Special Clinicopathological Features of Anaplastic Diffuse Large B-Cell Lymphoma: A Case Report and Literature Review
Received: 15-Nov-2022 / Manuscript No. JCEP-22-79797 / Editor assigned: 17-Nov-2022 / PreQC No. JCEP-22-79797 (PQ) / Reviewed: 01-Dec-2022 / QC No. JCEP-22-79797 / Revised: 17-Feb-2023 / Manuscript No. JCEP-22-79797 (R) / Published Date: 24-Mar-2023
Abstract
Anaplastic Diffuse Large B-Cell Lymphoma (A-DLBCL) is a mysterious and rare disease; its histopathological features have not been fully clarified today. This paper provides a case in the left axillary lymph node; there are a large number of pleomorphic centroblast like cells with anaplastic characteristics and HRS like cells in a nodular or follicular germinating center like growth, which is consistent with diffuse large B-cell lymphoma, non-special type, anaplastic subtype. The results of immunohistochemistry showed that these tumor cells were positive for CD20, CD79α, Pax5, LMO2, BCL6, IGD. Besides, CD21 showed FDC network and p53 was diffusely and strongly positive. Ki67 proliferation index was 80%~90%. Tumor cells were no association with EB Virus (EBV). Our case presents different morphological diversity from previously reported cases of A-DLBCL, which shows different clinicopathological features with common Diffuse Large B-Cell Lymphoma (DLBCL) and Anaplastic Large Cell Lymphoma (ALCL). Furthermore, we also review the most of article about ALCL and summarize the progress of its pathogenesis, treatment and prognosis in recent years.
Keywords: Anaplastic Diffuse Large B-Cell Lymphoma (A-DLBCL); Anaplastic Large Cell Lymphoma (ALCL); EB Virus (EBV); Clinicopathology; Proliferation index
Abbreviations
A-DLBCL: Anaplastic diffuse large B-cell lymphoma; EBV: EB virus; DLBCL: diffuse large B-cell lymphoma; ALCL: anaplastic large cell lymphoma; HRS: Hodgkin/Reed Sternberg; EBER-ISH: EBV encoded small nuclear early region in situ hybridization; FISH: Gene fluorescence in situ hybridization; CHL: classical Hodgkin lymphoma; IPI: international prognostic indicators; CR: complete remission
Introduction
In 1985 Stein proposed Anaplastic Large Cell Lymphoma (ALCL), also known as ki-1 lymphoma because it expresses CD30 (ki-1 antigen), then with the development of immunology, its cell lineage has B cells, T cells and null cells (its immunophenotype neither express T nor B cell markers),but TCR gene rearrangement supports that null cell are T cell lineage. The 1992 Kiel classification excludes B-cell derived ALCL from traditional ALCL and classifies it as Anaplastic Diffuse Large Cell Lymphoma (A-DLBCL), but the 1994 REAL classification doesn’t adopt this classification. In 2000, Italian scholars researched a group of ALK negative A-DLBCL diseases, they found that these lymphomas expressed B-cell markers, lacked T-cell markers and had no chromosomal (2;5) translocation, demonstrated that B-cell anaplastic large cell lymphomas are not associated with ALCL of ALK negative [1]. Since then, the 2001 WHO classification, the 2008 WHO classification and the 2017 WHO classification have followed classification of A-DLBCL.
Diffuse large B-cell lymphoma is a heterogeneous disease that includes morphologic variants, immunophenotypic and molecular subgroups, and distinct disease entities [2]. At the same time, DLBCL is also the most common Non-Hodgkin Lymphoma (NHL), according to morphology, it was classified into central cell type, immunoblastic type and anaplastic type according to morphology in 2017 WHO classification, the latter was characterized by large, pleomorphic and bizarre cells, which are usually similar to Hodgkin/Reed Sternberg (HRS) cells and hallmark cell of anaplastic large cell lymphoma, these tumor cells are cohesive and (or) sinusoidal pattern and may simulate malignant melanoma or undifferentiated cancer [3]. Based on COO immune molecular type, A-DLBCL was classified into Germinal Center type (GCB type) and Non-Germinal Center type (NGC/ABC type), most of A-DLBCL were NGC/ABC type 30/35 (86%) in study of Zhe Wang's group (2017) [4]. Since A-DLBCL is relatively rare, the pathological and genetic characteristics of A-DLBCL are not yet known and there are no consistent diagnostic criteria among pathologists, especially for GCB type, in order to provide richer clinicopathological and genetic features for the diagnosis, treatment and prognosis of this disease, this paper provides an unique case of A-DLBCL with morphological support for a GCB type, but Hans/Choi/Meyer or Tally immune molecular type considered it as NGC/ABC type of COO.
