Sophisticate Mechanisms and Unsolved Problems in the Resolution of Acute Gouty Arthritis
Received: 22-Mar-2016 / Accepted Date: 03-Jun-2016 / Published Date: 08-Jun-2016 DOI: 10.4172/2576-3881.1000107
Abstract
Only primates suffer from hyperuricemia and gout. Acute gouty attack with fulminate inflammation may recover within 7-10 days. The pathogenesis of monosodium urate monohydrate (MSU) crystal-induced acute gouty inflammation has been gradually elucidated. It is conceivable that MSU crystals possess both danger-associated pathological pattern (DAMP) and physical microcrystal properties that may activate innate immune cells IL-1 production and subsequent IL-6, IL-8 and TNF-α release from neighborhood cells. By contrast, the molecular basis of acute gouty inflammation resolution mostly remains unclear. Previous studiesdemonstrated that intracellular negative cytokine regulators CIS and SOCS-3 involved in the resolution of acute gouty inflammation in synergism with anti-inflammatory cytokine molecules (TGF-β1, IL-10 and soluble TNF-α receptors type 1 and 2). This commentary aims to dissect the potential mechanisms of induction and resolution of acute gouty attack. Besides, the unsolved problems in these issues are discussed.
Characteristics of monosodium urate crystal (MSU) induced gouty arthritis (GA)
Different from allantoin in avian species, the end-product of purine metabolism in human being is uric acid due to 2 non-sense mutations at codons 33 and 187 of urate oxidase during hominoid evolution [1,2]. The solubility of uric acid in the fluid milieu reaches a maximum of 6.8 mg/dl and becomes hyper-saturation status in plasma when over its solubility capacity. Many factors including [Na+], pH, temperature, oncostatic pressure, and in presence of nucleating factors/growth promoting factors may accelerate crystal formation in the joints, periarticular soft tissues, renal tubules or subcutaneous locations [3,4]. Thus, acute inflammation elicited by monosodium urate monohydrate (MSU) crystal deposition occurs usually in the low-temperature joints especially in the lower limbs. Usually, the acute inflammation in the joint of patients with acute attack may last a few days from 7-10 days. Then, spontaneous remission of the inflamed joint ensues after acute attack. Although gouty arthritis has been recognized since ancient age, the actual molecular basis of the acute attack and the subsequent spontaneous remission remain elucidation. In addition to the acute attack, part of the patients result in chronic tophaceous gout in the long-term course of hyperuricemia [5,6]. The etiopathogenesis of chronic gouty tophus formation also remain unclear. In 2011, Chen et al. [7] reported that not only intercellular anti-inflammatory cytokines TGF-β1 and IL-10, and soluble cytokine receptors TNF-α receptor type 1 (sTNF-R1) and type 2 (sTNF-R2), but intracellular cytokine negative regulators may involve in the spontaneous remission of acute gouty inflammation. The intracellular cytokine signaling inhibitors such as CIS and SOCS3 were considered the potent negative regulatory molecules for suppressing inflammatory cytokine-mediated acute gouty inflammation [8-12]. These findings are consistent with Martin et al. [13] that mononuclear phagocytes play a central role in both initiation and resolution of acute gouty inflammation. They showed that interplay between monocytes/macrophages and other elements of innate immune system including neutrophils and complement proteins are important. Despite a number of anti-inflammatory cytokines and endogenous cytokine signaling inhibitors have been found in the literatures, the connection between these cytokine inhibitors and resolution of acute gouty inflammation has never been reported. Figure 1 demonstrates a list of these potential cytokine inhibitors for controlling cytokine signaling.
