Case Report Open Access
Solid Pseudopapillary Neoplasm of the Pancreas and Concomitant Variation of the Common Hepatic Artery: Case Report and Literature Review
Hongchuan Zhao*, Shu Bo Pan, Zhi Gong Zhang and Xiao Ping Geng
Department of General Surgery, The First Affiliated Hospital, Anhui Medical University, Hefei 230022, Anhui Province, China
- *Corresponding Author:
- Hongchuan Zhao
Department of General Surgery
Anhui Medical University
Hefei 230022, Anhui Province, China
Tel: +86-551-62923191
Fax: +86-551-62922026
E-mail: zhc0117@sina.com
Received date: September 09, 2014; Accepted date: October 29, 2014; Published date: November 05, 2014
Citation: Zhao H, Pan SB, Zhang ZG, Geng XP (2014) Solid Pseudopapillary Neoplasm of the Pancreas and Concomitant Variation of the Common Hepatic Artery: Case Report and Literature Review. J Gastrointest Dig Syst 4:236. doi:10.4172/2161-069X.1000236
Copyright: © 2014 Zhao H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
A solid pseudopapillary neoplasm of the pancreas is a rare pancreatic tumor with a low malignant potential. It typically occurs in young women. Solid pseudopapillary neoplasm associated with extra-pancreatic and pancreatic anomalies are occasionally reported. We report a case of an solid pseudopapillary neoplasm with a concomitant variation of the common hepatic artery in a 43-year-old woman. The patient underwent pancreaticoduodenectomy; an Solid pseudopapillary neoplasm was confirmed with pathology findings. Neither recurrence nor metastases were present at the 19 months follow-up exam.
Keywords
Solid-pseudopapillary neoplasm; Whipple procedure; Anatomy; Variation of common hepatic artery; 18Ffluorodeoxyglucose
Abbreviations
SPN: Solid Pseudopapillary Neoplasm; CHA: Common Hepatic Artery; 18F-FDG-PET: 18F-Fluorodeoxyglucose Positron Emission Tomography
Introduction
A solid pseudopapillary neoplasm (SPN) is a rare exocrine tumor of the pancreas, with a 2-3% incidence rate among primary pancreatic tumors and a 10-15% incidence rate among cystic tumors of the pancreas [1,2]. The neoplasm was first described by Franz in 1959, and it was included in the World Health Organization classification in 1996 [3,4]. Because of its rarity, the literature contains few reports of this tumor; however, reports regarding SPN have increased significantly in the past 10 years [5]. We present radiological, 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET), and histological findings of a case of SPN with concomitant variation of the common hepatic artery (CHA).
Case Presentation
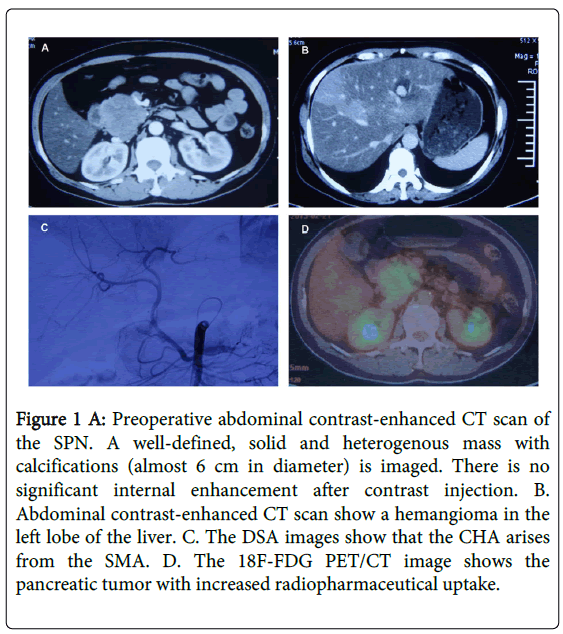
A 43-year-old woman with diabetes was admitted to our hospital in November 2012 for further evaluation and treatment of a pancreatic mass detected by abdominal ultrasonography (US) and abdominal computed tomography (CT scan) (Figure 1A) and magnetic resonance imaging (MRI )at a local hospital. Three months before presenting at our facility, her initial signs and symptoms were nonspecific abdominal pain and the physical examination was unremarkable. CT scan and MRI performed at a local hospital showed a circumscribed mass with solid and cystic areas located in the head of pancreas; it measured 6.0 × 5.6 × 5.4 cm with a clear margin. In addition, the body and tail of the pancreas were atrophic. The CT scan showed that the solid part of the tumor displayed peripheral enhancement, while there was no enhancement of the cystic portion. The pancreatic duct of the upper stream did not dilate. An MRI showed a cystic and solid tumor with low signal intensity on T1 and high signal intensity on T2; the pancreatic duct was normal. There also was a mass in the left lobe of the liver, measuring 2.5 × 2.5 cm. With enhanced abdominal CT scan (Figure 1B) and MRI, the liver mass exhibited a similar appearance as that of a hemangioma. Tumor markers (AFP, CA199, CEA, and CA125) were within normal limits. The clinical diagnosis was advanced pancreatic cancer and the tumor was incompletely resected. The patient underwent regional transarterial infusion chemotherapy with gemcitabine, oxaliplatin and 5-fluorouracil in local hospital. Interestingly, the patient presented with an artery variation in which the CHA arose from the superior mesenteric artery (SMA) and followed a prepancreatic route through the arteriography (Figure 1C). However the treatment failed. For further evaluation of the pancreatic tumor, the patient was admitted to our hospital and received an 18F-FDG-PET scan covering the area from the skull base to the upper part of the thigh. In regard to the PET findings, the pancreatic tumor showed increased radiopharmaceutical uptake (Figure 1D) in the mass at the head of the pancreas with a maximum standardized uptake value (SUVmax) of 11.0 at the initial imaging and 17.8 at the delayed imaging. At this time, the mass in the left liver was not observed. After a 18F-FDG-PET scan, the patient underwent a Whipple procedure.
