Some Physiological Insights of 2,4-D Sensitivity in an Aquatic Fern: Azolla pinnata R.Br
Received: 08-Jul-2016 / Accepted Date: 25-Jul-2016 / Published Date: 01-Aug-2016 DOI: 10.4172/2155-952X.1000235
Abstract
In an experiment with Azolla pinnata R.Br. under varying concentrations of herbicide (2,4-D), it reveals that the fern species has an wider ranges of physiological activities in moderation. Initially, the effects of the herbicide were reflected with minimum changes in dry matter accumulation in a dose dependent manner. The changes of membrane permeability had the maximum effects on 1000 μM of 2,4-D following the concentration gradient. However, the intermediate concentration of herbicide had not brought any significant changes in electrolytic conductivity. At initial concentration of herbicide treatments the electrolyte conductivity remains more stable. In support to osmotic stability plants recorded a significant change in water deficits that was experienced by proline accumulation in tissues. Thus, a steady increase in proline concentration was the feature of this species when interacts with 2,4-D. In another way the fern species had tolerated the loss of turgidity with significant accumulation of glycine-betaine with their maximum value at 1000 μM of 2,4-D. In further progress of effects of 2,4-D the plants were significantly characterised with an increase in ROS activities. Thus, ROS induced tissue lysis was detected by in vivo Evan's Blue staining of the root tissues with maximum intensity at 1000 μM of 2,4-D. The initial defence barrier of this species against ROS at cellular spaces was studied with in-gel analysis of enzyme like wallbound peroxidase. Plants registered a maximum peroxidase activities at influence of 2,4-D with 1000 μM. In addition to gene expression in Azolla a distinct polymorphism was observed as a function of 2,4-D treatments and that may be set as a bio indicator to herbicide interaction.
Keywords: Azolla, 2,4-D, Proline, Glycine-betaine, Evan's blue, Wall bound peroxidase
248956Abbreviations
2,4-D: 2,4-Dichlorophenoxy Acetic Acid; ROS: Reactive Oxygen Species; RH: Relative Humidity; EC: Electrical Conductivity; EL: Electrolyte Leakage; WPOD: Wall Bound Peroxidase; H2O2: Hydrogen Peroxide; MS Media: Murashige and Skoog’s Medium
Introduction
Herbicide sensitivity happens to be the most important and critical evaluation in sustenance of normal flora of soil. Acquisition of herbicide in soil by percolation or residual effects may hinder the nutrients availability to the non-target plants as well as to the other flora [1]. Plant species are found with their significant and adequate accumulation of herbicides in tissues by hyper accumulation. However, those species are marked with their tolerance to herbicide either by sequestering or quenching ability of the xenobiotic induced ROS [2]. Therefore, for both the cases physiological responses to herbicide tolerance are substantiated by few cellular funtionales. Previously reported the dis-functioning of water relationship is one of the important aspects of herbicide susceptibility in wild species. So, plants ought to stabilise the osmotic activities by development of compatible solutes in tolerant ones. This is further attributed with membrane activity for proper electrolyte transportation [3]. Whatever the strategies adopted by the plants, a minimal level of peroxidative denaturing of bio molecules are the obvious consequences for herbicidal action. This is more authenticated with the reactions by peroxidative herbicides in plants for impairment of cellular metabolism. Therefore, plants were evaluated with their extent of reactive oxygen species in engagement of peroxidation reactions. Certain peroxidase activities from enzymatic cascades for anti-oxidation are most important with their variability of isozymic profiles. The isozymic variability is markedly considered as bio indicator for a species coupling with its tolerance or susceptibility under stress condition. Herbicide sensitivity in higher plants are much explored in different phases of physiological and biochemical modules [4]. However, non-flowering, plants particularly those are aquatic species have not been focused with acute attention. Therefore, it would be imperative to decipher the cellular activities supporting the hyper-accumulation of herbicides in those species. This would be excercised by some cellular and physiological attributes in co-relation with tolerance and sensitivity to that herbicide. These may possibly serve the bio indices for tolerance to xenobiotics as herbicide by this species. This is with intention to employ those physiological attributes in environmental decontamination process with herbicidal pollution in soil. More so, the fate of such plant species if results in non-survival, then it would be granted for a good tester species for herbicidal sensitivity.
