Soil Net Nitrogen Mineralization at Different Ecosystem Development Stages after the Year 2000 Eruption on Miyakejima Island
Received: 02-Feb-2018 / Accepted Date: 19-Feb-2018 / Published Date: 27-Feb-2018 DOI: 10.4172/2157-7625.1000250
Abstract
Soil nitrogen (N) mineralization is a central process in the N cycle in terrestrial ecosystems. Previously many studies were conducted on soil N mineralization in terrestrial ecosystems, but those studies remain unclear due to large spatial and temporal variations. In present study soil N mineralization rates were measured in situ by using a resin core technique. The study reports the relationship of these rates with environmental factors at ten sites with various vegetation and soil properties which formed after the latest eruption in year 2000 on a volcanic island, Miyakejima. Miyakejima has diverse ecosystems, from grasslands with little soil organic matter to mature forests. With little damage from the year 2000 eruption, it is suitable for exploration of spatial and temporal variations in soil N mineralization. Annual soil N mineralization rates ranged from 0.9 to 52.5 kg N ha-1 yr-1 and were higher in the presence of the N-fixing vegetation Alnus sieboldiana. Present study data along with other data obtained from insitu observation suggested that soil C/N ratio can be a good indicator of annual soil N mineralization rate, like many previous studies pointed out; however, the relationship between the rate and soil C/N ratio was complicated due to some factors, such as existence of N-fixing vegetation and high sulfur dioxide gas exposure.
Keywords: Nitrogen mineralization; Spatial variation; Vegetation types, Nitrogen fixing plants; C/N ratio; Volcanic island; Miyakejima
Introduction
Nitrogen (N) is one of the vital nutrients for life [1]. It often limits primary production in terrestrial ecosystems [2,3], particularly in ecosystems that have newly established after severe disturbances, such as volcanic eruption [4] and glacial recession [5]. Although it is an abundant element of Earth’s atmosphere and soil, but it is present in the unavailable form to plants. Nitrogen becomes available for plants largely through N mineralization processes in which organic N is converted to inorganic forms, such as NH4+ and NO3- which can be taken up through plant roots. Thus, N mineralization is one of the most important processes for plant growth [6,7]. Because of the importance of N mineralization in the soil, recently soil N mineralization rates and the factors that control it have been measured in situ in various ecosystems: these in situ studies reported huge variations in both time and space [8-10].
Nitrogen mineralization occurs in series which is controlled by many biological processes. Like soil N mineralization rates have been considered to vary with abiotic factors in soil, such as soil temperature; soil moisture content [11,12]; and other soil properties, such as C:N ratio [13,14] and soil organic matter (SOM) [15]. Plants have been also recognized as important in the regulation of soil N mineralization rate because plants provide not only organic matter from both above and below-ground parts, but also habitat for soil microbes. For example, Björk et al. [16] investigated linkages between N turnover including N mineralization and plant community structure in an alpine tundra in Sweden: they showed an indirect effect of plant community on N mineralization. Bengston et al. [17] demonstrated a relationship between plant diversity, productivity, and soil gross N mineralization rate in a mixed beach-oak forest. In a wet grassland in France, Rossignol et al. [18] suggested that plant type altered soil N mineralization via regulation of soil microbial activity.
As for long-term variation in N mineralization rate, succession initiated by severe disturbances may be considered to be important factors, since soil and plant properties show drastic change over long time periods. Plant development and changes in the availability of soil nutrients along with succession have been described by many studies [5,6,19-22]. Vitousek et al. [21] reviewed various studies of soil N mineralization rate in primary successions that occur over decades to hundreds of years and demonstrated that soil N mineralization rate showed no constant change along successional gradients. Binkley et al. [23] reported that soil N mineralization rate on riverside terraces did not change directionally along a succession. Hence, long-term patterns of soil N mineralization rate along successional gradients and underlying mechanisms remain unclear due to insufficient in situ observation data.
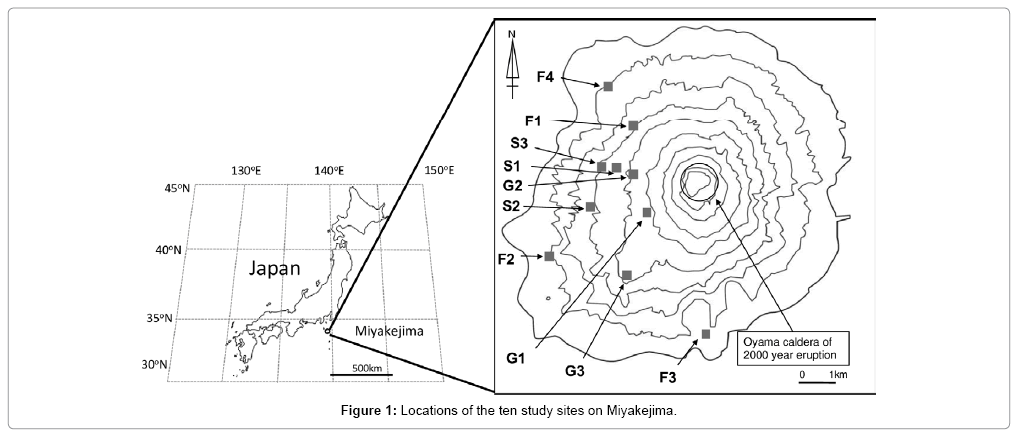
For present study, site was Miyakejima, a volcanic island near the western rim of the Pacific Ocean; it offers an opportunity to describe how soil N mineralization rate differs among diverse ecosystems from grasslands produced after the latest eruption in 2000 to mature forests. Historical records of eruptions of this island extend back to 1643, over which time there were seven eruptions [24]. These catastrophic disturbances initiated succession; consequently, on Miyakejima there are diverse ecosystems with various types of vegetation and soil properties [25,26]. Also there is a unique shrub, alder (Alnus sieboldiana), which is a N-fixing plant [5]. This shrub species can facilitate plant development [25] by increasing N mineralization and N input to the soil [27-29]. So it was hypothesized that soil N mineralization rate at the shrub dominant sites on the island would be higher than those at other sites.
The study was aimed to clarify the relationships between in situ soil N mineralization and environmental factors at various sites on Miyakejima. In this study, ten sites were established where soil N mineralization rate can be measured and so that factors controlling the mineralization can be explored. Using such sites level data, soil N mineralization rates were estimated at typical vegetation types, grassland, shrub land, and forest of Miyakejima and were compared the rates with those in other ecosystems.
