Sodium Oxybatefor Narcolepsy with Cataplexy– Cost-Effective Analysis
Received: 18-Apr-2014 / Accepted Date: 25-Jun-2014 / Published Date: 02-Jul-2014
Abstract
Objective: To assess the cost-effectiveness of sodium oxybate plus antidepressants and stimulants compared with the current standard treatment for narcolepsy with cataplexy in the UK. Methods: We developed a Markov model to assess the costs and benefits of sodium oxybate plus antidepressants and stimulants or current standard treatment based on their effects on the quality of life of patients. The main outcome was the incremental cost-effectiveness ratio (ICER) in terms of costs per additional qualityadjusted life year (QALY) gained over 5 years of treatment. One-way, multi-way and probabilistic sensitivity analyses were conducted to explore the impact of uncertainties on the findings. Results: The cost-effectiveness of sodium oxybate plus antidepressants and stimulants were very sensitive to the costs of sodium oxybate (price and dose) and differences in utilities between responders and non-responders. With current clinical evidence, it is recognised that measure of improvement in utilities used in the current model may be an underestimation of the reality. Conclusions: Our analysis suggests that, at the current UK list price, sodium oxybate would not represent good value for money for the NHS. This is unfortunate, since it appears that sodium oxybate is likely to benefit some patients in the management of these two conditions which affect about 0.03% of the UK population.
Keywords: Cost-utility analysis; Sodium oxybate; Narcolepsy; Cataplexy; Quality of life; Economic evaluation; Probability sensitivity analysis
411835Introduction
Narcolepsy is a sleep disorder, affecting about 0.02% of adults worldwide and around 0.04% of the UK population [1,2]. The condition often starts during childhood or early adolescence and is lifelong [1,3]. The main symptoms are Excessive Daytime Sleepiness (EDS) and cataplexy, which affects approximately 75% of those with narcolepsy [4] and is characterised by a sudden decrease of muscular tone, caused by emotion, typically laughter. Currently the most common treatment (standard care) is to use antidepressants as anti-cataleptics and stimulants for daytime sleepiness symptoms, but this combination of treatments has been observed to be inadequate for some patients [5].
Sodium oxybate, presented as an oral solution, is the official name for the sodium salt of gamma-hydroxybutyrate (GHB). In the UK it is classed as a controlled drug, which in practice puts few restrictions on its prescription. In the US GHB became subjected to more stringent controls which later became relaxed for medical products but retained for non-medical GHB. Sodium oxybate was licensed in the UK in 2005 for the treatment of cataplexy in adults with narcolepsy. Further clinical data permitted a change in the licence to “the treatment of narcolepsy with cataplexy in adult patients” in 2008 [6]. Sodium oxybate may improve quality of life (QoL) but studies indicate some patients fail to respond or find the treatment unacceptable due to side effects (e.g. nausea and vomiting, anorexia and dizziness) [6].
A number of trials [5,7-9] have demonstrated the effectiveness of sodium oxybate in this context but its cost-effectiveness has not been established. Our aim in this analysis was to assess the cost-effectiveness of sodium oxybate as an add-on to standard care (antidepressants as anti-cataleptics and stimulants for daytime sleepiness symptoms) compared with standard care alone for narcolepsy with cataplexy, taking into account the terms of a current Patient Access Scheme available to some funders in the UK. Patient Access Scheme states that the costs of sodium oxybate are refunded to the NHS by the manufacturer for patients who do not respond to sodium oxybate following the initial three months.