Figure 1 A: Preoperative abdominal contrast-enhanced CT scan of the SPN. A well-defined, solid and heterogenous mass with calcifications (almost 6 cm in diameter) is imaged. There is no significant internal enhancement after contrast injection. B. Abdominal contrast-enhanced CT scan show a hemangioma in the left lobe of the liver. C. The DSA images show that the CHA arises from the SMA. D. The 18F-FDG PET/CT image shows the pancreatic tumor with increased radiopharmaceutical uptake.
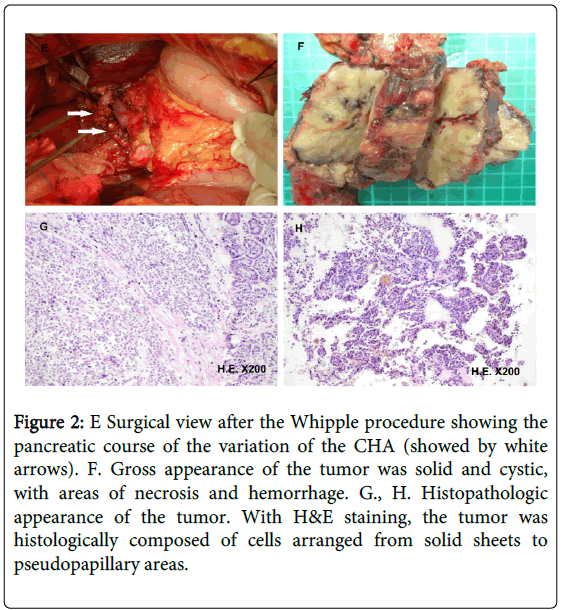
During surgery, the mass was discovered at the head of the pancreas; in addition, the patient had a hepatic arterial variation in which the CHA arose from the SMA and traversed the surface of the pancreatic tumor (Figure 2E). Without the aid of the preoperative imaging, this artery around the tumor would have been misinterpreted as a large gastroduodenal artery in its normal anatomic position, and would have been resected. By clamping the aberrant artery temporarily, no blood flow was measured in the hilar artery; therefore, the artery was preserved. The intervention continued normally via dissection of the tumor, which was challenging due to the past episode of regional transarterial infusion chemotherapy. Finally, the pancreaticojejunostomy, the choledochojejunostomy, and the gastrojejunostomy could be performed without difficulty. The postoperative recovery was uncomplicated and the patient was discharged on the 14th postoperative day.
Figure 2: E Surgical view after the Whipple procedure showing the pancreatic course of the variation of the CHA (showed by white arrows). F. Gross appearance of the tumor was solid and cystic, with areas of necrosis and hemorrhage. G., H. Histopathologic appearance of the tumor. With H&E staining, the tumor was histologically composed of cells arranged from solid sheets to pseudopapillary areas.
Gross examination of the surgical specimen revealed that the tumor was well encapsulated by a fibrous pseudocapsule and contained both solid and cystic components; it measured 8.0 × 5.0 × 3.5 cm (Figure 2F). The tumor margin was negative. Microscopically, the tumor was composed of sheets and nests of uniform polygonal epithelioid cells with round or oval nuclei and acidophilic cytoplasm divided by thin fibrovascular stroma. Degenerative changes were seen in some portions of the tumor. Invasion of the vascular space or perineural invasion was not identified. The proliferation marker Ki- 67 index was < 2% and cellular atypia was mild. With immunohistochemical analysis, the tumor cells showed a nuclear type of alpha-1-antichymotrypsin (++), progesteron receptor (++), vimentin (+), synaptophysin (+), neuron-specific enolase (+), Chromogranin A (+) and CD56 (+), but were negative for insulin (-), inhibin (-), α-fetoprotein (-), S-100 (-), Cytokeratin19 (-), Cytokeratin20 (-) and Epithelial Membrane Antigen (-). These finding supported a final diagnosis of SPN of the pancreas (Figure 2G and H). Neither recurrence nor metastases was found at the 19 months follow-up exam.
