-Llamas et al. [57] found an enhancement in the transport efficiency of species leaving the CE capillary towards the ICP. Therefore, the volatile species generation technique (VSG) was used. This technique increases the transport of the analyte and removes non volatile species (i.e. the matrix). Besides, the elemtents As, Hg, Se and Cd can be determined, too.
Conclusion and Outlook
This review gives an updated overview of studies dealing with metal speciation in various geological and environmental sample matrices. CE-ICP-MS has matured to an effective analytical tool developed for analysing and understanding metal speciation in the presence of natural organic and inorganic matter in geological and environmental samples. Doubtlessly, this hyphenated techniques will continue to grow during the next years. However, even at its present stage of development, CEICP- MS contributes significantly to the understanding of chemical mechanisms affecting the complexation behaviour of many relevant metals with NOM or inorganic matter in the environment. This knowledge is and will be very helpful to characterise and evaluate the true toxic potential of metal species present in or released to the environment.
Acknowledgements
This work was supported by funding from the German Federal Ministry of Economic Affairs and Energy (Bundesministerium für Wirtschaft und Energie, BMWi), Project Reference Number. 02E10196 and 02E10991.
References
- Wrobel K, Kannamkumarath S, Wrobel K, Caruso JA (2003) Environmentally friendly sample treatment for speciation analysis by hyphenated techniques. Green Chem 5: 250-259.
- Timerbaev AR (2000) Element speciation analysis by capillary electrophoresis. Talanta 52: 573-606.
- Kannamkumarath SS, Wrobel K, Wrobel K, B'Hymer C, Caruso JA (2002) Capillary electrophoresis-inductively coupled plasma-mass spectrometry: an attractive complementary technique for elemental speciation analysis. J Chromatogr A 975: 245-266.
- Alvarez-Llamas G, Fernandez de la Campa MR, Sanz-Medel A (2005) ICP-MS for specific detection in capillary electrophoresis. TrAC Trends in Analytical Chemistry 24: 28-36.
- Olesik JW, Kinzler JA, Olesik SV (1995) Capillary Electrophoresis Inductively Coupled Plasma Spectrometry for Rapid Elemental Speciation. Anal Chem 67: 1-12.
- Lustig S, Zang S, Michalke B, Schramel P, Beck W (1997) Platinum determination in nutrient plants by inductively coupled plasma mass spectrometry with special respect to the hafnium oxide interference. Fresenius J Anal Chem 357: 1157-1163.
- Dean JR, Munro S, Ebdon L, Crews HM, Massey RC (1987) Studies of metalloprotein species by directly coupled high-performance liquid chromatography inductively coupled plasma mass spectrometry. J Anal At Spectrom 2: 607-610.
- Hirner AV (2006) Speciation of alkylated metals and metalloids in the environment. Anal Bioanal Chem 385: 555-567.
- Li BH (2011) Rapid speciation analysis of mercury by short column capillary electrophoresis on-line coupled with inductively coupled plasma mass spectrometry. Anal Methods 3: 116-121.
- Michalke B (2000) CE-ICP-MS: Advantages and Improvements in Selenium Speciation. Spectroscopy 15: 30.
- Michalke B (2005) Capillary electrophoresis-inductively coupled plasma-mass spectrometry: a report on technical principles and problem solutions, potential, and limitations of this technology as well as on examples of application. Electrophoresis 26: 1584-1597.
- Kubán P, Timerbaev AR (2014) Inorganic analysis using CE: advanced methodologies to face old challenges. Electrophoresis 35: 225-233.
- Carter S, Fisher AS, Hinds MW, Lancaster S, Marshall J (2013) Atomic spectrometry update. Review of advances in the analysis of metals, chemicals and materials. J Anal At Spectrom 28: 1814-1869.
- Timerbaev AR, Timerbaev RM (2013) Recent progress of capillary electrophoresis in studying the speciation of actinides. TrAC - Trends in Analytical Chemistry 51: 44-50.
- Clough R, Harrington CF, Hill SJ, Madrid Y, Tyson JF (2013) Atomic spectrometry update. Elemental speciation review. J Anal Atom Spect 28: 1153-1195.
- Taylor A, Day MP, Hill S, Marshall J, Patriarca M, et al. (2013) Atomic spectrometry update. Clinical and biological materials, foods and beverages. J Anal Atom Spect 28: 425-459.
- Timerbaev AR (2013) Element speciation analysis using capillary electrophoresis: twenty years of development and applications. Chem Rev 113: 778-812.
- Pröfrock D, Prange A (2012) Inductively coupled plasma-mass spectrometry (ICP-MS) for quantitative analysis in environmental and life sciences: a review of challenges, solutions, and trends. Appl Spectrosc 66: 843-868.
- Meermann B, Sperling M (2012) Hyphenated techniques as tools for speciation analysis of metal-based pharmaceuticals: developments and applications. Anal Bioanal Chem 403: 1501-1522.
- Kubán P, Timerbaev AR (2012) CE of inorganic species--a review of methodological advancements over 2009-2010. Electrophoresis 33: 196-210.