This work was partially funded by National Institute for Health Research (NIHR) through The Peninsula Collaboration for Leadership in Applied Health Research and Care (PenCLAHRC), which is based at the University of Exeter. We thank Professor Adam Zeman from Peninsula College of Medicine & Dentistry of University of Exeter for his clinical opinions of this work. This article represents independent research and the views expressed are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Methods
Economic Evaluation
We estimate the cost-effectiveness of sodium oxybate comparing to the current treatment by following two cohorts of patients, one receiving standard treatment plus sodium oxybate and one continuing to receive standard treatment alone for a period of time. The economic evaluation estimates the average total cost for cohort of patients treated with sodium oxybate and the other cohort of patients with standard treatment, based on published data sources. Average quality-adjusted life years (QALYs) gained for the cohort treated with sodium oxybate and the cohort receiving standard treatment alone were based on the clinical trial data assessing QoL using scores obtained from the SF-36 instrument. We calculate the incremental cost-effectiveness ratio (ICER) to estimate the additional costs associated with the use of sodium oxybate to obtain a gain of one additional QALY when compared with standard treatment alone.
This ICER is given by the following formula:

We estimated the cost-effectiveness of sodium oxybate compared to standard treatment by assessing whether the ICER exceeded or remained below a willingness-to-pay threshold. If the ICER is no higher than a chosen threshold then sodium oxybate could be considered to be cost-effective when compared to standard treatment alone. In the UK, National Institute of Clinical Excellence (NICE) generally considers interventions with an ICER below the willingness-to-pay threshold of £20,000 per QALY gained to be cost-effective. Where the ICER is above £30,000 per QALY gained it is unlikely the intervention will be considered cost-effective, without additional exceptional factors supporting use (e.g. end-of-life care) [10].
Overview
In this economic evaluation, we developed a Markov model to follow patients from commencement of treatment with sodium oxybate to the end of a five-year time horizon. The state transition diagram of the Markov model is illustrated in Supplementary Figure1. To capture the differences in costs and utilities due to short-term events, such as non-response and patient withdrawal within the first three months, we used time-dependent parameters. The model takes an NHS perspective and incorporates utilities and costs associated with time spent on different treatments, time spent with significant side effects with sodium oxybate and death. We applied an annual discount rate of 3.5% to costs and benefits [10].
The population we considered consists of adults who suffer from narcolepsy with cataplexy and have derived inadequate benefit from treatment with antidepressants (as anti-cataleptic) and stimulants (for daytime sleepiness symptoms). Standard treatment used in clinical practice included using antidepressants such as clomipramine, fluoxetine and Venlafaxine for cataplexy symptoms, and stimulants including modafinil, dexamfetamine and methylphenidate for sleepiness symptoms. Sodium oxybate is assessed here as an add-on treatment i.e. used alongside stimulants and antidepressants. There were limitations in the data required for cost-effectiveness modelling reported in the published papers of sodium oxybate trials and so we have had to make a number of assumptions to permit comparison. These are detailed below.
We simulated two cohorts of patients, one receiving standard treatment plus sodium oxybate and one continuing to receive standard treatment alone, from the point of entry to the end of a five-year horizon using a Markov model (Supplementary Figure1). Three health states are defined in the model and we assume all patients, regardless of treatment, can only be in one state at any time-point. The three disease states defined are: (i) On Treatment: with maintained response (or in receipt of standard treatment alone and with ongoing inadequate response), (ii) Withdrawn from Treatment: patient withdrawal due to unsatisfactory response to sodium oxybate (for the group of patients with standard treatment, we assume the rate of withdrawal from standard treatment is zero throughout the comparison) and (iii) Dead: both cohorts of patients may die from other causes regardless of their treatment options and we have assumed likelihood of death is equal in each treatment arm. We have used a three-monthly cycle in the Markov model, where one month is equal to 30.4 (365/12) days.
There are short term events to be captured in the model for the first three months. In this period, for patients who receive sodium oxybate there are three possible transitions at the end of this first cycle:
They respond to sodium oxybate, continue receiving it, and stay in the “On Treatment” state;
They withdraw from treatment with sodium oxybate for one or both of two possible reasons: (1) side effects and (2) failure to respond to sodium oxybate (in which case costs are reimbursed under the Patient Access Scheme). In either case patients are assumed to continue with standard treatment alone afterwards;
They die from unrelated causes.