Case Presentation
On October 10, 2021, a man, 68 years old, was admitted to our hospital with paroxysmal colic in the lower and middle abdomen with vomiting for 1 month. Before 1 month ago, the patient had no obvious inducement to develop paroxysmal colic in the middle and lower abdomen accompanied by hyperactive bowel sounds and no obvious remission after body position changed, besides, normal diet can be taken during the break. There is no fever, chill, nausea, belching, etc. Blood routine examination showed that hemoglobin was 120.0 g/l, hematocrit was 38.8%, average erythrocyte hemoglobin was 26.4 pg, concentration of average erythrocyte hemoglobin was 309.0 g/l, platelet was 338 × 109/L, platelet distribution width (SD) was 9.7f1, the absolute value of lymphocytes was 1.01 × 109/L. Small intestinal CTE and chest CT showed that the small intestine in the right lower abdomen is locally thickened, the enhancement scan is significantly enhanced and the pipe wall is partially calcified, thus our doctor considered the possibility of malignant lesions. Mediastinum and bilateral axillary lymph nodes are enlarged; possibility of metastasis cannot be excluded. Giant examination site is the left axillary mass. Gross specimen showed a piece of grayish yellow grayish brown tissue with a diameter of 2.5 cm. The patient in this case was not accepted standard treatment after diagnosis, fortunately, he was still alive now, but his physical condition was poor.
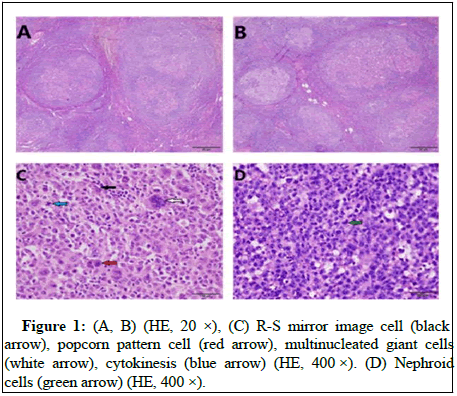
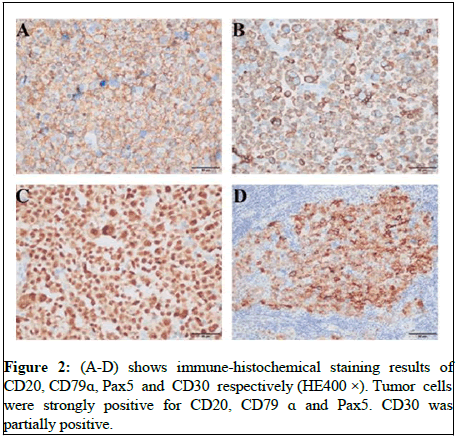
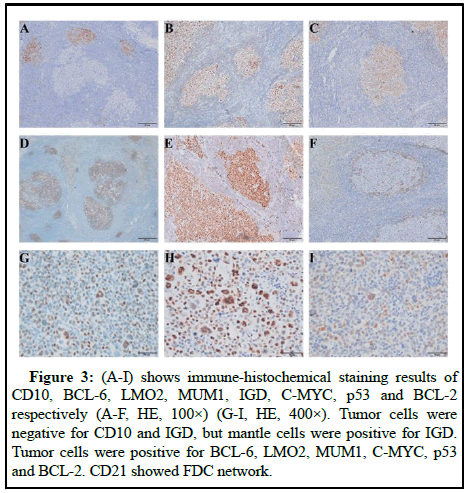
The lymph node capsule was complete and there are residual germinal centers in it. In the germinal center, there are a large number of pleomorphic centroblast like cells with anaplastic characteristics and HRS like cells grow in nodular like or follicular germinal center like, which is a rare growth pattern of A-DLBCL. Tumor cells have obvious anaplastic characteristic but absent of adhesion. Scattered mononuclear or multinuclear cells, HRS like cells and giant cells can be seen in lymph node architecture. Their cytoplasm was rich and oxyphilic, besides, kidney shaped nuclei can be seen, the chromatin was rough, the nucleolus and atypia were obvious and mitotic images were common. There are few background cells, which are composed of several small round cells, their cytoplasm is not obvious and mixed inflammatory cells can be seen in Figure 1. The overall histopathological appearance of this case was difficult to distinguish from follicular lymphoma, Hodgkin lymphoma, gray zone lymphoma and anaplastic large cell lymphoma, but can be differentiated by immune-histochemical results. Immunohistochemical results showed that the tumor cells were strongly positive for CD20, CD79α and Pax5, CD30 was partially positive (Figure 2). Tumor cells were negative for CD10 and IGD, but mantle cells were positive for IGD. Tumor cells were positive for BCL-6, LMO2, MUM1, C-MYC, p53 and BCL-2. CD21 showed FDC network (Figure 3). Ki67 proliferation index was 80%~90%. Based on EBV encoded small nuclear Early Region in Situ Hybridization (EBER-ISH), tumor cells were not related to EBV. Gene Fluorescence in Situ Hybridization (FISH) results showed that C-MYC gene breakage ratio: 1%, less than the threshold 15%; Bcl-6 gene breakage ratio: 3%, less than the threshold 15%; Bcl-2 gene breakage ratio: 2%, less than the threshold 15%; IRF4 gene breakage ratio: 3%, less than the threshold 15%.