Azolla (A. pinnata R.Br.), a duck weed fern is one of the important pteridophytic species belonging to fern groups which is important to control different xenobiotics. This species has been more exercised in hyper-accumulation of heavy and toxic metals showing their tolerance to various ROS [5]. The floating habitat and profuse root system makes it much feasible to adsorb metal ions or simpler organic moieties in different forms from contaminated water bodies. Some of the physiological characters have already been documented in this species with possible bio indication for particular metal and metalloids [6]. In the present experiment, we have evaluated few cellular activities in focusing the oxidative stress developed under a common herbicide, 2,4-D. It has already been established as an antagoniser in auxin mediated cellular functions of higher plants and thus is evident as a potential herbicide against broad leaf dicots [7]. Here, we show that the 2,4-D could induce some sort of changes for toxicity and its resistance pathways in some physiological modalities in Azolla.
Materials and Methods
A laboratory based experiment was conducted in the Plant Physiology and Plant Molecular Biology section, Department of Botany, University of Kalyani during summer season,2016. Azolla pinnata R.Br. free floating duckweed fern was selected as a plant material for the present experiment. The plants were collected from the Regional Fodder Station, Kanchrapara, W.B. India within University premises. Initially, plants were grown in fresh water for 7 days in the laboratory condition and thereafter, the plants were incubated in ¼ MS Medium [8] for 3 days to acclimatize. This was followed by treatment with varying concentrations (0 μM, 100 μM, 250 μM, 500 μM and 1000 μM) of 2,4-D (Sigma MB Grade). Plants were kept in such treatment for 7 days under ambient condition of 35 ± 1ºC of temperature, 60- 80% of RH and photoperiod of 13-11 h light and dark photoperiod. The solution for each treatment was renewed 3 days interval to steady supplementations of the plants.
Analysis of dry matter accumulation
Dry matter accumulation was determined from herbicide treated plants according to Hanway et al. [9]. Initially, the plant samples were randomly selected with three replications and their fresh weights were taken. Thereafter, the plant samples were dried in a hot air oven at 80ºC till it achieved to a constant weight.
Determination of electrolyte leakage
500 mg of fresh Azolla plants were chopped into small pieces and placed in test tubes containing 20 ml of deionised water. Then, the tubes were incubated in water bath at 30ºC for 2 h and initial electrical conductivity (EC1) of the medium was measured. Thereafter, the samples were autoclaved at 121ºC for 15 min to release all electrolytes; cooled to room temperature and the final electrical conductivity (EC2) was measured. The electrolyte leakage (EL) was determined as E=(EC1/ EC2) × 100 according to Dionisio and Tobita [10].
Estimation of proline content
Proline content from the treated plant was estimated according to Bates et al. [11]. 1.0 g of fresh Azolla fronds were homogenised in 3% (w/v) aqueous sulfosalicylic acid and the homogenate was centrifuged at 10,000x g for 15 min and it was mixed with 3% (w/v) glacial acetic acid and ninhydrin. Thereafter, the reaction mixtures were heated for 1 h in a water bath at 90ºC, cooled and mixed with 4 ml of toluene by constant shaking for 2 min. The toluene layer was aspirated out and the proline content was determined in reference to standard solutions of proline salt.
Estimation of glycine-betaine content
Glycine-Betaine content was estimated according to Greive et al. [12]. 1.0 g of plant sample was homogenised, dried and kept in incubator shaker with 15 ml of distilled water for 24 h at 30ºC. Thereafter, the samples were centrifuged at 10,000x g for 10 min and the homogenate was diluted using 2 N H2SO4 and stored at 4ºC. Then, the extract was reacted with potassium iodide-iodine reagent followed by vortexing and kept at 4ºC for overnight followed by centrifugation at 10,000x g for 10 min. The supernatant was mixed with 1,2-dichloroethane and incubated for 1 h. The glycine-betaine content was measured at 365 nm with UV-Visible spectrophotometer.