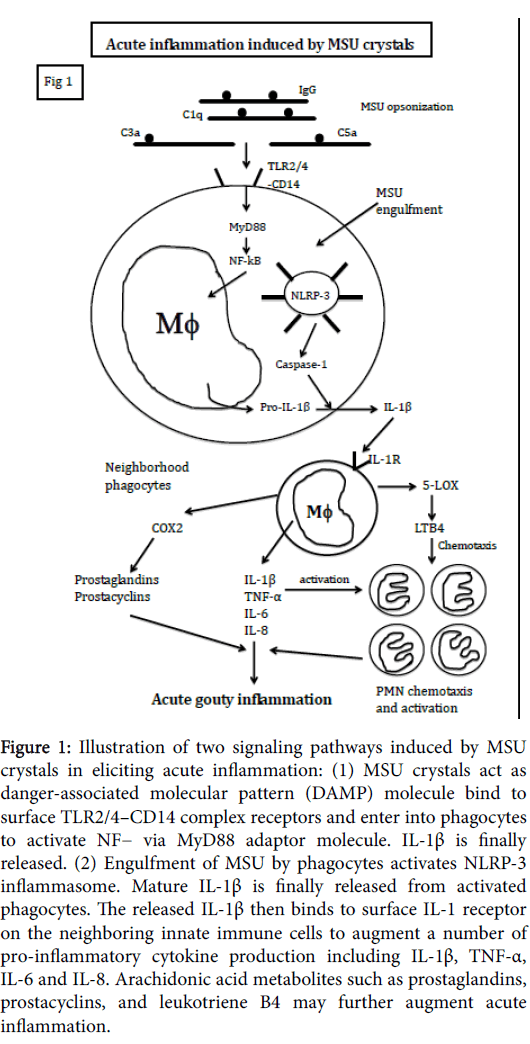
Figure 1: Illustration of two signaling pathways induced by MSU crystals in eliciting acute inflammation: (1) MSU crystals act as danger-associated molecular pattern (DAMP) molecule bind to surface TLR2/4–CD14 complex receptors and enter into phagocytes to activate NF− via MyD88 adaptor molecule. IL-1β is finally released. (2) Engulfment of MSU by phagocytes activates NLRP-3 inflammasome. Mature IL-1β is finally released from activated phagocytes. The released IL-1β then binds to surface IL-1 receptor on the neighboring innate immune cells to augment a number of pro-inflammatory cytokine production including IL-1β, TNF-α, IL-6 and IL-8. Arachidonic acid metabolites such as prostaglandins, prostacyclins, and leukotriene B4 may further augment acute inflammation.
Interplay among complements, phagocytes, proinflammatory cytokines and inflammatory mediators contribute to the initiation of MSU crystal-induced acute inflammation
As illustrated in Figure1, MSU crystals act dual roles in both danger-associated molecular pattern (DAMP) molecules and physical microcrystals in stimulating both mononuclear- and polymorphnuclear phagocytes. It is demonstrated that innate immune cells express Toll-like receptor (TLRs) on the cell surface and contain inflammasomes in the cytoplasm. As DAMP molecules, MSU crystals bind to TLR2/4-CD14 complex and are phagocytosed by macrophages in the synovial membrane and neutrophils in the synovial joints [14]. The MSU-engulfed phagocytes transduce signals to activate NF-kB via adaptor protein MyD88 and finally mature IL-1β release [15]. The released IL-1β then binds to IL-1 receptor on the neighborhood phagocytes to induce a bunch of potent pro-inflammatory cytokines (TNF-α, IL-6, IL-8) release. As physical microcrystals, direct phagocytosis of complements (C1q, C3 and C5)- and immunoglobulins-opsonized MSU crystals activates NLRP-3 inflammasome in macrophages. The produced IL-1β can further stimulate neighborhood innate immune cells via binding to IL-1 receptors to produce more and more proinflammatory cytokines [16]. Furthermore, IL-1β would stimulate COX-2 and 5-LOX pathways of the inflammatory cells to amplify acute inflammation with PMN recruitment and activation [17]. In conclusion, a rapid and vigorous inflammation is triggered by MSU crystals with orchestration of florid inflammatory cytokines, inflammatory mediators, and acute inflammatory cells accumulation in the joint.