There is a lack of published data on withdrawal rates from sodium oxybate over a longer time period and in our base case analysis we have assumed that there is a zero probability of withdrawal from sodium oxybate after the first three months. We also assume patients receiving standard treatment do not withdraw from the current treatment.
Clinical Effectiveness
Published evidence and specialist opinion indicates most patients develop narcolepsy symptoms when young [1,3]. The base case is thus assumed to be a 20-year-old cohort with clinically evident narcolepsy and cataplexy. There is no evidence suggesting narcolepsy with cataplexy affects overall survival, so the time horizon chosen is five years.
Patients generally receive an initial dose of 1.5 g of sodium oxybate at bedtime and an additional dose two-and-a-half and four hours later. Dose escalation happens according to response in steps of 1.5 g daily in two divided doses at intervals of one to two weeks up to a maximum of 9 g daily in two divided doses [6]. Studies OMC-SXB-21 or prescribed and OMC-GHB-3 report the average dose of sodium oxybate over a usage of several years or a 12-month period.6 The weighted mean dose of the two studies was 6.4 g and the median dose was 6 g. In our base case analysis we assumed patients with sodium oxybate receive an average daily dose of 6 g until drop out, death or the end of year five.
The scientific discussion in the European Public Assessment Report [6] from the European Medicines Evaluation Agency provides data from a number of studies, identified by the code numbers: SCRIMA, OMC-GHB-2, OMC-GHB-3, OMC-SXB-6, OMC-SXB-7, OMC-SXB-20 and OMC-SXB-21. Across these studies, 35 out of 421 patients who received sodium oxybate dropped out due to adverse events over a mean period of 219.7 days, and we used these figures to inform our probability of withdrawal due to adverse events within the first three months. In the base case we assumed no patients drop out from sodium oxybate after the first three months. The impact of withdrawal after month three is explored in sensitivity analyses.
There is no published evidence on the proportion of patients unresponsive to sodium oxybate so we have used expert opinion to inform the base case and assume that 25% patients would carry on with sodium oxybate after three months i.e. 75% would be non-responsive to sodium oxybate or withdraw due to adverse events.
Most published trials assess the short-term effectiveness of sodium oxybate and the longest reported follow-up was for one year. In the absence of evidence suggesting the long-term effectiveness of sodium oxybate differs from the short term effectiveness we assumed that if patients continue to use sodium oxybate at the end of month three then they would maintain their response until the end of year five (or until they died). The age-dependent rates of death due to other causes were estimated from national mortality statistics produced by the UK Office for National Statistics [11].
Quality of life
Sodium oxybate appears to improve QoL in responsive patients broadly in line with the dosing level [5]. The mortality rates in our model were assumed to be the same for all patients regardless of treatment options so the incremental QALYs required to calculate the ICER depend only on the difference between the utilities assumed for patients on either treatment. In other words, the model gives the same results regardless of the value of the baseline utility (utility for patients in inadequate response with standard treatment), as long as the difference in utility stays the same over time.
Although a number of studies have reported QoL in patients receiving standard treatment for narcolepsy and cataplexy there was only one study that reported QoL for patients undergoing treatment with sodium oxybate [12] This study reported SF-36 scores for patients prior to receiving sodium oxybate and after 6 months treatment with sodium oxybate. However, in this study, the reported SF-36 scores prior sodium oxybate therapies were distinctly different from the other studies that studied the QoL of patients without sodium oxybate therapy. Thus, we did not include data from this study in our assessment. As a result of excluding this study from our analysis, there was no data on QoL for patients undergoing treatment with sodium oxybate. We were also unable to find any information on utility changes associated with a reduction in cataplexy attacks following receipt of sodium oxybate.