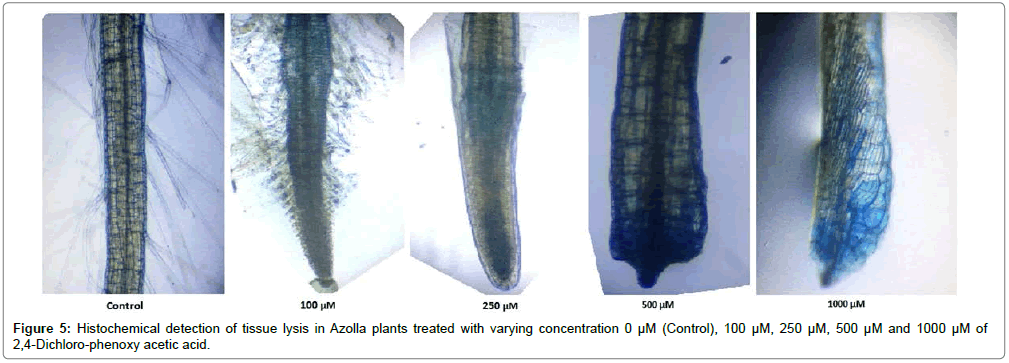
Histochemical detection of tissue lysis
Histochemical detection of tissue lysis was done from treated plant roots. Initially, the root tips were taken thoroughly washed with distilled water and incubated in 0.25% (w/v) Evan’s Blue staining solution for ½ h. Thereafter, the stained root tips were washed repeatedly to remove excess stain and observed under microscope (Olympus, CKX41, Japan).
Next, the root tips from the control and treated plants were incubated in N,N-dimethylformamide solution for 1/2 h with gentle agitation at room temperature and the intensity of stain absorbed was measured at 600 nm [13]. However, for this parameter plants have been worked upon in respective concentration of herbicide with three replications for each and the best images in observation were documented.
Assay of wall bound peroxidase activity
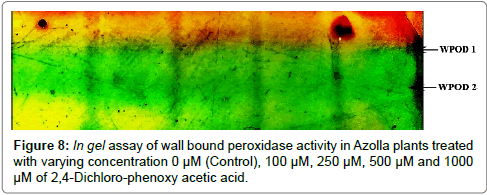
500 mg of fresh tissue samples were homogenised with Liquid Nitrogen and extracted with 20 mM Na-phosphate buffer (pH=6.5) at 4ºC. Thereafter, the homogenate was centrifuged at 12,000x g for 15 min at 4ºC. Initially, the pellet was collected and resuspended with equal volume of extraction buffer. Then the resuspended pellet was again centrifuged at 10,000x g for 15 min at 4ºC; supernatant was collected and the pellet and washed with 20 mM of phosphate buffer (pH=6.5) and incubated with 1 M NaCl for 1 h at 4ºC. After 1 h the solution was recentrifuged at 10,000x g for 10 min at 4ºC and finally, the supernatant containing the ionically bound enzyme was used for spectrophotometric assay according to Saroop et al. [14]. For isozymic analysis of WPOD the protein was loaded in a 10% native PAGE with of 200 mM Tris-HCl buffer (pH 8.8). Thereafter, the gel was stained for peroxidase activity within an assay mixture containing 20 mM Naphosphate buffer (pH 6.5), 0.05% H2O2 and 0.5 mM ortho-dianisidine (dissolved in methanol). The bands were resolved by transferring the gel to 5% of glacial acetic acid solution.