Figure 3: (A-I) shows immune-histochemical staining results of CD10, BCL-6, LMO2, MUM1, IGD, C-MYC, p53 and BCL-2 respectively (A-F, HE, 100×) (G-I, HE, 400×). Tumor cells were negative for CD10 and IGD, but mantle cells were positive for IGD. Tumor cells were positive for BCL-6, LMO2, MUM1, C-MYC, p53 and BCL-2. CD21 showed FDC network.
Discussion
At present, pathologic features of A-DLBCL have not been fully clarified. A-DLBCL is a disease with rare B cell phenotype and genotype variation, which have overlapping clinicopathological features between gray zone lymphoma and Classical Hodgkin Lymphoma (CHL) [5]. The most of A-DLBCL occurs in lymph nodes but also can occur in any part outside of it, such as tonsil, testis, posterior nasal cavity, retro peritoneum, prostate, liver and intestine, pancreas, thyroid, thymus, central nervous system, pleura, skin, etc. Otherwise, lesions involved with extra nodal organ or lymph node show the similar morphological characteristics; both were sinusoidal growth or diffuse adhesive cancer like growth [6-10]. But there may be different features in patients that occurred in outside of lymph nodes, such as the primary central nervous system of A-DLBCL have unique genetic and biological characteristics, often with MYC/BCL2 double expression, accompanied by MYC, BCL2 and/or Bcl6 gene abnormalities, besides, NF-κB pathway is constitutively activated, so prognosis is also more worse than universal A-DLBCL [11,12]. In addition, A-DLBCL can also be transformed from low grade mucosa associated lymphoid tissue gastric tissue lymphoma [13]. It needs more research about whether A-DLBCL occurred in the skin; mucosa and other sites have different characteristics.
In terms of morphology, the previously reported cases of A-DLBCL are sinusoidal growth, while our case provided in this paper are follicular or germinal center like growth. Due to the limitation of quantity, its mechanism is still unknown. The morphology of A-DLBCL is more diverse than ordinary DLBCL. A-DLBCL is often sinusoidal or solid, adherent and characterized by large, bizarre cells. These cells mixed with common immunoblastic cells and centroblastic cells of DLBCL and have bizarre nuclei that can be HRS like, horseshoe shaped, kidney shaped, doughnut like, which is similar to tumor cells of anaplastic large cell lymphoma. At present, the diagnosis of A-DLBCL mainly depends on morphology, especially HRS like cells, which always grow in sheets, in the study of professor Nirmeen A. Megahed and professor Zhe Wang et al, the probability of HRS like cells in A-DLBCL was 72% (13/18) and 51% (17/35) respectively, but whether the presence of HRS like cells in A-DLBCL is related to the pathogenesis of A-DLBCL and CHL still needs further study. Some scholars believe that EBV may play a role in the pathogenesis of some of these tumors [14].
About treatment, for patients with DLBCL, approximately 60%-70% of them can be cured by standard therapy, which contains rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). Although this patient was not accepted standard therapy, majority of tumor cells of this case are positive for CD30, it may demonstrate durable responses if treat with Brentuximab vedotin. Brentuximab vedotin is an antibody drug conjugate that targets CD30 with significant effects approved for the treatment of relapsed, refractory non-Hodgkin disease, but Brentuximab vedotin also has toxicity and side effects, such as peripheral neuropathy, which is a major reason why Brentuximab vedotin cannot be used for a long time, but this lesion is reversible and can be recovered after discontinuation [15-17]. Brentuximab vedotin was active in DLBCL with some level of CD30 expression, with an objective response in 44% of patients [18].