Materials and Methods
Site description
The study was conducted on Miyakejima (34°05′N, 139°31′E) near the western rim of the Pacific ocean, about 180 km south of Tokyo, Japan. The annual mean temperature and precipitation from 2005 to 2014 were: 17.7°C and 2948 mm. The island is an active volcano and the most recent eruption was in 2000; in the summer of that year, the eruption at the summit of Mt. Oyama produced total 9.3 × 106 m3 (dense-rock equivalent) deposits, then formed a new collapsed crater [30]. Harmful gases emission and volcanic ash deposition from the 2000 eruption severely damaged vegetation, especially around the crater [26]. Although the volcanic activity including emission of the harmful gases, such as sulfur dioxide (SO2) has continued, ecosystems damaged by the 2000 eruption have been undergoing restoration succession. Since the type and degree of damage differed among areas, there are various ecosystems on Miyakejima. Ten sites were selected that having various vegetation composition and coverage (Figure 1). All of the sites were located on the western half of Miyakejima to avoid high SO2 gas concentrations to the east. Vegetation distribution in each site was visually observed as coverage by each species from understory, mid-height, to canopy level during June to August 2013 (Table 1; Kamijo and others, unpublished data). In the context of this study, the ten sites were converted to three types of vegetation according to vegetation composition of each site; grassland, shrubland, and forest. Sites G1, G2 and G3 were classified as grassland, because Miscanthus condensatus dominated. At site G1, vegetation was only scattered M. condensatus patches with absence of litter and organic accumulation. Sites G2 and G3 were dominated by the grasses M. condensatus and Fallopia japonica. Sites S1, S2 and S3 were classified as shrubland. The dominant shrub in the shrubland was the N-fixing alder, Alnus sieboldiana. Sites F1, F2, F3 and F4 were classified as forest. The sites F1 and F2 were dominated by Machilus thunbergii trees. Sites F3 and F4 were dominated by Castanopsis sieboldii trees (Table 1). In each site, a 10 m × 10 m permanent plot was established in 2011, and this study was conducted in these plots. Site information including the dominant vegetation species, vegetation coverage, and soil properties were shown in Tables 1 and 2.
| Site | Main vegetation species | Summation of coverage (%) | ||
|---|---|---|---|---|
| G1 | Miscanthus condensatus (25) | Carex oshimensis* | Smilax china* | 25 |
| G2 | Miscanthus condensatus (33) | Reynoutria japonica (33) | Calamagrostis autumnalis (22) | 70 |
| G3 | Miscanthus condensatus (33) | Machilus thunbergii (22) | Eurya japonica (33) | 120 |
| S1 | Alnus sieboldiana (22) | Miscanthus condensatus (55) | Dryopteris caudipinna (22) | 125 |
| S2 | Alnus sieboldiana (55) | Machilus thunbergii (22) | Carex oshimensis (22) | 135 |
| S3 | Alnus sieboldiana (44) | Miscanthus condensatus (33) | Dryopteris caudipinna (22) | 185 |
| F1 | Machilus thunbergii (22) | Dendropanax trifidus (33) | Eurya japonica (22) | 135 |
| F2 | Machilus thunbergii (33) | Trachelospermum asiaticum (33) | Camellia japonica (33) | 160 |
| F3 | Castanopsis sieboldii (55) | Trachelospermum asiaticum (33) | Camellia japonica (33) | 180 |
| F4 | Castanopsis sieboldii (55) | Trachelospermum asiaticum (44) | Camellia japonica (33) | 205 |
Summation of coverage was calculated as the sum of total coverage of all vegetation species; Figures in brackets denote vegetation species coverage (%); * coverage < 1%.
Table 1: Vegetation character of study sites.
| Site | Frequency of SO2 concentration greater than 1 ppm (%) | VAD (cm) | ST (°C) | SP (%) | SOM (%) | TC (%) | TN (%) | C/N ratio |
|---|---|---|---|---|---|---|---|---|
| G1 | 19.7 | 28 | 15.8 (7.6) | 51.9 (3.9) | 2.1 (0.7) | 0.4 (0.3) | 0.1 (0.0) | 5.6 (3.3) |
| G2 | 6.7 | 43 | na | 59.4 (4.5) | 2.2 (0.1) | 0.5 (0.4) | 0.1 (0.0) | 6.6 (4.7) |
| G3 | 18.7 | 12 | 15.0 (7.1) | 69.9 (9.3) | 5.4 (6.0) | 1.9 (0.9) | 0.1 (0.0) | 13.6 (2.7) |
| S1 | 5.5 | 38 | na | 64.4 (9.5) | 6.1(1.9) | 2.3 (2.2) | 0.2 (0.1) | 11.3 (3.9) |
| S2 | 7.5 | 23 | 15.2 (6.4) | 76.9 (6.9) | 11.4 (4.5) | 4.8 (0.9) | 0.3 (0.1) | 14.6 (0.7) |
| S3 | 5.4 | 20 | 14.7 (6.7) | 61.2 (1.8) | 5.3 (4.5) | 1.7 (0.7) | 0.1 (0.1) | 11.3 (1.0) |
| F1 | 2.4 | 12 | 14.4 (6.6) | 74.6 (5.0) | 9.7 (1.7) | 3.6 (1.5) | 0.2 (0.1) | 16.2 (0.9) |
| F2 | 6.1 | 5 | 16.3 (6.7) | 76.0 (2.6) | 11.4 (5.5) | 5.1 (1.3) | 0.3 (0.0) | 16.0 (2.1) |
| F3 | 7.9 | 0 | 16.4 (6.7) | 76.7 (1.9) | 17.0 (3.3) | 8.5 (2.6) | 0.6 (0.2) | 15.2 (0.6) |
| F4 | 2 | 0 | 16.3 (6.3) | 75.7 (3.6) | 18.7 (1.4) | 9.1 (1.1) | 0.7 (0.1) | 14.0 (0.3) |
SO2 concentration is indicated as percentage of samples greater than 1 ppm;
VAD: Volcano Ash Depth; ST: Annual Mean Soil Temperature; SP: Soil Porosity; SOM: Soil Organic Matter; TC: Soil Total Carbon; TN: Soil Total Nitrogen; na: Not Available; C/N ratio by weight.
Because of the data logger fault, we missed part of data in G2 and S1sites; Data are mean with standard deviation (SD) in parentheses (n=3).
Table 2: Soil properties and SO2 concentration of study sites.