Results
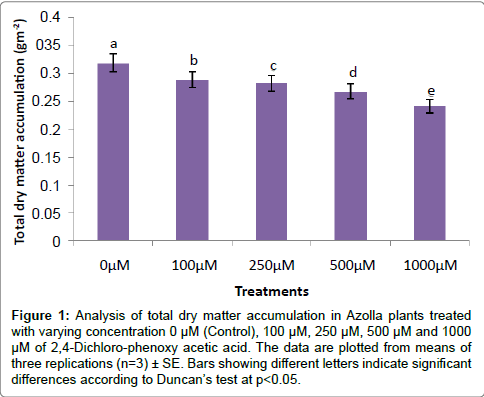
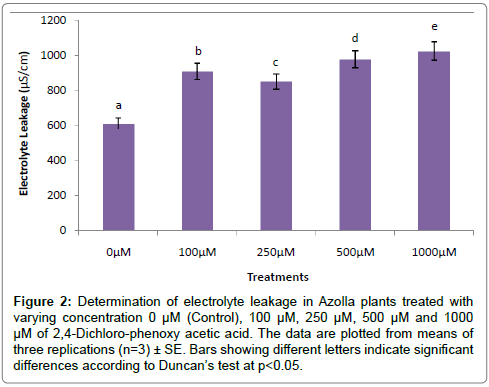
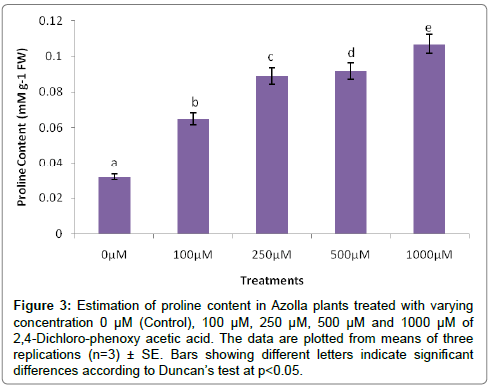
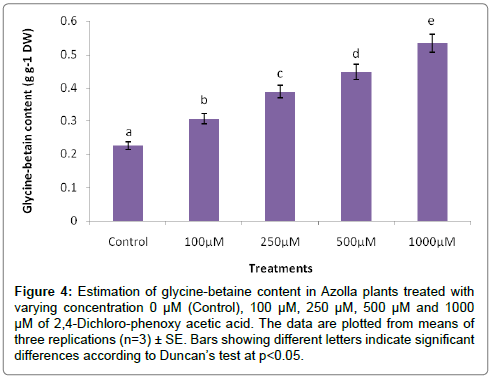
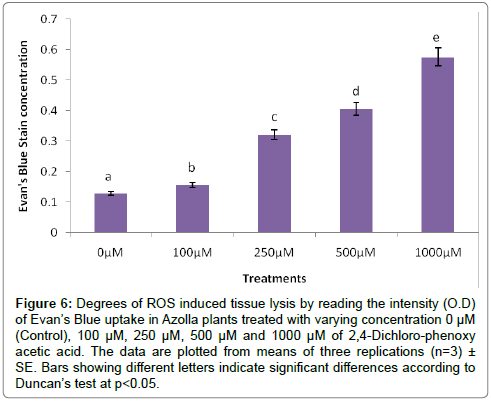
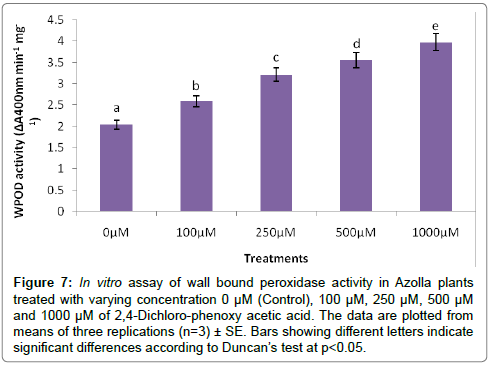
The evaluation of Azolla plants with respect to herbicidal sensitivity was done both at physiological and cellular responses. Plant’s reactions to xenobiotic stress are preliminarily observed on its phenotypic or morphological attributes. Initially, Azolla plants under varying concentration of 2,4-D treatments recorded hardly any significant changes such as the leaf colour and texture. However, the fronds showed some wilting nature through increasing concentration of 2,4-D (Data were not shown). Thus, an Azolla plant has also estabilised their growth by hardly any significant differences in total dry matter accumulation. Still, the maximum decline in dry matter accumulation was 10.38% less in comparison to control under 1000 μM of 2,4-D concentration (Figure 1). In accordance to dry matter accumulation with influence of any abiotic factors, plants must have faced some changes in water relation. The loss of membrane permeability of the Azolla species is evident from its electrolyte leakage activity over the cellular membrane. The electrolyte leakage recorded a consistent trend except at 250 μM of herbicide treatment. The maximum electrolyte leakage of the membrane as recorded 1.67 fold over control which indicates a substantial loss of ions upon herbicide treatment (Figure 2). 2,4-D, a potent herbicide has effectively proved its activities in development of water stress. Thus, in the present experiment, the development of two compatible solutes proline and glycine-betaine are the citing examples. For both the cases plants register a linear increase of osmolytes accumulation in a dose dependent manner of herbicides. The maximum accumulation of proline and glycine-betaine were found to have 3.34 fold and 2.38 fold under highest concentration as compared to control (Figures 3 and 4). Still, proline is found to be more over expressed than glycinebetaine in comparison with 1.40 fold when 2, 4-D was used at highest concentration. It is mentioned that herbicide stress is compounded with both osmotic deficit as well as specific cellular effects. The later in this case may be intruded generation of free radical or