In the study of biomarker of prognosis in DLBCL, CD30 has always been a research hotspot, which is of great significance for the diagnosis of A-DLBCL, but it is not a necessary condition. Compared with other immunophenotype, the positive rates of CD30 of anaplastic variants are higher, which was often less than 90%. Moreover, its positive signal was always located in the tumor cell membrane, unlike the classic ALCL; it is more located in the tumor cell membrane and Golgi region [19]. In 2001, New Zealand scholars proposed that the expression of CD30 in A-DLBCL may be related to its anaplastic features and survival rate [20]. In previous studies, it is still controversial about whether CD30 is related to the prognosis of patients. Hao et al, believed that the expression of CD30 was related to B cell symptoms, non-germinal center immunophenotype and poor prognosis, Noorduyn LA, et al, thought that the expression of CD30 was not related to the survival rate of patients, while Hu and Slack et al, reported that CD30 positive DLBCL had superior 5 years overall survival rate and progression free survival rate in GCB and N-GCB subtypes [20-24]. Therefore, whether the expression of CD30 is related to prognosis remains to need further studied. But compared with CD30 negative A-DLBCL, CD30 positive A-DLBCL has a higher p53 mutation rate trend, which may suggest that the expression of CD30 may have an adverse impact on the prognosis.
A-DLBCL has different genetic changes and biological characteristics from ordinary DLBCL. There are literature suggested that A-DLBCL may biologically mimic gray zone or intermediate lymphoma between DLBCL and Classic Hodgkin Lymphoma (CHL) [25,26]. The main prognostic factors of DLBCL are closely related to GCB type or non-GCB type and prognosis of the former may be better [27-29]. About 30% of DLBCL cases have MYC and BCL2 overexpression, which is called double expression lymphoma, compared with MYC or BCL2 single or no overexpression, prognosis of the former is worse, while double expression lymphoma is more common in ABC subtype, which may affect the prognosis to some extent [30-33]. However, patients of DLBCL with MYC and Bcl6 rearrangement or co-expression do not always have a poor prognosis. MYC protein plays an important role in a variety of cellular processes, including cell proliferation and differentiation, cell cycle progression, metabolism and apoptosis and MYC is usually overexpressed in human cancer. However, it has not been elucidated about whether MYC overexpression alone is associated with the prognosis of A-DLBCL. Prognostic factors for A-DLBCL may include the origin of tumor cells, genetic alterations, etc. Compared with ordinary DLBCL, A-DLBCL are more prone to have high stage disease, extra lymph nodes involvement, elevated serum LDH and high International Prognostic Indicators (IPI), however, the incidence of Complete Remission (CR) during chemotherapy is low (P<0.05). Factors affecting the prognosis of A-DLBCL include the source of tumor cells, genetic changes, etc. Zhe Wang group in 2017 found that patients with A-DLBCL have a bigger probability to be non-GCB immune-phenotype, have expression of CD30, p53 mutation, abnormalities of MYC, BCL2 and (or) BCL6 simultaneously (P<0.05). Overexpression of C-MYC and Bcl-2 protein, as well as TP53 mutation may lead to poor prognosis. Expression of Bcl-6 may be related to the development of primary central nervous system lymphoma, but it is also a potential and independent favorable prognostic marker. The prognosis of A-DLBCL and its factors need to be studied further.
Conclusion
In summary, our case showed and revealed some clinicopathological features of A-DLBCL classified as GCB by morphology, which can grow as sinusoidal, nodular or follicular germinal center like. These characteristics strengthened our understanding of this mysterious disease. In addition, A-DLBCL has different features and prognosis with other type of DLBCL. There are many factors influence its pathogenesis, such as the origin of tumor cells, genetic alterations, etc. We still need more research to clarify A-DLBCL.
Availability of Data and Materials
All the data regarding the findings are available within the manuscript.
Acknowledgement
The authors would like to thank Chongqing medical university for granting this project.
Funding
This study was supported by Guizhou provincial science and technology projects (grant no. ZHYX202104).
Author Contributions
Resources: Min Zhao, Lixing Wang, Juan He, Xingyu Wang, Kuai Yu, Ying Huang, Jie Xian. Writing original draft: Min Zhao. Writing-review and editing: Min Zhao, Dan Li. All the authors have read and approved the final manuscript.