- Dressler VL, Antes FG, Moreira CM, Pozebon D, Duarte FA (2011) As, Hg, I, Sb, Se and Sn speciation in body fluids and biological tissues using hyphenated-ICP-MS techniques: A review. International Journal of Mass Spectrometry 307: 149-162.
- Pröfrock D (2010) Progress and possible applications of miniaturised separation techniques and elemental mass spectrometry for quantitative, heteroatom-tagged proteomics. Anal Bioanal Chem 398: 2383-2401.
- Tholey A, Schaumlöffel D (2010) Metal labeling for quantitative protein and proteome analysis using inductively-coupled plasma mass spectrometry. TrAC - Trends in Analytical Chemistry 29: 399-408.
- Timerbaev AR (2010) Inorganic species analysis by CE--an overview for 2007-2008. Electrophoresis 31: 192-204.
- Jiang C, Armstrong DW (2010) Use of CE for the determination of binding constants. Electrophoresis 31: 17-27.
- Kubán P, Pelcová P, Margetínová J, Kubán V (2009) Mercury speciation by CE: an update. Electrophoresis 30: 92-99.
- Mounicou S, Szpunar J, Lobinski R (2009) Metallomics: the concept and methodology. Chem Soc Rev 38: 1119-1138.
- Pitois A, Aldave de Las Heras L, Betti M (2008) Determination of fission products in nuclear samples by capillary electrophoresis-inductively coupled plasma mass spectrometry (CE-ICP-MS). Int J Mass Spectrom 270: 118-126.
- Kuczewski B, Marquardt CM, Seibert A, Geckeis H, Kratz JV, et al. (2003) Separation of plutonium and neptunium species by capillary electrophoresis-inductively coupled plasma-mass spectrometry and application to natural groundwater samples. Anal Chem 75: 6769-6774.
- Stöbener N, Amayri S, Gehl A, Kaplan U, Malecha K, et al. (2012) Sensitive redox speciation of neptunium by CE-ICP-MS. Anal Bioanal Chem 404: 2143-2150.
- Topin S, Aupiais J, Moisy P (2009) Direct determination of plutonium(V) and neptunium(V) complexation by carbonate ligand with CE-ICP-sector field MS. Electrophoresis 30: 1747-1755.
- Topin S, Aupiais J, Baglan N, Vercouter T, Vitorge P, et al. (2009) Trace metal speciation by capillary electrophoresis hyphenated to inductively coupled plasma mass spectrometry: sulfate and chloride complexes of Np(V) and Pu(V). Anal Chem 81: 5354-5363.
- Sonke JE, Salters VJM (2004) Determination of neodymium–fulvic acid binding constants by capillary electrophoresis inductively coupled plasma mass spectrometry (CE-ICP-MS). J Anal Atom Spectrom 19: 235-240.
- Sonke JE, Salters VJM (2006) Lanthanide - humic substances complexation. I. Experimental evidence for a lanthanide contraction effect. Geochim Cosmochim Acta 70: 1495-1506.
- Kautenburger R, Nowotka K, Beck HP (2006) Online analysis of europium and gadolinium species complexed or uncomplexed with humic acid by capillary electrophoresis-inductively coupled plasma mass spectrometry. Anal Bioanal Chem 384: 1416-1422.
- Kautenburger R, Beck HP (2007) Complexation studies with lanthanides and humic acid analyzed by ultrafiltration and capillary electrophoresis-inductively coupled plasma mass spectrometry. J Chromatogr A 1159: 75-80.
- Möser C, Kautenburger R, Philipp Beck H (2012) Complexation of europium and uranium by humic acids analyzed by capillary electrophoresis-inductively coupled plasma mass spectrometry. Electrophoresis 33: 1482-1487.
- Kautenburger R (2009) Influence of metal concentration and the presence of competing cations on europium and gadolinium speciation with humic acid analysed by CE-ICP-MS. J Anal At Spectrom 24: 934-938.
- Kautenburger R, Hein C, Sander JM, Beck HP (2014) Influence of metal loading and humic acid functional groups on the complexation behavior of trivalent lanthanides analyzed by CE-ICP-MS. Anal Chim Acta 816: 50-59.
- Mendes M, Hamadi S, Le Naour C, Roques J, Jeanson A, et al. (2010) Thermodynamical and structural study of protactinium(V) oxalate complexes in solution. Inorg Chem 49: 9962-9971.
- Lustig S, Michalke B, Beck W, Schramel P (1998) Platinum speciation with hyphenated techniques: high performance liquid chromatography and capillary electrophoresis on-line coupled to an inductively coupled plasma-mass spectrometer – application to aqueous extracts from a platinum treated soil. Fesenius J Anal Chem 360: 18-25.
- Lustig S, De Kimpe J, Cornelis R, Schramel P, Michalke B (1999) Platinum speciation in clinical and environmental samples: scrutiny of data obtained by using electrophoresis techniques (flatbed and capillary). Electrophoresis 20: 1627-1633.
- Wuilloud RG, Shah M, Kannamkumarath SS, Altamirano JC (2005) The potential of inductively coupled plasma-mass spectrometric detection for capillary electrophoretic analysis of pesticides. Electrophoresis 26: 1598-1605.