ROS inducing cellular damages. With this concept we elucidate the in vivo detection of tissue lysis by staining the roots of Azolla by specific stain with Evan’s Blue (Figure 5). A distinct colorization with increasing intensity as a function of herbicide concentration simply refers the sensitivity of root tissue for oxidative stress (Figure 6). Moreover, the abscission of root hairs more with higher concentration of 2,4-D has made it more evident for oxidative damages of the roots. Azolla, a free floating aquatic hydrophyte is expected to be affected from dehydration stress. When herbicide concentrations were increasing in order, it seems making the cellular environment more negative in water potential. Thereby, plants are expected to elevate moisture tension which might be transmitted to the internal tissue system to resist the maximum tension on the cell wall. Azolla would have also been developed with more rigidity in the tissues. In the present experiment, WPOD activity suggests that plants were induced for more lignifications on the cell wall. A linear rise of WPOD which was highest in 1000 μM of 2,4-D concentration against control with the value of 1.95 fold is the clear indication of the fact (Figure 7). The promotion of enzymatic activity under 2,4-D stress has also been established from polymorphic variants of WPOD. With this isolated cell wall proteins of plant in their in vitro activity system with distinct two possible isoforms were recorded (WPOD1 and WPOD2). Of those WPOD1 appears to be more contributing in lignifications through intense concentration and shown its possibility to be a cellular marker against herbicide sensitivity (Figure 8).
Figure 1: Analysis of total dry matter accumulation in Azolla plants treated with varying concentration 0 μM (Control), 100 μM, 250 μM, 500 μM and 1000 μM of 2,4-Dichloro-phenoxy acetic acid. The data are plotted from means of three replications (n=3) ± SE. Bars showing different letters indicate significant differences according to Duncan’s test at p<0.05.
Figure 2: Determination of electrolyte leakage in Azolla plants treated with varying concentration 0 μM (Control), 100 μM, 250 μM, 500 μM and 1000 μM of 2,4-Dichloro-phenoxy acetic acid. The data are plotted from means of three replications (n=3) ± SE. Bars showing different letters indicate significant differences according to Duncan’s test at p<0.05.