Ethics Declarations
Ethics approval and consent to participate this case report was approved by the ethics committee of the affiliated hospital of Chongqing medical university. Written informed consent was obtained from the patient for publication of this clinical case report.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- Haralambieva E, Pulford KAF, Lamant L (2000) Anaplastic large cell lymphomas of B-cell phenotype are Anaplastic Lymphoma Kinase (ALK) negative and belong to the spectrum of diffuse large B-cell lymphomas. Br J Haematol 109: 584-591.
[Crossref] [Google Scholar] [PubMed]
- Megahed NA, Kohno K, Sakakibara A (2019) Anaplastic variant of diffuse large B-cell lymphoma: Reappraisal as a nodal disease with sinusoidal involvement. Pathol Int 69: 697-705.
[Crossref] [Google Scholar] [PubMed]
- Li M, Liu Y, Wang Y (2017) Anaplastic variant of diffuse large B-cell lymphoma displays intricate genetic alterations and distinct biological features. Am J Surg Pathol 41: 1322-1332.
[Crossref] [Google Scholar] [PubMed]
- AbdullGaffar B, Seliem R (2021) Anaplastic diffuse large B-cell lymphoma cytologically diagnosed in pleural effusion: Report of two cases. Diagn Cytopathol 49: E307-E311.
[Crossref] [Google Scholar] [PubMed]
- Singh N, Sood R, Agrawal N (2019) Anaplastic diffuse large b cell lymphoma: A single center experience. Indian J Hematol Blood Transfus 35: 557-560.
[Crossref] [Google Scholar] [PubMed]
- Asano H, Imai Y, Ota S (2010) CD30 positive anaplastic variant diffuse large B cell lymphoma: A rare case presented with cutaneous involvement. Int J Hematol 92: 550-552.
[Crossref] [Google Scholar] [PubMed]
- Bruno R, Giannasio P, Bellitti P (2010) A case of anaplastic diffuse large B-cell thyroid lymphoma: Unique immunophenotype and unusual clinical presentation. J Endocrinol Invest 33: 767-768.
[Crossref] [Google Scholar] [PubMed]
- Xu T, Jia Q, Wang Y (2019) Rare cases of primary central nervous system anaplastic variant of diffuse large B-cell lymphoma. Diagn Pathol 14: 45.
[Crossref] [Google Scholar] [PubMed]
- Kim S, Nam SJ, Kwon D (2016) MYC and BCL2 overexpression is associated with a higher class of memorial Sloan-Kettering cancer center prognostic model and poor clinical outcome in primary diffuse large B-cell lymphoma of the central nervous system. BMC Cancer 16: 363.
[Crossref] [Google Scholar] [PubMed]
- Shendler Y, Delgado B, Delgado J (2004) Diffuse large B-cell lymphoma with anaplastic features and focal low grade mucosa associated lymphoid tissue lymphoma component of the stomach. Ann Diagn Pathol 8: 36-38.
[Crossref] [Google Scholar] [PubMed]
- Lai R, Medeiros LJ, Dabbagh L (2000) Sinusoidal CD30 positive large B-cell lymphoma: A morphologic mimic of anaplastic large cell lymphoma. Mod Pathol 13: 223-228.
[Crossref] [Google Scholar] [PubMed]
- Babashov V, Begen MA, Mangel J (2017) Economic evaluation of Brentuximab vedotin for persistent Hodgkin lymphoma. Curr Oncol 24.
[Crossref] [Google Scholar] [PubMed]
- Mahevas T, Ram-Wolff C, Battistella M (2019) Dramatic response to Brentuximab vedotin in refractory non-transformed CD30 (-) mycosis fungoides allowing allogeneic stem cell transplant and long term complete remission. Br J Dermatol 180: 1517-1520.
[Crossref] [Google Scholar] [PubMed]
- Vaklavas C, Forero-Torres A (2012) Safety and efficacy of Brentuximab vedotin in patients with Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Ther Adv Hematol 3: 209-225.
[Crossref] [Google Scholar] [PubMed]
- Jacobsen ED, Sharman JP, Oki Y (2015) Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 125: 1394-1402.
[Crossref] [Google Scholar] [PubMed]
- Maes B, Anastasopoulou A, Kluin-Nelemans JC (2001) Among diffuse large B-cell lymphomas, T-cell rich/histiocyte rich BCL and CD30+ anaplastic B-cell subtypes exhibit distinct clinical features. Ann Oncol 12: 853-858.