Net N mineralization rate measurement
In this study, annual N mineralization rate (ANMR) in the soil (expressed as kg N ha-1 yr-1) was defined as the net change in mass of inorganic N forms (NH4+-N, NO3--N and NO2--N) before and after a one-year incubation period from 28 Aug 2013 to 27 Aug 2014. The ANMR in the soil was estimated by the resin core technique [31,32]. The resin core is composed of three polyvinyl chloride (PVC) pipes containing the soil column and resin bags. Briefly, one of the PVC pipes (5.8 cm diameter, 5 cm length) was inserted into the ground to a depth of 5 cm to keep the soil core intact. Both sides of this long PVC pipe were connected to two short PVC pipes (5.8 cm diameter, 1 cm length) with vinyl tape. Resin bags containing 10 g of wet mixed H+ and OH- ion exchange resin were placed tightly into both the top and bottom short PVC pipes. The two short PVC pipes were then covered with polypropylene nets and fastened by vinyl tape to the pipes to minimize disturbance. The upper resin bag traps external inorganic N infiltrating into the core from above, and the lower resin bag traps internal N transported downward from the soil core. For each site, nine replicate resin cores were set up, then reinserted back into the ground and covered with leaf on the surface for in situ field.
Soil properties
Soil temperature (ST) at 5 cm depth was monitored hourly from Aug 2013 to Aug 2014 by sensors (TidbiTv2 Temperature Data Logger, Onset Computer Corporation, Bourne, USA). While setting the resin core, additional three undisturbed soil samples were collected i.e., soil samples per site at 5 cm depth to determine soil organic matter content (SOM), soil total C (TC), total N (TN), and soil porosity (SP). SOM content was determined by loss on ignition with heating of the soil samples to 550°C for 4 h [33]. Total soil C and N contents were determined by a fully automatic CN analyzer (SUMIGRAPH NC- 900, SCAS, Ltd, Tokyo, Japan). The air, water and solid volume were determined using a three-phase meter (DIK-1130, Daiki Rika Kogyo Co., Ltd, JPN). The SP is then calculated as the fraction of the air and water volume in total soil volume. Vegetation character in 2013, SO2 concentration at 1 m height during 2011 to 2012, and volcanic ash depth in 2012 and 2013 were obtained by cooperative study (Kamijo and others, unpublished data).
Statistical analysis
The significance differences of soil ANMR among sites and ANMR, soil SOM, TC, TN and C/N ratio according to vegetation types were test by one-way ANOVA. Different groups were determined by the Duncan’s multiple range tests, and similar groups were designated with the same letters and different groups with different letters. The significance of relationships between ANMR and environmental factors was tested by Pearson bivariate correlation analysis. Statistical analyses were performed with SPSS Statistics, version 22 (IBM Corp, Armonk, NY, USA).
Results
Environmental variables
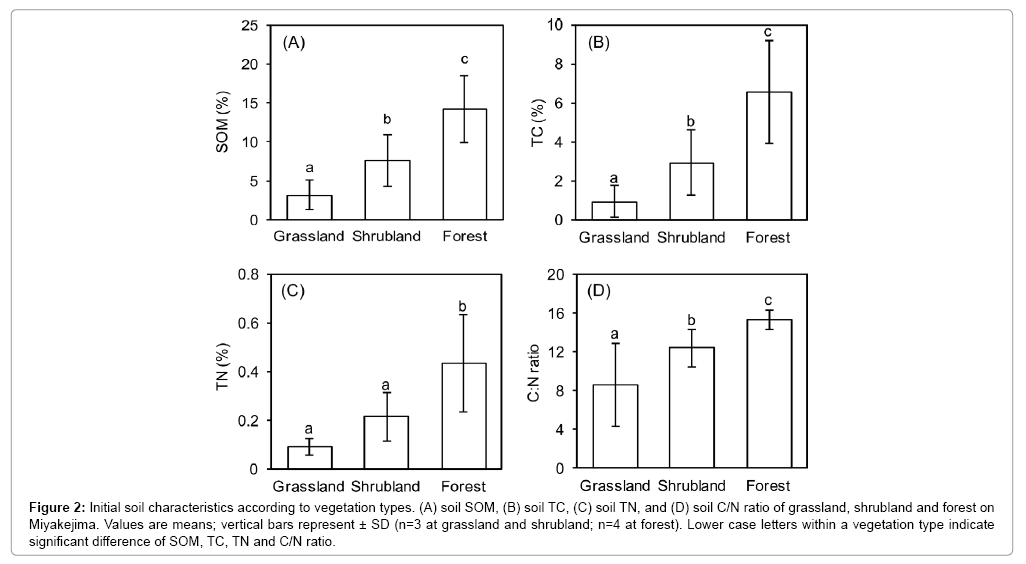
Total vegetation coverage tended to increase from grassland to forest, from G1 to F4 (Table 1). Among sites, SO2 concentrations were more frequently greater than 1 ppm at G1 and G3 than at other sites; consequently, these two sites were more disturbed by the harmful gas (Table 2). Annual mean soil temperature at 5 cm depth (ST) varied slightly among sites (Table 2). The depth of volcanic ash (VAD) varied greatly from 0 cm at F3 and F4, far from the crater, to 43 cm at G2, near the crater. Soil properties, such as SOM, total C, total N, and soil C to N ratio (C/N) also varied greatly among sites (Table 2). SOM was highest at F3 and F4 (18.7% and 17.0%) and lowest at G1 and G2 (2.1, and 2.2%, respectively; Table 2). The patterns of total C and total N were similar to that of SOM. Soil C/N ratios were quite low at G1 and G2 (Table 2). Among the three vegetation types, SOM, TC, TN and C/N ratios gradually increased from grassland to forest (Figure 2).
Figure 2: Initial soil characteristics according to vegetation types. (A) soil SOM, (B) soil TC, (C) soil TN, and (D) soil C/N ratio of grassland, shrubland and forest on Miyakejima. Values are means; vertical bars represent ± SD (n=3 at grassland and shrubland; n=4 at forest). Lower case letters within a vegetation type indicate significant difference of SOM, TC, TN and C/N ratio.