Figure 3: Estimation of proline content in Azolla plants treated with varying concentration 0 μM (Control), 100 μM, 250 μM, 500 μM and 1000 μM of 2,4-Dichloro-phenoxy acetic acid. The data are plotted from means of three replications (n=3) ± SE. Bars showing different letters indicate significant differences according to Duncan’s test at p<0.05.
Figure 4: Estimation of glycine-betaine content in Azolla plants treated with varying concentration 0 μM (Control), 100 μM, 250 μM, 500 μM and 1000 μM of 2,4-Dichloro-phenoxy acetic acid. The data are plotted from means of three replications (n=3) ± SE. Bars showing different letters indicate significant differences according to Duncan’s test at p<0.05.
Figure 6: Degrees of ROS induced tissue lysis by reading the intensity (O.D) of Evan’s Blue uptake in Azolla plants treated with varying concentration 0 μM (Control), 100 μM, 250 μM, 500 μM and 1000 μM of 2,4-Dichloro-phenoxy acetic acid. The data are plotted from means of three replications (n=3)± SE. Bars showing different letters indicate significant differences according to Duncan’s test at p<0.05.
Figure 7: In vitro assay of wall bound peroxidase activity in Azolla plants treated with varying concentration 0 μM (Control), 100 μM, 250 μM, 500 μM and 1000 μM of 2,4-Dichloro-phenoxy acetic acid. The data are plotted from means of three replications (n=3) ± SE. Bars showing different letters indicate significant differences according to Duncan’s test at p<0.05.
Discussion
Xenobiotics have already been evident as an affective hazardous agent for environment and ecosystem. If herbicides are granted under the category of xenobiotic series then its residual affect after leaching from plant parts as residual fractions in soil definitely be a botheration for distortion of biodiversity. Therefore, it is the plant genotype that has unique ability to quench significant amount of the residual herbicide that would be granted as an effective material for bio remediation. On that basis the physiological characteristics in attribution for sensitivity to 2,4-D is relevant for effective biomarkers against the herbicide deposition in soil. Therefore, the present study may also be relevant in documentation to report the potential of aquatic fern species in quenching of herbicides as well as bioremediation of aquatic environment. The most distinguishable features of herbicide sensitivity in Azolla are found not to have some significant reduction on total dry matter accumulation. Interestingly, it reflects the plant’s endurance or resistance in inhibition either of photosynthetic activities or/and translocation of photo assimilates over loss of respiratory carbon dioxide. In most of the cases the broad leaf plants (more with dicotyledonous species) are shown with their suppression of growth as a function of herbicide sensitivity in the developmental stages [15]. In earlier studies the dry matter accumulation in plants was also found to have strong co-relation with tissue hydration status. 2,4-D has also been proved to execute its effect on curtailing of water relation leading to water deficit stress [16]. Therefore, Azolla plants might have faced with water deficits following its effect on photosynthetic activity of the fronds. This is more illustrated with an evaluation of tissue water potential and its adjustment through osmolyte accumulation. Biosynthesis of proline in terms of water stress induction or hydrolysis of protein could serve as a reliable index even in this species also [17]. Plants could register osmotic adjustment by another compatible solutes like glycine-betaine etc. The later being a quaternary amine is not readily served with cellular metabolism. However, de novo synthesis of glycine-betaine and its tissue specific accumulation could support the osmotic adjustment in other way. In the present experiment, both proline and glycine-betaine accumulation together may constitute a better adaptive system with 2,4-D stress in terms of water relation. The analysis of the data for proline and glycine-betaine are significantly corroborated with the earlier findings in other fern species induced by xenobiotics for tolerance [18]. Therefore, 2,4-D is also proved as a herbicide with additional induction of water deficit stress along with suppression of auxin activity. Azolla plants have also tuned its osmotic system by employing these two compatible solutes in respect of plant water relationship. In accordance to maintenance of water balance to any metals or xenobiotics plants have to drop a substantial amount of ions over the cellular membrane [19]. Potassium (K+) being the most important ions which shows a severe depletion out of various abiotic stress inducing dehydration in tissues. Likewise, an adequate and significant release of electrolytes from roots of Azolla as studied by electrolyte leakage under different 2,4-D concentrations registered plants failure in ionic balance. Still, the sustenance of tissue hydration with executions of solute or ion is definitely employed with some mechanisms on cell membrane [20]. Azolla in the present experiment, however, is not examined in regaining of electrolyte loss by expense of any such cellular responses.