In the absence of relevant data on the change in utility using treatment with sodium oxybate we used the effect of treatment on the Epworth Sleepiness Scale (ESS) scores to estimate changes in utility. Analyses reported in Dodel et al. [13] failed to detect an association between QoL and either improvement in cataplexy symptoms or in nocturnal sleep quality. Dodel and colleagues found the only impact on utility came from excessive daytime sleepiness and continuous sleep, and daytime sleepiness is usually assessed using the ESS. A number of other studies (Table 1) have also reported sleepiness as the main symptom of narcolepsy with cataplexy, and this is in keeping with expert opinion.
| Study (sample size) | Median ESS change |
Dose | Median ESS for current treatment | Median ESS for treatment with sodium oxybate |
| US Xyrem Multicentre Study Group 2002 [7] (120) |
1 | 3g | 17 | 16 |
| 4 | 6g | 18 | 14 | |
| 5 | 9g | 17 | 12 | |
| US Xyrem Multicentre 2003 [8] (118) |
2 | 3g – 9g | 15 | 13 |
| International Xyrem Study Group 2005 [9] (228) |
3 | 3g | 19 | 16 |
| 3 | 6g | 18 | 15 | |
| 7 | 9g | 19 | 12 | |
| Black and Houghton 2006 [14] (270) |
4 | 3g – 9g | 15 | 11 |
Table 1: ESS change due to sodium oxybate at different dosing levels.
As presented in Table 1, our selection of a four-point improvement in ESS associated with receipt of a daily dose of 6g of sodium oxybate represents a favourable estimate of the effects of sodium oxybate but may fail to capture the effects on QoL of a reduction in frequency or severity in cataplexy attacks or of improvement in nocturnal sleep quality.
We used the results of a regression model relating changes of ESS to changes in utility values 15 to convert changes in ESS to changes in utility due to treatment. This found a one point reduction in ESS equates to an increase of 0.0097 (95% Confidence Interval 0.0019 to 0.0175) in EQ-5D utility. This study was based on treatment for obstructive sleep apnoea–hypopnoea syndrome We have no reason to believe that the relationship between ESS and utility change is disease-specific so we have assumed the relationship between ESS change and utility change is similar for patients with narcolepsy.
Patients who drop out due to adverse events are likely to have had poorer QoL than patients who respond to sodium oxybate but there is no evidence to confirm this. In the base case we assumed that, up to the point of drop-out, QoL for these patients is the same as patients who respond. We assessed the impact of QoL reductions for these patients in sensitivity analyses.
Resource use and Costs
The resources and costs considered were the costs of sodium oxybate and the standard treatment of stimulants (modafinil, dexamfetamine and methylphenidate) and antidepressants (Clomipramine, Fluoxetine and Venlafaxine), and costs of consultant outpatient clinic attendence. We assumed that no additional costs are associated with adverse events for either treatment.
We found no published data on frequency of clinic visits for patients using standard treatment or sodium oxybate. We assumed, therefore, that patients on sodium oxybate require more frequent clinic visits initially, but once patients are stabilised on treatment there is no predictable difference in the pattern of clinic visits for patients receiving either treatment.
The values of the parameters used in the model are shown in Table 2.