Soil net N mineralization
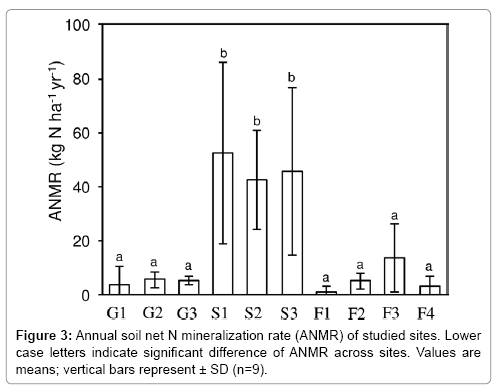
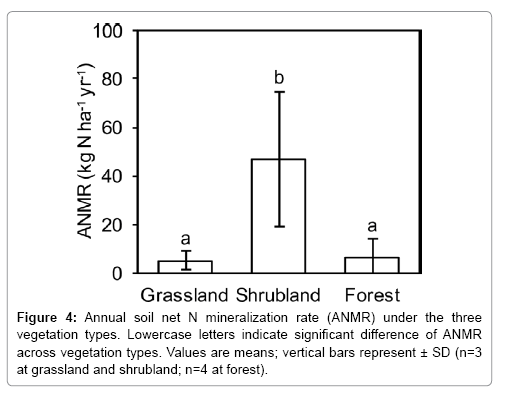
ANMR in the upper 5 cm of soil varied widely among the sites, from approximately 0.9 to 52.5 kg N ha–1 yr–1 (Figure 3). Among all sites, ANMR was significantly higher at S1, S2, and S3 where the N-fixing alder A. sieboldiana was present; ANMR was lowest at F1, where M. thunbergii dominated, but this lowest ANMR did not differ significantly from the other six sites. In addition to the high magnitude of ANMR at the shrubland sites, there was large variation of ANMR within each of these sites, as shown by Figure 3. Mean ANMR among three vegetation types, grassland, shrubland, and forest, ranged from 5 kg N ha–1 yr–1 in grassland to 47 kg N ha–1 yr–1 in shrubland. Mean ANMR was significantly higher in shrubland (Figure 4), but the values in the other two vegetation types did not differ significantly, despite very different environmental factors between the two vegetation stands.
Correlation analyses of ANMR and environmental factors
ANMR was not significantly correlated with VAD, ST, SP, SOM, TN, TC, C/N ratio, or the frequencies of high SO2 concentrations, neither at the site level nor by vegetation types (Table 3). This lack of correlation suggests that rather than a single factor, complex factors regulated ANMR under field conditions on Miyakejima, or other environmental factors would be important for ANMR.
| Frequency of SO2concentration greater than 1 ppm | VAD | ST | SP | SOM | TC | TN | C/N ratio | |
|---|---|---|---|---|---|---|---|---|
| Sites level | -0.233ns | 0.389ns | -0.325 ns | -0.086ns | -0.113ns | -0.132ns | -0.116ns | 0.978ns |
| Vegetation types | -0.397ns | 0.459ns | -0.855ns | -0.022ns | -0.096ns | -0.145 ns | -0.219ns | 0.434ns |
SO2 concentration is indicated as percentage of samples greater than 1 ppm;
VAD: Volcano Ash Depth; ST: Annual Mean Soil Temperature; SP: Soil Porosity; SOM: Soil Organic Matter; TC: Soil Total Carbon; TN: Soil Total N; C/N ratio by weight; ns No significant relationship.
Table 3: Pearson correlations coefficients between ANMR and environmental factors of sites level and vegetation types.
Discussion
Soil N mineralization at site level on Miyakejima varies widely
Various vegetation with different soil properties are fundamental features of Miyakejima (Tables 1 and 2). In the latest eruption, a large amount of volcanic ash and emission harmful gas SO2 disturbed the ecosystems on the upper elevations of the island [34]. Hence, the landscape was reduced almost to bareland (such as at G1) near the crater, but forests far from the crater (such as at F3 and F4) were only minimally damaged; all the sites have been changed continuously. The study reported that the ANMR changed with vegetation and soil conditions at site level (Figure 3 and Table 2). However, ANMR was significantly higher at only three sites (G1, G2, and G3), and the differences among the low values at the other seven sites were not significant (Figure 3). These results indicate that although vegetation coverage and its composition are important controlling factors for ANMR, one-directional ecosystem development from grassland to forest could not sufficiently explain the spatial variation in ANMR on Miyakejima.
Considering the vegetation and soil properties of each site, it was hypothesised that the three sites i.e., S1, S2, and S3 are “hot-spots” for ANMR is because of the presence of N-fixing A. sieboldiana. The results of this study are consistent with those of previous studies which also reported that soil N mineralization beneath stands of such vegetation was high compared with that beneath stands of non-Nfixing vegetation [27,28,35]. Although the mechanisms of higher soil ANMR beneath N-fixing vegetation remain obscure, there are two possible mechanisms. First, N-fixing vegetation, such as alder, can increase input into the soil of easily available organic N obtained from atmospheric inactive N2. Second, the N-fixing vegetation considerably contribute to the supply of organic substrates that are easily decomposed and thereby decrease C/N ratios [36,37]. Thus, the N-fixing vegetation provides both accessible and plenty of resources for soil microbes, and facilitate N mineralization in the soil. Additionally, it was reported that there were quite low ANMR points even under the A. sieboldiana in the shrubland type. The actual range of ANMR from incubated samples from sites S1, S2 and S3 was 7.9 to 107, 14.3 to 67.8, 10.1 to 114.8 kg N ha–1 yr–1, respectively. Such high spatial variation over small distances (cm level) suggests the importance of existence of litter or root nodules of A. sieboldiana due to uneven distribution of the litter fall and root nodules in both above-and under-ground parts. Further study will be needed on the impact of litter and root nodules from N-fixing vegetation, which directly affects ANMR through changes in available N and organic matter at the soil surface, in addition to the impact on ANMR of underground parts of the N-fixing vegetation and related microbes.
Many factors may contribute to the lower range of ANMR at the other seven sites, since soil and vegetation properties differed considerably among the sites. Among these seven sites, two sites (G1 and G2) would be bareland rather than grassland due to low vegetation coverage (Table 1) with low SOM, total C and total N (Table 2). Thus, these two sites can be assumed as the early stage of succession. A number of studies have indicated that net N mineralization rates in early successional stages are low, probably due to scarcity of organic matter and lower substrate quantity [21,38]. Yoshitake et al. [39] demonstrated that soil microbial biomass in the Mt. Fuji volcanic desert in Japan was very low in the early successional stage and strongly correlated with soil total C, total N, and SOM contents. Thus, possible reasons for the very low ANMR at sites G1 and G2 are insufficient resources for N mineralization and lower soil microbial biomass than those at the other sites. But, the reason why ANMR was low in the G3 site with relatively high SOM was different from that in G1 and G2 sites. Although this study could not clarify the mechanisms of lower ANMR in the G3 site, high frequency of high SO2 concentrations might account for the low ANMR at G3 (Table 2). Previous studies have demonstrated that exposure to high SO2 concentrations could inhibit both ammonification [40] and nitrification in acid soil [41]. However, the direct effects of high SO2 concentrations on N mineralization processes were not assessed in the present study; further studies of this are needed.