- Yang GD, Xu XQ, Shen M, Wang W, Xu LJ, et al. (2009) Determination of organophosphorus pesticides by capillary electrophoresis-inductively coupled plasma mass spectrometry with collective sample-introduction technique. Electrophoresis 30: 1718-1723.
- Michalke B, Schramel P (1998) Application of capillary zone electrophoresis-inductively coupled plasma mass spectrometry and capillary isoelectric focusing-inductively coupled plasma mass spectrometry for selenium speciation. J Chromatogr A 807: 71-80.
- Zhao YQ, Zheng JP, Yang MW, Yang GD, Wu YN, et al. (2011) Speciation analysis of selenium in rice samples by using capillary electrophoresis-inductively coupled plasma mass spectrometry. Talanta 84: 983-988.
- Casiot C, Donard OFX, Potin-Gautier M (2002) Optimization of the hyphenation between capillary zone electrophoresis and inductively coupled plasma mass spectrometry for the measurement of As-, Sb-, Se- and Te-species, applicable to soil extracts. Spetrochim Acta B 57: 173-187.
- Li BH, Tian BJ (2012) High Throughput Speciation Analysis of Mercury in Water Environment by Short Column Capillary Electrophoresis On-Line Coupled with Inductively Coupled Plasma Mass Spectrometry. Advan Mat Res 383-390: 790-795.
- Zhao YQ, Zheng JP, Yang MW, Fu FF (2011) Speciation analysis of arsenic in seaweeds by capillary electrophoresis-inductively coupled plasma mass spectrometry. Chin J Chrom 29: 111-114.
- Zhao Y, Zheng J, Fang L, Lin Q, Wu Y, et al. (2012) Speciation analysis of mercury in natural water and fish samples by using capillary electrophoresis-inductively coupled plasma mass spectrometry. Talanta 89: 280-285.
- Silva da Rocha M, Soldado AB, Blanco E, Sanz-Medel A (2001) Speciation of mercury using capillary electrophoresis coupled to volatile species generation-inductively coupled plasma mass spectrometry. J Anal At Spectrom 16: 951-956.
- Meermann B, Bartel M, Scheffer A, Trümpler S, Karst U (2008) Capillary electrophoresis with inductively coupled plasma-mass spectrometric and electrospray time of flight mass spectrometric detection for the determination of arsenic species in fish samples. Electrophoresis 29: 2731-2737.
- Yang GD, Xu JH, Zheng JP, Xu XQ, Wang W, et al. (2009) Speciation analysis of arsenic in Mya arenaria Linnaeus and Shrimp with capillary electrophoresis-inductively coupled plasma mass spectrometry. Talanta 78: 471-476.
- Yeh CF, Jiang SJ (2005) Speciation of arsenic compounds in fish and oyster tissues by capillary electrophoresis-inductively coupled plasma-mass spectrometry. Electrophoresis 26: 1615-1621.
- Lee TH, Jiang SJ (2005) Speciation of lead compounds in fish by capillary electrophoresis-inductively coupled plasma mass spectrometry. J Anal At Spectrom 20: 1270-1274.
- Alvarez-Llamas G, Fernandez de la Campa MR, Sanz-Medel A (2003) Sample stacking capillary electrophoresis with ICP-(Q)MS detection for Cd, Cu and Zn speciation in fish liver metallothioneins. J Anal At Spectrom 18: 460-466.
- Alvarez-Llamas G, Fernandez de la Campa MR, Sanz-Medel A (2005) An alternative interface for CE–ICP–MS cadmium speciation in metallothioneins based on volatile species generation. Anal Chim Acta 546: 236-243.
- Koellensperger G, Nurmi J, Hann S, Stingeder G, Fitz WJ, et al. (2002) CE-ICP-SFMS and HPIC-ICP-SFMS for arsenic speciation in soil solution and soil water extracts. J Anal At Spectrom 17: 1042-1047.
- Yeh CF, Jiang SJ (2004) Speciation of V, Cr and Fe by capillary electrophoresis-bandpass reaction cell inductively coupled plasma mass spectrometry. J Chromatogr A 1029: 255-261.
- Chen FR, Zheng L, Wang ZG, Sun J, Han LH, et al. (2014) [Determination of arsenic speciation in scomberomorus niphonius by capillary electrophoresis-inductively coupled plasma mass spectrometry]. Guang Pu Xue Yu Guang Pu Fen Xi 34: 1675-1678.
- Liu L, He B, Yun Z, Sun J, Jiang G (2013) Speciation analysis of arsenic compounds by capillary electrophoresis on-line coupled with inductively coupled plasma mass spectrometry using a novel interface. J Chromatogr A 1304: 227-233.
- Chen Y, Huang L, Wu W, Ruan Y, Wu Z, et al. (2014) Speciation analysis of lead in marine animals by using capillary electrophoresis couple online with inductively coupled plasma mass spectrometry. Electrophoresis 35: 1346-1352.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16052
- [From(publication date):
December-2014 - Dec 02, 2024] - Breakdown by view type
- HTML page views : 11512
- PDF downloads : 4540