Discussion
SPN is an uncommon neoplasm of the pancreas; it is predominantly observed in female patients in their second or third decade [1]. Historically, SPN has been reported to account for only 2-3% of all pancreatic tumors [2]. With the growing clinical and pathological recognition of this tumor, numerous recent studies suggest that there may be a higher percentage of the solid-pseudopapillary pancreatic tumor. Presenting symptoms of SPN are nonspecific because the lesion grows slowly and rarely invades adjacent structures, such as the common bile duct; this characteristic is similar to that of some nonfunctional neuroendocrine tumors [6]. In the present case, the presenting symptom of the patient was nonspecific abdominal pain and was typical in terms of radiological features and pathological findings, but not in terms of demographic features. It occurred in a 43-year-old woman; thus, it does not represent one found in the typical population. Previous studies have shown that SPN significantly affects young females aged 20 to 40 years [1]. However, it exhibited the typical radiological features on CT images, such as a regular shape, a well-defined margin, and inhomogeneous appearance, consistent with a solid and cystic composition [7].
Those radiological features of SPN are very different from those of ductal adenocarcinoma, as the latter is often infiltrative with poorly defined margin and is invasive to the surrounding structures. The results of light microscopy and immunohistologic analysis of our case were typical. The tumor is characterized by a surrounding fibrous capsule and varied portions of solid and cystic components showing hemorrhagic changes. Under light microscopy, the characteristic findings are the pseudopapillary structures and degenerative changes, such as necrosis, hemorrhage, cholesterol clefts and foamy macrophages. In addition, the tumor cells are characteristically uniform with mild atypia and rare mitoses, indicating that it is a benign entity [8]. Based on these histological features, it is relatively easy to differentiate SPN from ductal adenocarcinoma, as the latter is often more moderately or poorly differentiated and is composed of haphazardly arranged glands admixed with a dense desmoplastic stroma [9]. Cystic neoplasms, such as mucinous or serous cystic neoplasms, can also be easily differentiated from SPN, because they lack communication with the pancreatic duct system and have no mucinous or serous epithelium, which is typically supported by an “ovarian” stroma [10]. Neuroendocrine tumors, especially the well-differentiated ones,are the most important entities in the differential diagnosis of SPN; this is because they may display similar light microscopic features, and neuroendocrine markers are variably expressed in SPN [8]. Except for the consistently negative results for chromogranin A, expressions of other neuroendocrine markers such as synaptophysin, neuron-specific enolase, and CD56 at various levels have been demonstrated [11]. Recently, the nuclear type of β-catenin has been regarded to be a unique immunohistochemical feature of SPT because it underlies the genetic mutation of catenin, which is found in more than 90% of SPNs [12]. Abnormal nuclear labeling of β-catenin strongly supports the diagnosis of SPN. SPN is usually benign, based on its pathological features. However, interestingly, it always shows hyper-metabolism of FDG on 18F-FDG-PET scan, which is a characteristic feature of malignant tumors. The high cellular density, rich mitochondria and the hyper-vascular nature as shown in radiological findings have been thought to contribute to the FDG accumulation [13]. The curative treatment is complete surgical resection and transarterial infusion chemotherapy is not recommended despite some reports that noted that the tumor disappeared after the treatment [14]. In rare cases, SPN metastasizes to liver or seeds the peritoneum. In this patient, 18F-FDG-PET staged the tumor to be confined without metastases, and she had safely undergone Whipple procedure.
In regard to the anomalies of the arterial hepatic supply, it was firstly described by Haller in 1756; in addition, a classification of the various anatomic variants was proposed by Hiatt in 1994. Only 50–80% of cases have the so-called normal vascular anatomy [15-17]. In our case, presenting with the CHA originating from the SMA, the anatomic variant corresponds to a type IX with Michel’s classification and a type V with Hiatt’s classification. The high risk of misinterpretation is the key point of this case during surgery; this risk was minimized by careful preoperative imaging during the arteriography that revealed the aberrant CHA. Furthermore, it could have been misinterpreted as an artery supplying the tumor. Preoperative imaging from the angiography allows for the precise identification of hepatic vascularization; thus, facilitating intraoperative management. At present, angio-CT or angio-MRI is superior to angiography for vascularization assessment. In particular, angio-CT is of significant value for the evaluation of the resectability of pancreatic cancer (80% of cases) [18]. In the presence of the hepatic vascularization, the tumor is resectable; however, there is a high risk of arterial injury during its removal if the surgeon does not recognize the anatomic variant before or during the procedure. A safe dissection along the entire course of the artery along its entire course around the tumor without vascular injury is technically difficult. During the surgery, we clamped the artery and, via ultrasonography, determined that there was no blood in the hilar artery. Therefore, we preserved the artery. Without the vascular supply from the CHA, there would be a high risk of long-term biliary ischemia, or even necrosis of a hepatic segment. Conversely, the case presented another challenge due to the past episode of transarterial infusion chemotherapy because it would increase the risk of fatal hemorrhage during the procedure.