The other four sites, F1, F2, F3, and F4, including forest type can be regarded as mature ecosystems because of relatively high vegetation coverage, SOM, total C, and total N (Table 2). Another common feature of the four sites was high C/N ratio (16.2, 16.0, 15.2, and 14.0 in F1, F2, F3 and F4, respectively), which may be the reason for the low measured ANMR. Several authors reported that substrate C/N ratio strongly influences N mineralization [14,42]. Booth et al. [43] also suggested that increase in C/N ratio decreases N mineralization. Among other controlling factors leading to the lower ANMR at F3 and F4, the large proportion of mature trees in the total vegetation would be important: the proportion of mature C. sieboldini, one of the climax species on the island, was more than half at F3 and F4 (Table 1). In general, large, mature trees contain a large fraction of recalcitrant substances, such as lignin, which decompose slowly [44]. Hence, in addition to the quantity of substrates for N mineralization, the quality and composition of each substrate may affect ANMR.
Soil N Mineralization at three vegetation types on Miyakejima
The sampling sites on Miyakejima were categorized by vegetation coverage and composition, and soil properties (Tables 1 and 2) can be regarded as three vegetation types. Mean ANMR among the three vegetation types on Miyakejima ranged from 3.0 to 47.0 kg N ha–1 yr–1. With variation in ANMR at site level, the highest ANMR in the shrubland was found in which the N-fixing A. sieboldiana (alder) was the dominant species (Table 1). Since statistical tests show that there were no significant controlling factors (Table 3), the highest ANMR in shrubland could be due to the existence of A. sieboldiana. Many previous studies also reported differences in net N mineralization rate at various vegetation types, such as cropland, grassland, shrubland, and forest [27,28,45,46]. By chronosequences approach using unique sites, such as riverside terrace [47] and volcanic mountain side [48] where can speculate successional change of ecosystem structure, some of the previous studies mentioned about successional change of the net N mineralization rate [23,49]. In present study, if different vegetation type were assumed along with sequential vegetation types in succession then there will be more chance of predicting the long-term change of ANMR with succession, often initiated by volcanic eruption in Miyakejima. Since limited data and information are available at present, further studies will be needed to understand not only spatial but also temporal variation in ANMR related to succession, a common phenomenon, in Miyakejima.
Characteristics of soil N mineralization on Miyakejima
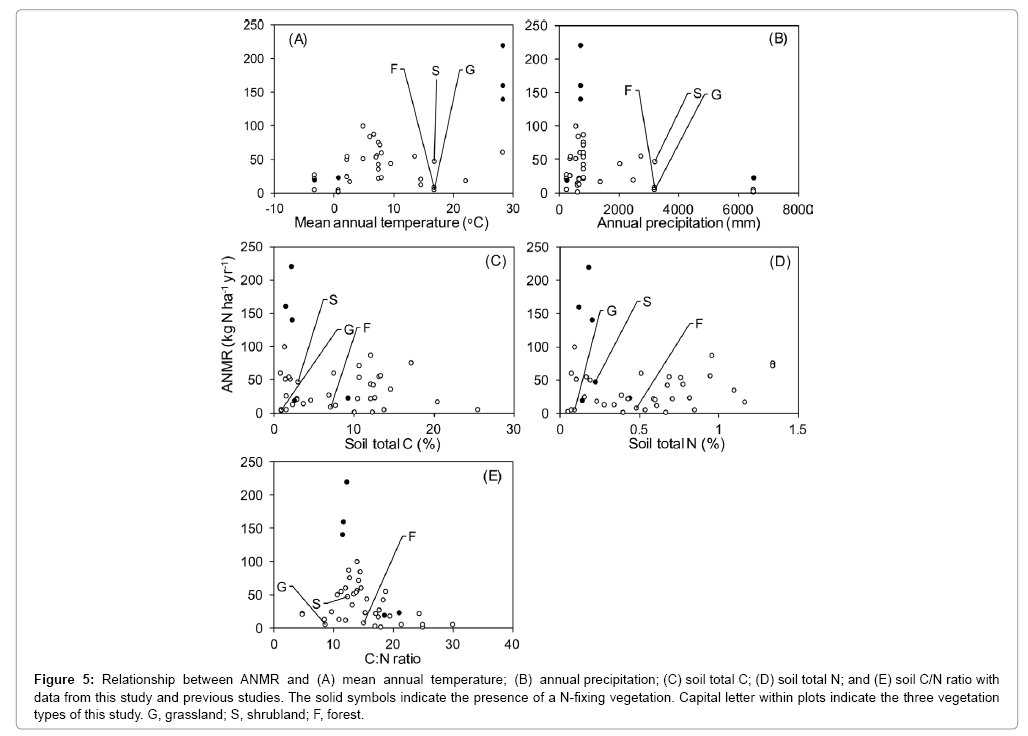
ANMR was reviewed in previous studies and this study (Table 4). Not only the magnitude of ANMR on Miyakejima, but also the range of ANMR are comparable to those from other studies in various ecosystems; also similar were the large spatial variation in ANMR at both plot and vegetation-scale. Similar to the Miyakejima results, ANMR in most stands of N-fixing vegetation are higher than those in stands of non-N- fixing vegetation (Table 4). In plots of ANMR versus environmental factors with data from both previous studies and this study, no significant controlling factors for ANMR were apparent (Figure 5). ANMR tended to increase with annual mean temperature from sub-zero to 30°C, but decrease with annual mean precipitation from 0 to ca. 6200 mm yr–1 (Figure 5). These relationships between N mineralization and temperature and moisture conditions are considered to be common in various processes related to decomposition of organic matter and respiration [50-52]. As for soil N mineralization rate, some studies reported that N mineralization rate tends to decrease under conditions of excess soil moisture [53,54]. Thus, the relatively lower ANMR values on Miyakejima except for that from shrubland were probably due to the high precipitation, ca. more than 3000 mm yr–1.