| Parameter | Value | Source/Comments | ||||||
| Base Case | Distribution | |||||||
| Utilities | ||||||||
| Inadequate response to current treatment alone | 0.763 | Beta (23,7) | Use the SF-36 scores reported in Teixeira et al. [15] and apply mapping algorithm from Ara and Brazier [16] to obtain the EQ-5D utilities; Beta (α,β) |
|||||
| No response to sodium oxybate | 0.763 | Beta (23,7) | The same as the utility of inadequate response to current treatment alone | |||||
| In response to sodium oxybate | 0.8059 | - | Use the baseline utility for patients in inadequate response to current treatment alone and add the utility increase corresponding to ESS change | |||||
| Difference in utilities between in response and no response to sodium oxybate | 0.0429 | Beta (96,2134) | Use the change in utility for 1 unit change in ESS estimated in a York economic model of CPAP in OSAH [17], assuming 4 units of ESS change [7] in the base case and adjust for age | |||||
| Increase in EQ-5D utility for 1 unit decrease in ESS | 0.0097 | - | York economic model of CPAP in OSAH [15] | |||||
| Change in ESS scores | 4 | - | The mean change in ESS scores for patients receiving a mean daily dose of 6 g of sodium oxybate [7] | |||||
| Costs for clinic visits | ||||||||
| Costs per specialist visit | £131 | Normal (131,3.24) | Consultant led follow-up attendance non- admitted face to face (neurology) [18]; Normal (μ,σ) | |||||
| Costs for drugs | ||||||||
| Sodium oxybate | £4 per gram | - | [19] | |||||
| Average daily dose | 6 g | Gamma (11.11,0.54) | Weighted average dose calculated from trials OMC- SXB-21, OMC-GHB-3 & OMC- GHB-4 and adjusted for common daily dose [6] |
|||||
| Average monthly costs of sodium oxybate | £730 | - | [6,19] | |||||
| Average monthly costs of stimulants | £181.28 | - | The same for both cohorts | |||||
| Average monthly costs of antidepressants | £27.10 | - | Mainly three drugs: clomipramine, Fluoxetine and Venlafaxine (specialist’s opinion) | |||||
| Possibilities of response to sodium oxybate in the first cycle | ||||||||
| Patients with Sodium Oxybate | Probability of a good Response | 0.25 | Beta (75,224) | Specialist’s opinion | ||||
| Probability STOP due to AE | 0.0339 | Beta (97,2749) | [6] | |||||
| Probability No Response | 0.7161 | - | The residual of the other two possibilities | |||||
| Transition probabilities per cycle | ||||||||
| Patients with Sodium Oxybate | From in response to withdrawal | 0 | - | From cycle 2 onwards | ||||
| From response withdrawal death | in or to | 0.0001-0.0838 | - | Age dependent [11] | ||||
| Patients treatment with current | From in response to withdrawal | 0 | - | |||||
| From in response or withdrawal to death |
in or to | 0.0001-0.0838 | - | Age dependent [11] | ||||
Table 2: Values of parameters used in the decision model.
Uncertainty in key parameter
The value for the baseline utility is not important for the calculation of the ICER, as described above, but is of significance for some sensitivity analyses. To explore the sensitivity of our calculated ICER to changes in utility we need to estimate the biggest possible change and to do this we must first estimate a baseline utility. Several studies assess QoL in patients with narcolepsy but only one reported EQ-5D utility weights [13]. Most of the remaining studies report SF-36 scores [12,16,20-22] and one reports total Functional Outcomes of Sleep Questionnaire scores (FOSQ) [5]. The mean EQ-5D index score for 75 patients with a mean age of 48.9 years given in Dodel et al. [13] is 0.87, which we considered too high since it is higher than the utility value for the age-matched UK general population (0.85) [23]. We calculated baseline utilities for patients receiving standard treatment by applying the mapping algorithm from Ara and Brazier [16] to SF-36 scores from the study by Teixeira et al. [15]. We used these data because this was a UK-based study and because the participants’ characteristics were the closest to the population of interest: all participating patients suffered from narcolepsy with cataplexy. We note this study reports the lowest QoL among the candidate studies (sample size was 49).
The baseline utility is for patients at age 47 because this was the mean age in the patient group of the study by Teixeira et al. [15] from which we took SF-36 scores (Supplementary Table 1). We adjusted these values for the modelled cohorts by assuming the ratios of the utilities between different age groups of the population of interest are the same as those between utilities for different age groups in the general UK population [23].