Induction of phenotype transformation, intracellular negative cytokine signaling regulators, and probably apoptosis in macrophages by MSU crystals involve in the resolution of acute gouty inflammation
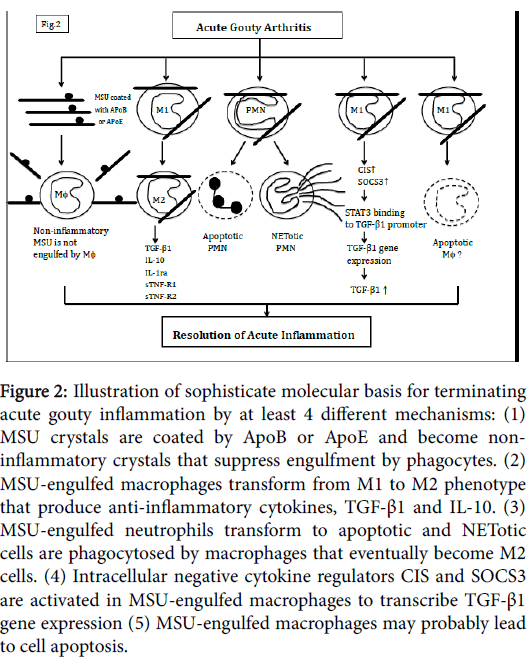
Acute gouty arthritis is generally self-limited and underlies spontaneous remission without 7-10 days. It has been demonstrated that low density lipoprotein (more specifically apolipoprotein B in the LDL) is elevated with acute inflammation [13,18]. This particular lipoprotein coats MSU crystals and renders the crystals noninflammatory to prevent further engulfment by phagocytes (Figure 2). In the mean time, the MSU-engulfed inflammatory macrophages(M1) transform to M2 phenotype that conversely produces antiinflammatory cytokines TGF-β1, IL-1ra or IL-10 [19]. On the other hand, the MSU-engulfed PMNs in synovial joints transform to apoptotic and NETotic cells are phagocytosed by M1 macrophages that eventually transform to M2 phenotype [20]. The highlight findings of Chen et al. [7] suggest that MSU-engulfed macrophages not only produced proinflammatory cytokines but spontaneously transcribed CIS/SOCS3 gene expression immediately to enhance TGF-β1 as well as sTNF-α receptors release by innate immune cells. It is also possible that MSU crystals-engulfed macrophages proceed to apoptosis. These sophisticate and potential mechanisms involving in acute gouty inflammation resolution are schemmed in Figure 2.
Figure 2: Illustration of sophisticate molecular basis for terminating acute gouty inflammation by at least 4 different mechanisms: (1) MSU crystals are coated by ApoB or ApoE and become noninflammatory crystals that suppress engulfment by phagocytes. (2) MSU-engulfed macrophages transform from M1 to M2 phenotype that produce anti-inflammatory cytokines, TGF-β1 and IL-10. (3) MSU-engulfed neutrophils transform to apoptotic and NETotic cells are phagocytosed by macrophages that eventually become M2 cells. (4) Intracellular negative cytokine regulators CIS and SOCS3 are activated in MSU-engulfed macrophages to transcribe TGF-β1 gene expression (5) MSU-engulfed macrophages may probably lead to cell apoptosis.
Unsolved problems in spontaneous remission of acute gouty inflammation
Almost of the studies suggest that IL-1β production is the milestone and initiator for MSU-induced acute inflammation. However, Martin et al. [21] have demonstrated that the production of IL-6 by MSUstimulated peritoneal exudate macrophages is much higher than IL-1β or TNF-α. Chen et al. [7] also found that IL-1β production by MSUstimulated mouse macrophage cell line was less than TNF-α after 24h incubation. It is deduced that the levels and activities of IL-1β and TNF-α in gout synovial fluid can be counteracted by large amount of IL-1ra and soluble TNF-α receptor 1. Undoubtedly, the comparative roles of IL-1β, IL-6 and TNF-α in acute gouty inflammation need further investigations.
It is conceivable that CIS/SOCSs family is a potent intracellular cytokine negative regulator to suppress cytokine signaling by preventing STATs phosphorylation [22]. However, both CIS and SOCS3 are not endogenous inhibitors for terminating IL-1β- or TNF- α-induced signaling pathway. In contrast, IL-6 signaling can be inhibited by SOCS3. Thus, the anti-inflammatory roles of TGF-β1 and IL-10 in the remission of acute gouty inflammation seem more realistic than CIS/SOCS family but more investigations are required to solve the problem [23]. Furthermore, the molecular basis of MSU-induced NETosis in the resolution of acute gouty inflammation as suggested by Mitroulis et al. [24] and MSU-induced macrophage apoptosis warrants intensive investigation.
References
- Wu XW, Muzny DM, Lee CC, Caskey CT (1992) Two independent mutational events in the loss of urate oxidase during hominoid evolution. See comment in PubMed Commons below J MolEvol 34: 78-84.
- Oda M, Satta Y, Takenaka O, Takahata N (2002) Loss of urate oxidase activity in hominoids and its evolutionary implications. See comment in PubMed Commons below MolBiolEvol 19: 640-653.
- De Yoreo JJ, Vekilov PG (2003) Principles of crystal nucleation and growth. Rev Mineral Geochem 54: 57-93.