| Study area | AMT(oC) | AMP(mm) | Method | Dominant vegetation | ANMR (kg ha-1 y-1) |
|---|---|---|---|---|---|
| Temperate climate zone, Japan (Present study) | 16.8 | 3188 | R method (5 cm) |
Miscanthus condensatus Alnus Sieboldiana* Machilus thunbergii and Castanopsis sieboldii |
5.0 47.0 6.1 |
| Subarctic watershed, USA [8] | 0.7 | 650 | R method (7.5 cm) |
Alnus Tenuifolia* Empetrum Nigrum and Vaccinium uliginosum Sphagnum spp. Piceaglauca |
22.5 0.5 4.7 2.7 |
| Boreal ecosystem, Estonia [35] | 6 | 653 | R method (10 cm) |
Alnusincana (L.) Moench* | 84.0 |
| Subarctic, cool temperate zone, warm temperate zone and subtropical, Japan [9] | 2.6- 22.0 |
1391 2047 2737 2498 |
R method (5 cm) |
Tsuga Diversifolia and Larix Kaempferi Cryptomeria japonica and Faguscrenata Chamaecyparis obtuse and Castanopsis sieboldii Castanopsis sieboldii |
16.2 43.1 54.9 18.2 |
| Semiarid subtroptical savanna zone, USA [62] | 28.3 | 720 | R method (10 cm) |
Herbaceous Prosopis glandulosa * (Clusters) Prosopis glandulosa * (Groves) Prosopis glandulosa * (Woodland) |
60.0 220.0 160.0 140.0 |
| Continental climate zone, USA [63] | −3.3 | 269 | B method (10 cm) |
Salix interior; etc Alnus tenuifolia * Populus balsamifera Picea glauca Picea mariana |
5.0 19.0 26.5 21.5 5.0 |
| Cool temperatures, low precipitation and temporal soil freezing, Japan [10] | 6.2 | 821 | B method (10 cm) |
Quercus crispula and Acer pictumsubsp. mono Larix kaempferi |
42.0 62.5 |
| High altitude ecosystem, Turkey [64] | 3.0- 28.0 | 615 | B method (5 cm) |
Aegilops umbellate Juniperus sabina Pinus nigra ssp. pallasiana |
13.2 10.8 0.6 |
| Boreal ecosystem, Estonian [65] | 4.8 | 575 | B method (10 cm) |
Betula. Pendula Elytrigia repens; etc |
99.0 51.0 |
| Subtropical zone, Japan [66] | 14.0 | 3410 | B method (10 cm) |
Cryptomeria japonica | 51.8 |
| Mediterranean climate zone, Turkey [67] | 14.6 | 697 | B method (5 cm) |
Ouercusrobur L. subsp. robur (natural) Ouercusrobur L. subsp.robur (thinned) Pinuspinea L |
21.0 20.0 12.0 |
| Semiarid monsoon zone, China [68] | 2.1 | 385 | P method (10 cm) |
Stipa krylovii and Artemisia frigida Artemisia scoparia Zea mays and Linumusitatissimum |
24.6 50.0 55.0 |
| USA [69] | M method | Grassland High elevation forests Low elevation low temperature forests Low elevation high temperature |
14.3 22.6 55.6 82.9 |
R:Resin core method; B: Buried bag method; P: PVC tube incubation method; M: Model estimate method; Values in parentheses show the incubation depth in soil.
*Nitrogen-fixing vegetation.
Table 4: ANMR in this study compared to those from other ecosystems.
Figure 5: Relationship between ANMR and (A) mean annual temperature; (B) annual precipitation; (C) soil total C; (D) soil total N; and (E) soil C/N ratio with data from this study and previous studies. The solid symbols indicate the presence of a N-fixing vegetation. Capital letter within plots indicate the three vegetation types of this study. G, grassland; S, shrubland; F, forest.
Soil C/N ratio is commonly considered to be a good indicator of N mineralization [55]. Higher soil C/N ratios are generally thought to hinder mineralization [56]. When the C/N ratio of substrate is low (12- 20:1) decomposers are not N limited, and a net release of inorganic N to the soil solution occurs [57]. The majority of soil C/N ratios that were measured on Miyakejima, are within this range and often N was mineralized in these soils. The relationship between ANMR and soil C/N ratios would show an appealing single-peak curve, which is ANMR was also low under low soil C/N ratios less than ca. 10. This is different from the general understanding that low soil C/N ratios promote N mineralization [58]. The mechanisms that keep low N mineralization under low soil C/N ratios remain unclear, even though several factors seem to be involved, such as microbial properties [59,60], vegetation properties [61-69], and extraordinary disturbance like high SO2 gas exposure in volcanic area of current study.
In-situ data demonstrated that ANMR in the Miyakejima, reported the huge spatial variation and suggest that the spatial variation could not be explained by single environmental factor though, could be partly explained by some unique factors in the volcanic island, such as existence of N-fixing and high SO2 gas exposure.
Conclusions
In this study soil N mineralization rate were in-situ at a series of sites that were disturbed to various extents by the latest 2000 eruption on the volcanic island Miyakejima. ANMR varied greatly among sites, from about 0.9 to 52.5 kg N ha–1 yr–1. The highest rates were at sites where the N-fixing vegetation A. sieboldiana was present as the dominant species. Low ANMR was observed in grassland and forest-vegetation type; the former would be due to lower SOM, high SO2 concentration, or both and the latter would be due to relatively high C/N ratio. Compared to previously published studies of other locales, the relatively lower ANMR were observed on Miyakejima (except for those at shrubland) were due to the high precipitation. A unique pattern between ANMR and soil C/N ratio, which ranged from about 5 to 30, suggests that the soil C/N ratio can be a good indicator for in situ ANMR.
Acknowledgments
The authors would like to acknowledge the Ministry of the Environment, Japan. We also thank the Miyakejima Nature Center, Akakokko station, and Miyakejima village office for valuable information.
References
- Sterner RW, Elser JJ (2002) Ecological stoichiometry: The biology of elements from molecules tobiosphere. Princeton (NJ): Princeton University Press.
- Vitousek PM, Howarth R(1991)Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 13: 87-115.
- LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystemsis globally distributed. Ecology 89: 371-379.
- Vitousek PM, Walker LR, Whiteaker LD, Matson PA (1993) Nutrient limitations to plant growth during primary succession in Hawaii Volcanoes National Park. Biogeochemistry 23:197-215.
- Chapin FS, Walker LR, Fastie CL, Sharman LC (1994) Mechanisms of primary succession followingdeglaciation at Glacier Bay, Alaska. Ecol Monogr 64: 149-175.