Results
Base case analysis
The base case was assumed to be a 20-year-old cohort with narcolepsy and cataplexy. In the base case analysis, using the values presented in Table 2, we estimated the ICER for sodium oxybate plus current treatment vs. current treatment alone to be approximately £210,000 per QALY gained (Table 3).
| Sodium Oxybate + Standard Treatment | Standard Treatment | Incremental | |
| QALYs (discounted) | 3.6119 | 3.5616 | 0.0502 |
| Costs (discounted) | |||
| Drug costs | £21,933 | £11,673 | £10,260 |
| Costs for clinic visits | £1,511 | £1,223 | £288 |
| Total | £23,444 | £12,896 | £10,548 |
| Cost/QALY | £210,000 |
Table 3: Results summary for the base-case analysis.
Sensitivity analyses
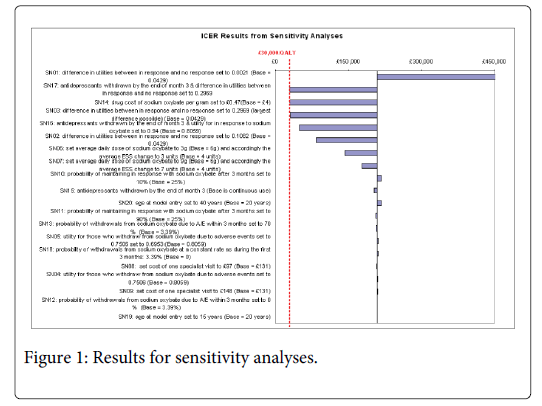
The results of one-way and multi-way sensitivity analyses are presented in Figure 1 and suggest, not surprisingly, that the ICER is very sensitive to differences in the utilities between patients who respond to sodium oxybate and those who have no response (see results of SN01 – SN05). As shown in SN03, if the differences between these utilities were as large as 0.2969 (0.6431 for no response vs. 0.94 for those in response) for 20-year-old patients then the ICER would be reduced from £210,000 to just over £30,000 per QALY gain. A change in utility of 0.2969 can be achieved only by assuming the utility of patients who respond to sodium oxybate is as high as the utility value of 20 year-olds in the UK general population (0.94), implying a total resolution of symptoms and at the same time assuming the utility of patients with standard treatment is the lower bound of the 95% Confidence Interval (CI) of the estimated utility for patients with an inadequate response to standard treatment (0.6431).
The model is also sensitive to the price and dosage of sodium oxybate. If the price of sodium oxybate dropped to 12% of the current price then the resultant ICER, using our base case assumptions, would be less than £30,000 per QALY gained (SN14). The model is not sensitive to the costs of clinic visits, which is as expected since the total costs of clinic visits are less than 10% of the total costs for both groups (SN8-SN9).
The model is relatively insensitive to the proportion of patients who continue on sodium oxybate after the initial three months (SN10-SN11). Through the Patient Access Scheme the manufacturer offers a refund of the costs of sodium oxybate for patients who do not respond. In the base case the cost of drugs in the first three months is only about 5.5% of the total drug costs and less than 0.0001% of total costs so the ICER is relatively insensitive to the proportion of patients who do not respond to sodium oxybate and the proportion of patients who stop taking sodium oxybate due to adverse events (SN12-SN13).
Scenarios 15 to 17 explore the impact of assuming that patients may withdraw from anti-depressants at the end of month three if they respond to sodium oxybate. The resultant ICERs are lower than the results of corresponding scenarios with no withdrawal from anti-depressants. However, given usual patterns of sodium oxybate use and possible issues associated with withdrawal from anti-depressants, these results should be interpreted with caution.
There is also a possibility that patients might lose response after a period of responsiveness to sodium oxybate and as a result might drop out from sodium oxybate treatment after the first three months. The impact of possible drop outs after the first three months is explored in SN18 and the ICER is not very sensitive to this uncertainty. This is in line with the close association between sodium oxybate level and improvement in utility in responsive patients: drop out from sodium oxybate brings down total costs but total QALYs gained also decrease.
