Editorial Open Access
Solid Oxide Fuel Cell
Mridula Biswas*
Korea Institute of Science and Technology, Seoul, South Korea
- *Corresponding Author:
- Mridula Biswas
Korea Institute of Science and Technology
Seoul, South Korea
Tel: +919836050800
E-mail: luckymridula@gmail.com
Received Date: July 17, 2013; Accepted Date: July 18, 2013; Published Date: July 19, 2013
Citation: Biswas M (2013) Solid Oxide Fuel Cell. J Powder Metall Min 2:e114. doi: 10.4172/2168-9806.1000e114
Copyright: © 2013 Biswas M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Engineers, scientists and environmentalists have been working on the development of clean energy technology. Now-a-days widely available clean energy sources are solar panels and wind mills. The problem associated with them is the stability of the source since solar energy and wind energy cannot be available in night time and cloudy days, and windless days, respectively. Therefore, researchers are always in search of another energy source. In 1962, Scientists at Westinghouse Electric Corporation first announced that electricity can be successfully generated using solid electrolyte. The new device using this solid electrolyte is known as solid oxide fuel cell (SOFC).
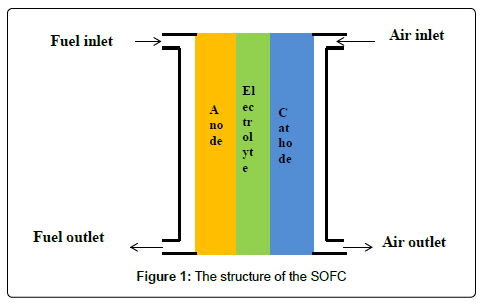
SOFC is a solid electrochemical conversion device which directly converts chemical energy of fuel into electrical energy. In this device, fuel is oxidized with the help of oxygen; air is used as the source of oxygen. The advantage of this fuel cell is that it does not contain liquid electrolyte, so there is no chance of liquid flooding. The primary structure of unit cell consists of anode, cathode and electrolyte; interconnect is necessary for assembling many cells together and sealant is another optional component for planner type of SOFC to prevent mixing of fuel and air.Electrolyte is nonporous to avoid mixing of fuel and air directly while the electrodes are porous to allow fuel and air to come in contact with triple phase boundary (TPB) where reaction takes place between fuel gas and oxidant in presence of electrode and electrolyte; electrolyte must be ion conducting along with electronically non-conducting while the electrodes must be both ionic and electronically conductive to facilitate current collection (in cathode) and catalytic activities.
There are several types of SOFC. Based on the cell support, SOFC is divided into three types: electrolyte, cathodeand anode-supported. In case of electrolyte supported cell, electrolyte must be thick enough to hold the load of the whole cell. On the other hand, thick electrolyte provides huge amount of ohmic resistance which reduces cell performance. Therefore, researchers are more interested in electrode supported cell. Cathode-supported cell is not also finding potential application because of its poor support. Oxygen is passed through cathode; volume of oxygen molecule is higher than that of hydrogen. Therefore, larger pores are required in cathode than in anode. Large pore is not desirable for the component which is intended to provide mechanical strength to the cell. Therefore, cathode supported cell is almost abandoned. Anode-supported SOFCs are widely used. Hydrogen molecule passes through anode;since its molecular size is too small it can easily pass through very small pores. Therefore, anode with small pores can provide good mechanical strength to the cell. Based on the design consideration, SOFCs are divided into several categories: planner, tubular (sealant not required), single chamber, symmetric, honeycomb, thin film, etc (Figure 1).
The role of electrolyte is crucial for ion conduction. Electrolyte conducts either O2- ion or H+ ion.Based on the type of ion conduction through electrolyte, SOFCs are divided into two categories: O-SOFC (oxygen ion conducting) and P-SOFC (proton conducting). As per the type of ion, direction of ion flow is decided. If the conducting ion be O2-, it will flow from cathode to anode side; therefore reaction site will be at the junction of electrolyte and cathode. The phenomenon is reversed if the electrolyte conducts H+ ion.