- Chapin FS, Matson PA, Vitousek PM (2011) Principles of terrestrial ecosystem ecology. New York (NY):Springer-Verlag.
- Pastor J, Aber JD, McClaugherty CA, Melillo JM (1984) Aboveground production and N and P cycling along a nitrogen mineralization gradienton Black hawk Island, Wisconsin. Ecology 65: 256-268.
- Hart SC, Gunther AJ (1989) In situ estimates of annual net nitrogen mineralization and nitrification in asubarctic watershed. Oecologia 80: 284-288.
- Hirai K, Kaneko S, Takahashi M (2007) Nitrogen mineralization of forest soil along the climate in Japan:estimation of rate of nitrogen mineralization in the field by soil properties, temperature and soil type. Jpn J For Environment 49: 123-131 (in Japanese).
- Hishi T, Urakawa R, Tashiro N, Maeda Y, Shibata H (2013) Seasonality of factors controlling Nmineralization rates among slope positions and aspects in cool-temperate deciduous natural forests andlarch plantations. Biol Fertil Soils 50: 343-356.
- Gonçalves JLM, Carlyle JC (1994) Modelling the influence of moisture and temperature on net nitrogenmineralization in a forested sandy soil. Soil Biol Biochem. 26: 1557-1564.
- Schütt M, Borken W, Stange CF, Matzner E (2014) Substantial net N mineralization during the dormantseason in temperate forest soils. J Plant Nutr Soil Sci 177: 566-572.
- Kitayama K (1996) Soil nitrogen dynamics along a gradient of long-term soil development in a Hawaiianwet montane rainforest. Plant Soil 183: 253-262.
- Bengtsson G, Bengtson P, MÃ¥nsson KF (2003) Gross nitrogen mineralization-, immobilization-, andnitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol Biochem 35: 143-154.
- Hofman G, Ossemerct C, Ide G, Van Ruymbeke M (1986) Nitrogen supply from some soil types withvarious organic-matter treatments. Plant Soil 91: 411-415.
- Björk RG, Klemedtsson L, Molau U, Harndorf J, Ödman A, et al. (2007) Linkages between N turnoverand plant community structure in a tundra landscape. Plant Soil 294: 247-261.
- Bengtson P, Falkengren-Grerup U, Bengtsson G (2006) Spatial distributions of plants and gross Ntransformation rates in a forest soil. J Ecol 94: 754-764.
- Rossignol N, Bonis A, Bouzillé JB (2006) Consequence of grazing pattern and vegetation structure on thespatial variations of net N mineralisation in a wet grassland. Appl Soil Ecol 31: 62-72.
- Walker LR, del Moral R (2003) Primary succession and ecosystem rehabilitation. Cambridge (UK):Cambridge University Press.
- Tilman D (1987) Secondary succession and the pattern of plant dominance along experimental nitrogengradients. Ecol Monogr 57: 189-214.
- Vitousek PM, Matson PA, Van Cleve K (1989) Nitrogen availability and nitrification during succession:Primary, secondary, and old-field seres. Plant Soil 115: 229-239.
- Boggs K, Weaver T (1994) Changes in vegetation and nutrient pools during riparian succession. Wetlands14: 98-109.
- Binkley D, Suarez F, Stottlemyer R, Caldwell B (1997) Ecosystem development on terraces along theKugururok River, northwest Alaska. Ecoscience 4: 311-318.
- Kato T, Kamijo T, Hatta T, Tamura K, Higashi T (2005) Initial soil formation processes of volcanogenousregosols (scoriacious) from Miyake-jima island, Japan. Soil Sci Plant Nutr 51: 291-301.
- Kamijo T, Kitayama K, Sugawara A, Urushimichi S, Sasai K (2002) Primary succession of the warm-temperate broad-leaved forest on a volcanic island, Miyake-jima, Japan. Folia Geobot 37: 71-91.
- Kamijo T, Hashiba K (2003) Ecosystem and vegetation dynamics before and after the 2000-year eruptionon Miyake-jima Island, Japan, with implications for conservation of the Island’s ecosystem. Glob EnvironRes 7: 69-78.
- Hart SC, Binkley D, Perry DA (1997) Influence of red alder on soil nitrogen transformations in twoconifer forests of contrasting productivity. Soil Biol Biochem 29: 1111-1123.
- Myrold DD, Huss-Danell K (2003) Alder and lupine enhance nitrogen cycling in a degraded forest soil inNorthern Sweden. Plant Soil 254: 47-56.
- Perakis SS, Matkins JJ, Hibbs DE (2012) N2-fixing red alder indirectly accelerates ecosystem nitrogencycling. Ecosystems 15: 1182-1193.
- Nakada S, Nagai M, Kaneko T, Nozawa A, Suzuki-Kamata K (2005) Chronology and products of the2000 eruption of Miyakejima Volcano, Japan. Bull Volcanol 67: 205-218.
- DiStefano JF, Gholz HL (1986). A proposed use of ion exchange resins to measure nitrogenmineralization and nitrification in intact soil cores. Commun Soil Sci Plant Anal 17: 989-998.
- Shibata H, Urakawa R, Toda H, Inagaki Y, Tateno R, et al. (2011) Changes in nitrogen transformation in forest soil representing the climate gradient of the Japanese archipelago. J For Res 16: 374-385.
- Heiri O, Lotter AF, Lemcke G (2011) Loss on ignition as a method for estimating organic and carbonatecontent in sediments: reproducibility and comparability of results. J Paleolimnol 25: 101-110.
- Nakada S, Nagai M, Yasuda A, Shimano T, Geshi N, et al. (2001) Chronology of the Miyakejima 2000eruption: Characteristics of summit collapsed crater and eruption products. J Geogr 110: 168-180 (inJapanese).
- Uri V, Lõhmus K, Tullus H (2003) Annual net nitrogen mineralization in a grey alder (Alnus incana (L.)moench) plantation on abandoned agricultural land. For Ecol Manage 184: 167-176.
- Zavitkovski J, Newton M (1971) Litterfall and litter accumulation in red alder stands in western Oregon.Plant Soil 35: 257-268.
- Clein JS, Schimel JP (1995) Nitrogen turnover and availability during succession from alder to poplar inAlaskan taiga forests. Soil Biol Biochem 27: 743-752.
- Berendse F, Lammerts EJ, Olff H (1998) Soil organic matter accumulation and its implications fornitrogen mineralization and plant species composition during succession in coastal dune slacks. Plant Ecol137: 71-78.