The in vivo detection of tissue lysis by Evan’s Blue staining is really implicative of the tissue sensitivity with 2,4-D action. A significant indication for abscission of root hair all through the doses of herbicides established the activity of ROS on cellular degeneration. Physiologically the development of root hairs on the epiblema is a sure sign of proper root physiology facilitating root hydraulic conductance for water absorption. Interestingly, abscission of root hair coupled with intense staining of Evan’s Blue within the cortical zone may also be supportive in degeneration of root system in Azolla. Therefore, it would not be less to presume that 2,4-D could also induce some abscission phenomenon related with ethylene biosynthesis. Thus, loss of root hairs following generation of lipid peroxidation or membrane hydrolysis may ensure the prime sensitivity of Azolla at cellular level under herbicide treatment.
At physiological level plants have to endure the oxidative stress through modification of the cell wall with lignifications [21]. Among the ROS, it is the H2O2 content and its involvement both in degenerative and constitutive functions is in question. For the later, specifically lignification is the key to add cell wall residues by specific reaction with higher level of H2O2 in apoplastic spaces. It is the signalling cascade that H2O2 could trigger the lignifications process by few specific enzymes under peroxidase category. These specifically include the wall-bound peroxidase (WPOD). Those are the most important one with variability in phenolic residues as electron donor for oxidation of H2O2 into free anions. In earlier experiments water ferns like Salvinia and Marsilea shown their affinity for both chloro-napthol and ortho-dianisidine as phenolic residues in contribution of reduction of H2O2 under metal stress [22]. It also favours the lignifications process of cell wall in those fern species for more mechanical rigidity. Still, in the present experiment, Azolla recorded a well sensitivity with ortho-dianisidne in such process employing WPOD. The in-gel staining of proteins for polymorphism of WPOD is clear indication of lignification process [23]. Cell wall modification with lignifications, bonding and other chemical processes has been noticeable for stress tolerance in plants more under water deficit condition. Since, 2,4-D is already proven as inducer in water deficit, the expression of WPOD with various isoforms and its over activity compared to control should be relevant. In the present experiment, WPOD with its variability in different substrates is the sign of herbicidal tolerance in different modes in fern.
Conclusion
The responses of Azolla plants to 2,4-D treatments for 7 days recorded some physiological alteration proving the efficacy of the herbicide. It is admitted that the critical value for its survival to this herbicide has not been determined at least within the doses considered in this work. In anticipation of herbicidal affectivity in general 2,4-D is hypothesised to act on existing auxin metabolism. Actually, in other species the synthesis and turn-over of auxin by specific paths is in a homeostasis and thus plants are favoured in auxin mediated growth. However, it may be postulated that 2,4-D may either suppress the auxin biosynthesis or induced its lysis making plants in a state of dispossession of growth and development. Still, this needs more exploration to probe this question. The cellular responses starting from membrane permeability to peroxidase activity through accumulations of ROS in different degrees undoubtedly indicates the Azolla species as sensitive to 2,4-D. Despite the loss of electrolytes in plants the adjustment of water relation with maintenance adequate dry matter accumulation also proves the probable tolerance of this fern to herbicide. Therefore, at least at preliminary level Azolla species could be hypothesized as herbicide tolerant and quencher by alteration of its cellular processes through ROS accumulation. Still, in depth studies are required to establish its underline mechanism in more details of herbicide tolerance in a consistent manner.
Acknowledgement
This work is financially supported by DST-PURSE programme, 2016, DST, Govt. of INDIA, New Delhi to University of Kalyani.