Because it is an indolent entity, the overall 5-year survival rate of an SPN is approximately 95% [19]. Although the malignant potential of SPN is low, about 10-15% of patients develop metastatic disease; the liver and peritoneum are most often involved. Aggressive behavior can be unpredictable, and a number of different criteria are proposed by some investigators [8]. However, there currently is no agreement whether gender and prognosis is relevant to the aggressive behavior. Therefore, long time follow-up is indicated for all SPN patients; this is more important than determining whether the tumor is malignant.
References
- Adams AL, Siegal GP, Jhala NC (2008) Solid pseudopapillary tumor of the pancreas: a review of salient clinical and pathologic features. AdvAnatPathol 15: 39-45.
- Allen PJ, D'Angelica M, Gonen M, Jaques DP, Coit DG, et al. (2006) A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg 244: 572-582.
- Frantz V. (1959) Tumors of the pancreas. In: Atlas of tumor pathology. Washington, DC: US Armed Forces Institute of Pathology 32-33.
- Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. (1996) Histological typing of tumors of the exocrine pancreas. 2nd ed. (WHO international histological classification of tumors). Berlin, Heidelberg, New York: Springer 15-21.
- Yang F, Jin C, Long J, Yu XJ, Xu J, et al. (2009) Solid pseudopapillary tumor of the pancreas: a case series of 26 consecutive patients. Am J Surg 198: 210-215.
- Surlin V, Ramboiu S, Ghilusi M, Plesea IE. (2012) Incidental intraoperative discovery of a pancreatic neuroendocrine tumor associated with chronic pancreatitis. DiagnPathol 29: 132-6.
- Yin Q, Wang M, Wang C, Wu Z, Yuan F, et al. (2012) Differentiation between benign and malignant solid pseudopapillary tumor of the pancreas by MDCT. Eur J Radiol 81: 3010-3018.
- Kim CW, Han DJ, Kim J, Kim YH, Park JB, et al. (2011) Solid pseudopapillary tumor of the pancreas: can malignancy be predicted? Surgery 149: 625-634.
- Shi C1, Daniels JA, Hruban RH (2008) Molecular characterization of pancreatic neoplasms. AdvAnatPathol 15: 185-195.
- Li P, Wang Y, Zhang Q, Liu Y, Lv Y, et al. (2012) A noninvasive mucinous cystic neoplasm with intermediate-grade dysplasia of the pancreas and extensive quamous metaplasia: a case report with clinicopathological correlation. DiagnPathol 7: 89-93.
- Liu BA, Li ZM, Su ZS, She XL (2010) Pathological differential diagnosis of solid-pseudopapillary neoplasm and endocrine tumors of the pancreas. World J Gastroenterol 16: 1025-1030.
- Cavard C, Audebourg A, Letourneur F, Audard V, Beuvon F, et al. (2009) Gene expression profiling provides insights into the pathways involved in solid pseudopapillary neoplasm of the pancreas. J Pathol 218: 201-209.
- Dong A, Wang Y, Dong H, Zhang J, Cheng C, et al. (2013) FDG PET/CT findings of solid pseudopapillary tumor of the pancreas with CT and MRI correlation. ClinNucl Med 38: e118-124.
- Canzonieri V, Berretta M, Buonadonna A, Libra M, Vasquez E, et al. (2003) Solid pseudopapillarytumour of the pancreas. Lancet Oncol 4: 255-256.
- Michels NA (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 112: 337-347.
- Adamthwaite JA, Pennington N, Menon KV (2007) Anomalous hepatic arterial anatomy discovered during pancreaticoduodenectomy. SurgRadiolAnat 29: 269-271.
- Spanknebel K, Conlon KC (2001) Advances in the surgical management of pancreatic cancer. Cancer J 7: 312-323.
- Papavramidis T1, Papavramidis S (2005) Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am CollSurg 200: 965-972.
- Campbell F, Azadeh B (2008) Cystic neoplasms of the exocrine pancreas. Histopathology 52: 539-551.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14294
- [From(publication date):
November-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9875
- PDF downloads : 4419