- Yoshitake S, Fujiyoshi M, Watanabe K, Masuzawa T, Nakatsubo T, et al. Successional changes in the soilmicrobial community along a vegetation development sequence in a subalpine volcanic desert on MountFuji, Japan. Plant Soil 364: 261-272.
- Lettl A (1984) The effect of atmospheric SO2 pollution on the microflora of forest soils. Folia Microbiol29: 455-475.
- Labeda DP, Alexander M (1978) Effects of SO2 and NO2 on nitrification in soil. J Environ Qual 7: 523-526.
- Mack MC, D’Antonio CM (2003) Exotic grasses alter controls over soil nitrogen dynamics in a Hawaiianwoodland. Ecol Appl 13: 154-166.
- Booth MS, Stark JM, Rastetter E (2005) Controls on nitrogen cycling in terrestrial ecosystems: a syntheticanalysis of literature data. Ecol Monogr 75: 139-157.
- McGee GG, Mitchell MJ, Leopold DJ (2007) Relationships among forest age, composition and elementaldynamics of Adirondack northern hardwood forests. J Torr Bot Soc 134: 253-268.
- Sánchez L F, GarcÃa-Miragaya J, Chacón N (1997) Nitrogen mineralization in soils under grasses andunder trees in a protected Venezuelan savanna. Acta Oecol 18: 27-37.
- Yang LL, Zhang FS, Mao RZ, Ju XT, Cai XB, et al. (2008) Conversion of natural ecosystems to croplandincreases the soil net nitrogen mineralization and nitrification in Tibet. Pedosphere 18: 699-706.
- Walker LR, Zasada JC, Chapin III FS (1986) The role of life history processes in primary succession on anAlaskan floodplain. Ecology 67: 1243-1253.
- del Moral R, Chang CC (2015) Multiple assessments of succession rates on Mount St. Helens. Plant Ecol216:165-176.
- Riley RH, Vitousek PM (1995) Nutrient dynamics and nitrogen trace gas flux during ecosystem development in montane rain forest. Ecology 76: 292-304.
- Fujita Y, van Bodegom PM, Olde Venterink H, Runhaar H, Witte JPM (2013) Towards a properintegration of hydrology in predicting soil nitrogen mineralization rates along natural moisture gradients.Soil Biol Biochem 58: 302-312.
- Cook FJ, Orchard VA (2008) Relationships between soil respiration and soil moisture. Soil Biol Biochem40: 1013-1018.
- Dessureault-Rompré J, Zebarth BJ, Georgallas A, Burton DL, Grant CA, et al. (2010)Temperaturedependence of soil nitrogen mineralization rate: comparison of mathematical models, referencetemperatures and origin of the soils. Geoderma 157: 97-108.
- Cassman KG, Munns DN (1980) Nitrogen mineralization as affected by soil moisture, temperature, anddepth. Soil Sci Soc Am J 44: 1233-1237.
- Quemada M, Cabrera ML (1997) Temperature and moisture effects on C and N mineralization fromsurface applied clover residue. Plant Soil 189: 127-137.
- Tate RL (1995) Soil microbiology. New York (NY): John Wiley & Sons, Inc.
- Fernandez IJ, Simmons JA, Briggs RD (2000) Indices of forest floor nitrogen status along a climategradient in Maine, USA. For Ecol Manage 134: 177-187.
- Vitousek P (1982) Nutrient cycling and nutrient use efficiency. Am Nat 119: 553-572.
- Bonito GM, Coleman DC, Haines BL, Cabrera ML (2003) Can nitrogen budgets explain differences insoil nitrogen mineralization rates of forest stands along an elevation gradient? For Ecol Manage 176: 563-574.
- Zak DR, Grigal DF (1991) Nitrogen mineralization, nitrification and denitrification in upland and wetlandecosystems. Oecologia 88: 189-196.
- Alef K, Beck T, Zelles L, Kleiner D (1988) A comparison of methods to estimate microbial biomass andN-mineralization in agricultural and grassland soils. Soil Biol Biochem 20: 561-565.
- Fornara DA, Bardgett R, Steinbeiss S, Zak DR, Gleixner G, et al. (2011) Plant effects on soil Nmineralization are mediated by the composition of multiple soil organic fractions. Ecol Res 26: 201-208.
- Hibbard KA, Archer S, Schimel DS, Valentine DW (2001) Biogeochemical changes accompanyingwoody plant encroachment in a subtropical savanna. Ecology 82: 1999-2011.
- Kielland K, Olson K, Ruess RW, Boone RD (2006) Contribution of winter processes to soil nitrogen fluxin taiga forest ecosystems. Biogeochemistry 81: 349-360.
- Guleryuz G, Gucel S, Ozturk M (2010) Nitrogen mineralization in a high altitude ecosystem in themediterranean phytogeographical region of Turkey. J Environ Biol 31: 503-14.
- Uri V, Lõhmus K, Kund M, Tullus H (2008) The effect of land use type on net nitrogen mineralizationonbandoned agricultural land: silver birch stand versus grassland. For Ecol Manage 255: 226-233.
- Tateno R, Suzuki Y, Hamada T, Hidaka K (2010) Effects of slope position on soil nitrogen mineralizationin Cryptomeria japonica plantations in southern Kyushu, Japan. Res Bull Kagoshima Univ For 37: 129-136 (in Japanese).
- Arslan H, Güleryüz G, Kırmızı S (2010) Nitrogen mineralisation in the soil of indigenous oak and pineplantation forests in a Mediterranean environment. Eur J Soil Biol 46: 11-17.
- Zhang X, Wang Q, Li L, Han X (2008) Seasonal variations in nitrogen mineralization under three land usetypes in a grassland landscape. Acta Oecologica 34: 322-330
- Chapman LY, McNulty SG, Sun G, Zhang Y (2013) Net nitrogen mineralization in natural ecosystemsacross the conterminous US. Int J Geosci 4: 1300-1312.
Citation: Cui J, Hirota M, Kamijo T, Yoshitake S, Katoh K (2018) Soil Net Nitrogen Mineralization at Different Ecosystem Development Stages after the Year 2000 Eruption on Miyakejima Island. J Ecosys Ecograph 8: 250. DOI: 10.4172/2157-7625.1000250
Copyright: © 2018 Cui J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6971
- [From(publication date): 0-2018 - Apr 26, 2025]
- Breakdown by view type
- HTML page views: 6047
- PDF downloads: 924