[Crossref] [Google Scholar] [PubMed]
- Xu T, Chai J, Wang K (2021) Tumor immune microenvironment components and checkpoint molecules in anaplastic variant of diffuse large B-cell lymphoma. Front Oncol 11: 638154.
[Crossref] [Google Scholar] [PubMed]
- Hao X, Wei X, Huang F (2015) The expression of CD30 based on immunohistochemistry predicts inferior outcome in patients with diffuse large B-cell lymphoma. PLoS One 10: e0126615.
[Crossref] [Google Scholar] [PubMed]
- Noorduyn LA, Bruin PCd, Heerde Pv (1994) Relation of CD30 expression to survival and morphology in large cell B cell lymphomas. Clin Pathol 47: 33-37.
[Crossref] [Google Scholar] [PubMed]
- Hu S, Xu-Monette ZY, Balasubramanyam A (2013) CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: A report from the international DLBCL rituximab-CHOP consortium program study. Blood 121: 2715-2724.
[Crossref] [Google Scholar] [PubMed]
- Slack GW, Steidl C, Sehn LH (2014) CD30 expression in de novo diffuse large B-cell lymphoma: A population based study from British Columbia. Br J Haematol 167: 608-617.
[Crossref] [Google Scholar] [PubMed]
- Sakakibara A, Kohno K, Kuroda N (2018) Anaplastic variant of diffuse large B-cell lymphoma with hallmark cell appearance: Two cases highlighting a broad diversity in the diagnostics. Pathol Int 68: 251-255.
[Crossref] [Google Scholar] [PubMed]
- Megahed NA, Kato S, Asano N (2011) Anaplastic variant of diffuse large B-cell lymphoma: Re-apprasial as extra mediastinal grey zone lymphoma intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma. Blood 118: 3660.
- Lenz G, Wright G, Dave SS (2008) Stromal gene signatures in large B-cell lymphomas. N Engl J Med 359: 2313-2323.
[Crossref] [Google Scholar] [PubMed]
- Li X, Huang Y, Bi C (2017) Primary central nervous system diffuse large B-cell lymphoma shows an activated B-cell like phenotype with co-expression of C-MYC, BCL-2 and BCL-6. Pathol Res Pract 213: 659-665.
[Crossref] [Google Scholar] [PubMed]
- Scott DW, Mottok A, Ennishi D (2015) Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin fixed paraffin embedded tissue biopsies. J Clin Oncol 33: 2848-2856.
[Crossref] [Google Scholar] [PubMed]
- Cho YA, Hyeon J, Lee H (2021) MYC single hit large B-cell lymphoma: Clinicopathological difference from MYC negative large B-cell lymphoma and MYC double hit/triple hit lymphoma. Hum Pathol 113: 9-19.
[Crossref] [Google Scholar] [PubMed]
- Johnson NA, Slack GW, Savage KJ (2012) Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone. J Clin Oncol 30: 3452-3459.
[Crossref] [Google Scholar] [PubMed]
- Sehn LH, Salles G (2021) Diffuse large B-cell lymphoma. N Engl J Med 384: 842-858.
[Crossref] [Google Scholar] [PubMed]
- Valera A, Lopez-Guillermo A, Cardesa-Salzmann T (2013) MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica 98: 1554-1562.
[Crossref] [Google Scholar] [PubMed]
- Ye Q, Xu-Monette ZY, Wang (2016) Prognostic impact of concurrent MYC and BCL6 rearrangements and expression in de novo diffuse large B-cell lymphoma. Oncotarget 7: 2401-2416.
[Crossref] [Google Scholar] [PubMed]
- Posternak V, Cole MD (2016) Strategically targeting MYC in cancer. F1000Res 5: 408.
[Crossref] [Google Scholar] [PubMed]
- Spranger S, Gajewski TF, Kline J (2016) MYC-a thorn in the side of cancer immunity. Cell Res 26: 639-640.
[Crossref] [Google Scholar] [PubMed]
Citation: Zhao M, Wang L, Wang X, He J, Li D, et al. (2023) Special Clinicopathological Features of Anaplastic Diffuse Large B-Cell Lymphoma: A Case Report and Literature Review. J Clin Exp Pathol 13: 431.
Copyright: © Zhao M, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1008
- [From(publication date): 0-2023 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 770
- PDF downloads: 238