The impact of patient age group has also been assessed. Scenarios 19-20 show that the cost-effectiveness of sodium oxybate in patients aged 15 or 40 at outset differs little from that in patients aged 20.
Probabilistic Sensitivity Analyses (PSA)
In our PSA, the mean ICER was estimated to be £235,200 per QALY gained. The cost-effectiveness plane is given in Supplementary Figure 2. Using a threshold of £30,000 per QALY, the probability that sodium oxybate plus standard treatment is cost effective for patients with narcolepsy and cataplexy at age 20 compared to standard treatment was 1.3% (Supplementary Figure 3). As shown in Supplementary Figure 2, the costs associated with sodium oxybate were always higher than the standard treatment alone. This is as expected as sodium oxybate is used as add on to the standard treatment, i.e. both cohorts generate costs associated with standard treatment. We can also see from Supplementary Figure2 that the sodium oxybate-treated cohort was more likely to have higher QALY gains, which is also as expected as patients responding to sodium oxybate have better quality of life.
Discussion
Our deterministic base case estimate of the ICER for sodium oxybate for narcolepsy with cataplexy is approximately £210,000 per QALY gained. The mean ICER of the PSA was estimated to be £235,200 per QALY. Both of these estimate places this intervention some way beyond NICE’s guideline cost-effectiveness range for the UK: £20,000-£30,000 per QALY gained.
Our modelling suggests this estimate is sensitive to differences between the utilities of patients with the two different treatments and to the price of sodium oxybate. Extreme estimates of utility change or large reductions in price could each bring the ICER close to £30,000 per QALY. The 0.30 difference in utilities required in the first instance is possible only if we assume the utility for patients who respond to sodium oxybate is as high as that for the age-matched general population of the UK, which would mean that all symptoms were fully resolved, and that the utility for patients with standard treatment alone is the lower bound of the 95% CI of the baseline utility estimate, i.e. the worst possible scenario in relation to the condition based on current, albeit it limited, evidence. In the second instance, the price of sodium oxybate would have to be reduced to just over 10% of its current price.
The model is insensitive to the proportion of patients who continue with sodium oxybate after the initial three months, the proportion of patients who do not respond to sodium oxybate, and the proportion of patients who stop taking sodium oxybate at the end of the first three months due to adverse events. The lack of sensitivity related to these parameters allows us to have more confidence in our base case as lack of data means there is substantial uncertainty around these three parameters.
Strengths and Weaknesses
Our model captures all relevant material from existing studies on this topic and combines it with expert input. Our model is limited by uncertainties associated with key parameters due to lack of data. It is therefore important to consider the sensitivity of the resultant ICERs to the parameters included in the decision model.
One limitation of our model is that the estimation of utility changes based on changes in ESS. This was based on a study which used a regression model derived from three sets of patient-level data for patients with obstructive sleep apnoea-hypopnoea syndrome. In addition, our model does not differentiate between sodium oxybate and standard treatment in terms of the disutility associated with the side effects of each treatment.
We noted the data reporting a utility difference in patients before and after treatment with sodium oxybate [12]. However in comparison with five other papers we found which assessed utility scores in patients with narcolepsy the scores reported appeared so different that we considered there was some doubt as to the reliability of these. However, we also noted that the difference between the pre-treatment and on-treatment score was also quite small (0.025). This difference is less that the difference we obtained by applying the ESS–utility relationship regression equation. Thus, we consider that there are currently no data which would suggest that this method yields unreliable results for narcolepsy patients.
In our model we incorporated only one outcome measure from the clinical trial data, the effect of treatment on ESS. It is likely other measures reported in the trials could impact on QoL, such as number of cataplexy attacks per week or nocturnal sleep quality, but we found no information allowing us to relate such findings to impact on cataplexy (or of resolution of cataplexy or improvement in nocturnal sleep quality), which could be related to QALY changes. Our sensitivity analyses show outcomes associated with assuming the widest possible range of utilities associated with this condition, going from the most severe end of reported utilities to total resolution. It is unlikely that the inclusion of additional data on cataplexy would represent cases not already assessed in the sensitivity analyses but the absence of these data introduces additional uncertainty to our model.