References
- Paoletti MG, Pimentel D (1996) Genetic engineering in agriculture and the environment: Assessing risks and benefits. BioScience 46: 9.
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant PhysiolBioch 48: 909–930.
- Trapp S (2004) Plant uptake and transport models for neutral and ionic chemicals. ESPR-Environ SciPollut Res 11: 33-39.
- Kichey T, Heumez E, Pocholle D, Pageau K, Vanacker H, et al. (2006) Combined agronomic and physiological aspects of nitrogen management in wheat highlight a central role for glutamine synthetase. New Phytol 169: 265–278.
- Mudgal V, Madaan N, Mudgal A (2010) Heavy metals in plants: Phytoremediation: Plants used to remediate heavy metal pollution. AgricBiol J N Am 1: 40-46.
- Benaroya RO, Tzin V, Tel-Or E, Zamski E (2004) Lead accumulation in the aquatic fern Azolla?liculoides. Plant PhysiolBioch 42: 639–645.
- Peterson MA, McMaster SA, Riechers DE, Skelton J, Stahlman PW (2016) 2,4-D past, present and future: A review. Weed Technol 30: 303-345.
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15: 473–497.
- Hanway JJ, Weber CR (1971) Dry matter accumulation in eight soybeans (Glycine max (L.) Merrill) varieties. Agron J 63: 227-230.
- Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1-9.
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39: 205–207.
- Greive CM, Grattan SR(1983) Rapid assay for determination of water-soluble quaternary amino compounds. Plant Soil 70: 303-307.
- Baker CJ, Mock NM (1994) An improved method for monitoring cell deathin cell suspension and leaf disc assays using evan’s blue. Plant Cell Tissue Organ Cult 39: 7–12.
- Saroop S, Chanda S V, Singh Y D (2002) Changes in soluble and ionically bound peroxidase activities during Brassica juncea seed development. Bulg J Plant Physiol 28: 26–34.
- Wright T R, Shan G, Walsh T A, Lira J M, Cui C, et al. (2010) Robust crop resistance to broadleaf and grass herbicides provided by aryl-oxy-alkanoatedioxygenase transgenes. ProcNatlAcadSci 107: 20240–20245.
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: Effects, mechanisms and management. Agron Sustain Dev29: 185-212.
- Vogel HJ, Davis BD (1952) Glutamic- d-semialdehyde and Δ'-pyrroline-5-carboxylic acid, intermediates in the biosynthesis of proline. J Am ChemSoc 74: 109-112.
- Ashraf M, Foolad MR (2007) Role of glycine-betaine and proline in improvement plant abiotic stress tolerance. Environ Exp Bot 59: 206–216.
- Blokhina O, Virolainen E,Fagerstedt KV (2013) Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann Bot 91: 179- 194.
- Basu S, Roychoudhury A, Saha PP, Sengupta DN (2010) Acomparative analysis of some biochemical responses of three indica rice varieties during polyethylene glycol-mediated water stress exhibits distinct varietal differences. ActaPhysiologiaePlantarum 32: 551-563.
- Claeys H, Inze D (2013) Theagony of choice: How plants balance growth and survival under water-limiting conditions. Plant Physiol 162: 1768–1779
- Mandal C, Ghosh N, Dey N, Adak MK (2013) Physiological responses of Salvinianatans L. to aluminium stress and its interaction with putrescine. J Stress PhysiolBiochem 9: 163-179.
- Swaroop S, Chanda SV,Singh YD (2002) Changes in soluble and ionically bound peroxidase activities during Brassica juncea seed development. Bulg J Plant Physiol 28: 26–34.
Citation: De AK, Dey N, Adak MK (2016) Some Physiological Insights of 2,4-D Sensitivity in an Aquatic Fern: Azolla pinnata R.Br. J Biotechnol Biomater 6:235. DOI: 10.4172/2155-952X.1000235
Copyright: © 2016 De AK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12476
- [From(publication date): 9-2016 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 11478
- PDF downloads: 998