- Pascual E, Addadi L, Andrés M, Sivera F (2015) Mechanisms of crystal formation in gout-a structural approach. See comment in PubMed Commons below Nat Rev Rheumatol 11: 725-730.
- Chhana A, Dalbeth N (2015) The gouty tophus: a review. See comment in PubMed Commons below CurrRheumatol Rep 17: 19.
- Cronstein BN, Terkeltaub R (2006) The inflammatory process of gout and its treatment. See comment in PubMed Commons below Arthritis Res Ther 8 Suppl 1: S3.
- Chen YH, Hsieh SC, Chen WY, Li KT, Wu CH, et al. (2011) Spontaneous resolution of acute gouty arthritis is associated with rapid induction of the anti-inflammatory factors TGFß1, IL-10 and soluble TNF receptors and intracellular cytokine negative regulators CIS and SOCS3. Ann Rheum Dis 70: 1655-1663.
- Heeg K, Dalpke A (2003) TLR-induced negative regulatory circuits: role of suppressor of cytokine signaling (SOCS) proteins in innate immunity. See comment in PubMed Commons below Vaccine 21 Suppl 2: S61-67.
- Rakesh K, Agrawal DK (2005) Controlling cytokine signaling by constitutive inhibitors. See comment in PubMed Commons below BiochemPharmacol 70: 649-657.
- Yoshimura A, Nishinakamura H, Matsumura Y, Hanada T (2005) Negative regulation of cytokine signaling and immune responses by SOCS proteins. See comment in PubMed Commons below Arthritis Res Ther 7: 100-110.
- Shuai K (2006) Regulation of cytokine signaling pathways by PIAS proteins. See comment in PubMed Commons below Cell Res 16: 196-202.
- Strebovsky J, Walker P, Dalpke AH (2012) Suppressor of cytokine signaling proteins as regulators of innate immune signaling. See comment in PubMed Commons below Front Biosci (Landmark Ed) 17: 1627-1639.
- Martin WJ, Harper JL (2010) Innate inflammation and resolution in acute gout. See comment in PubMed Commons below Immunol Cell Biol 88: 15-19.
- Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R (2005) Innate immunity conferred by Toll-like receptor 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 52: 2936-2946.
- Busso N, Ea HK (2012) The mechanisms of inflammation in gout and pseudogout (CPP-induced arthritis). See comment in PubMed Commons below Reumatismo 63: 230-237.
- Martinon F (2010) Update on biology: uric acid and the activation of immune and inflammatory cells. See comment in PubMed Commons below CurrRheumatol Rep 12: 135-141.
- Amaral FA, Costa VV, Tavares LD, Sachs D, Coelho FM, et al. (2012) NLRP3 inflammasome-mediated neutrophil recruitment and hyper- nociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum 64: 474-484.
- Cronstein BN, Sunkureddi P (2013) Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis. See comment in PubMed Commons below J ClinRheumatol 19: 19-29.
- Steiger S, Harper JL (2014) Mechanisms of spontaneous resolution of acute gouty inflammation. See comment in PubMed Commons below CurrRheumatol Rep 16: 392.
- Schett G, Schauer C, Hoffmann M, Herrmann M (2015) Why does the gout attack stop? A roadmap for the immune pathogenesis of gout. See comment in PubMed Commons below RMD Open 1: e000046.
- Martin WJ, Walton M, Harper J (2009) Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal induced murine peritoneal model of acute gout. Arthritis Rheum 60: 281-289.
- Krebs DL, Hilton DJ (2001) SOCS proteins: negative regulators of cytokine signaling. See comment in PubMed Commons below Stem Cells 19: 378-387.
- Sabat R1, Grütz G, Warszawska K, Kirsch S, Witte E, et al. (2010) Biology of interleukin-10. See comment in PubMed Commons below Cytokine Growth Factor Rev 21: 331-344.
- Mitroulis I, Kambas K, Ritis K (2013) Neutrophils, IL-1? and gout: is there a link? SeminImmunopathol 35: 501-512.
Citation: Hsieh SC, Yu CL (2016) Sophisticate Mechanisms and Unsolved Problems in the Resolution of Acute Gouty Arthritis. J Cytokine Biol 1: 107. DOI: 10.4172/2576-3881.1000107
Copyright: © 2016 Hsieh SC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12624
- [From(publication date): 8-2016 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 11712
- PDF downloads: 912