Nowadays, all the major three components of SOFC are engineered to be composite, either layered or particulate. This type of composite is called functionally graded material. The state-of-the-art materials for O-SOFC areyttria stabilized zirconia (YSZ) as electrolyte, lanthanum strontium manganite (LSM) as cathode and YSZ-Ni cermet as anode. YSZ requires high operation temperature (1073-1273K) for conducting sufficient amount of oxygen ion (around 0.1S/cm), which causes severe problems such as electrode sintering resulting in the reduction of TPB for reaction, formation of electronically insulating phase such as pyrrochlore (La2Zr2O7) due to the interfacial diffusion between electrolyte and lanthanum containing cathode, and mechanical and thermal stress. The advantage of YSZ material is that it can withstand a wide range of oxygen partial pressure (1-10-21atm). Another potential electrolyte material is doped ceria; they are application because of high ionic conductivities (0.128S/cm to 0.199S/cm at 1023 K); but the disadvantage is that these get reduced under low oxygen partial pressure(10-19atm at 973K) and at high temperature (873-1073K). Therefore, it is difficult to use ceria at the anode side, i, e., oxygen deficient side due to almost no oxygen partial pressure. Therefore, to utilize the advantages of both the materials, the concept of composite electrolyte has come into the picture. YSZ is used as a dense layer at the anode side while doped ceria electrolyte, especiallygadolinia doped ceria (GDC), is used as second layer of electrolyte on the cathode side. To avoid thermal and mechanical stresses generated out of difference in coefficients of thermal expansion (CTE) of two different materials, cathode has been engineered to be a bi-layer composite: 1st layer: GDCLSM and 2nd layer: LSM. GDC-LSM provides more TPB for reaction as well as CTE matching to GDC as well as LSM.Recent trend is to modify the surface of cathode materials with some catalysts since surface is responsible for reduction of oxygen to oxide ion.This modification is carried out with the infiltration of catalyst solution to the cathode layer. Anode is also composed of two layers: anode support and functional layers in case of anode supported cell. Since support layer should have strong enough to support the whole cell, as well as porous to facilitate gas permeation, the support layer should be thicker. On the other hand, there must be good network among Ni particles to facilitate electronic conduction; therefore, anode functional layer becomes necessary.
The other two additional components are interconnect and sealant. Interconnect material connects anode of one cell to the cathode of other cell. Interconnect materials must conduct electron, but no ion. In the operating temperature and environment, they must retain their shape, mechanical properties and microstructure. Since these materials are exposed to both oxidizing and reducing atmospheres, they should not undergo any oxidative or reductive reaction. They must be impermeable to gas to prevent mixing of fuel and oxygen directly. They should be inert enough not to diffuse into adjacent fuel cell components. Ferritic stainless steel and doped lanthanum chromates are most widely used interconnect materials.
Sealant has the most important role in preventing the dangerous water formation reaction inside the stack. It prevents mixing of fuel and oxidant inside the stack and leakage from the stack. Besides these, it also isolates cell in the stack. It must be chemically compatible to other components and structurally stable.
The main drawback of O-SOFC is its high operational temperature. Therefore, researchers are searching for alternative materials, processing technology and modification of materials properties. The major polarization loss components come from electrolyte and cathode materials. Nowadays scientists are putting lot of efforts in making electrolyte as thin as possible and trying to modify the surface characteristic of cathode. Protonic ceramic fuel cell (PCFC or P-SOFC) is one of the alternatives.
In case of P-SOFC, electrolyte materials areyttria doped barium cerate (BCY) and barium zirconate (BZY). BCY (0.014S/cm at 973K) has higher conductivity than BZY (7.9 × 10-3S/cm at 873K). But the basic problem with BCY is that it is not stable in air because of carbon dioxide while BZY is stable in air. BZY powder has very low sinterability; therefore, sintering aids such as CuO, ZnO, NiO, etc. are necessary. These sintering aids also causes second phase formation which lowers down the conductivity. Therefore, the development of this SOFC has become challenge for the scientists. The advantage of this SOFC is that it can generate electricity at lower temperature than can O-SOFC. However, for this SOFC, the state-of-the-art materials are BZY-Ni as anode, BZY as electrolyte and lanthanum strontium cobalt ferrite (LSCF) as cathode. These three components are also bilayer composite. The cell is made of six layers: anode support (BZY-Ni), anode functional layer (BZY-Ni), electrolyte (BZY, BCY-two separate layer), cathode functional layer (BCY-LSCF) and cathode current collector layer (LSCF).
Various esteemed research groups are worldwideworking on this technology. NSERC solid oxide fuel cell Canada strategic research network has been formed in 2008. This network includes 21 research groups from Canada. Pacific Northwest National Laboratory (PNNL, USA) has announced that SOFC can achieve 57% efficiency in 2012while the efficiency reported previously was 30-50%. Risø DTU National Laboratory, Denmark has long been working in this field. Bloom Energy is commercializing SOFC in a cost effective way. GE Global Research team gave demonstration on 3-10kW SOFC system to be a highly efficient and future reliable power plant. Besides these, there are several other research laboratories such as Naval Materials Research Laboratory-India, CSIR-Central Glass and Ceramic Research Institute- India, Bhabha Atomic Research Centre-India, International Advanced Research Centre for Powder Metallurgy and New Materials-India, Korea Institute of Science and Technology-South Korea, World Class University-South Korea, National Institute of Materials Science-Japan, Max Planck Institute for Solid State Research-Germany, Lawrence Berkeley National Laboratory-USA, Center for Innovative Fuel Cell and Battery Technologies, Georgia Institute of Technology, Global Climate and Energy Program of Stanford University, Massachusetts Institute of Technology, California Institute of Technology, King Abdullah University of Science and Technology-Saudi Arabia, etc. Submit
--Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 14213
- [From(publication date):
September-2013 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9645
- PDF downloads : 4568