Implications
On the basis of current costs, sodium oxybate is unlikely to be cost-effective as an add-on treatment for patients who respond inadequately to standard treatment with stimulants and antidepressants. Although, it is recognised that measure of improvement in utilities used in the current model may be an underestimation of the reality.
There remains substantial uncertainty about the quality of life impact of this chronic rare condition and further research is necessary to permit future analyses to consider the cost-effectiveness of sodium oxybate. Current understanding of the impact of cataplexy is particularly limited, as is available evidence on the long-term course of the condition.
References
- Dauvilliers Y, Arnulf I, Mignot E (2007) Narcolepsy with cataplexy. Lancet 369: 499-511.
- Ohayon MM, Priest RG, Caulet M, Guilleminault C (1996) Hypnagogic and hypnopompic hallucinations: pathological phenomena? Br J Psychiatry 169: 459-467.
- UCB Pharma Ltd. Sodium oxybate, 500mg/ml oral solution (Xyrem) No. (246/06) - Resubmission to Scottish Medicines Consortium2007 10 August 2007.
- Weaver TE, Cuellar N (2006) A randomized trial evaluating the effectiveness of sodium oxybate therapy on quality of life in narcolepsy. Sleep 29: 1189-1194.
- European Medicines Evaluation Agency (2007) Scientific discussion of the European Public Assessment report for Xyrem®.
- The U.S. Xyrem® (2002) Multicenter Study Group. A Randomized, Double Blind, Placebo-Controlled Multicenter Trial Comparing the Effects of Three Doses of Orally Administered Sodium Oxybate with Placebo for the Treatment of Narcolepsy. Sleep25:42-49.
- The U.S. Xyrem® (2003) Multicenter Study Group. A 12-month, Open-Label, Multicenter Extension Trial of Orally Administered Sodium Oxybate for the Treatment of Narcolepsy. Sleep 26:31-35.
- The Xyrem® (2005) International Study Group. A Double-Blind, Placebo-Controlled Study Demonstrates Sodium Oxybate Is Effective for the Treatment of Excessive Daytime Sleepiness in Narcolepsy. Journal of Clinical Sleep Medicine1:391-397.
- National Institute for Health and Clinical Excellence (2008) Guide to the Methods of Technology Appraisal. London: NICE.
- Office for National Statistics (ONS). Interim Life Tables, United Kingdom.
- Hayduk R, Mitler M (2001) Sodium Oxybate Therapy Improves the Quality of Life of Narcolepsy Patients. Sleep24 abstract supplement:A328.
- Dodel R, Peter H, Spottke A, Noelker C, Althaus A, et al. (2007) Health-related quality of life in patients with narcolepsy. Sleep Med 8: 733-741.
- Black J, Houghton WC (2006) Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep 29: 939-946.
- Teixeira VG, Faccenda JF, Douglas NJ (2004) Functional status in patients with narcolepsy. Sleep Med 5: 477-483.
- Ara R, Brazier J (2008) Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health 11: 1131-1143.
- McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, et al. (2009) Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess 13:1-274.
- Department of Health (UK) NHS Reference Costs 2009. London: Department of Health.
- Joint Formulary Committee (2010) British National Formulary No. 59. London: British Medical Association and Royal Pharmaceutical Society of Great Britain.
- Beusterien KM, Rogers AE, Walsleben JA, Emsellem HA, Reblando JA, et al. (1999) Health-related quality of life effects of modafinil for treatment of narcolepsy. Sleep 22: 757-765.
- Daniels E, King MA, Smith IE, Shneerson JM (2001) Health-related quality of life in narcolepsy. J Sleep Res 10: 75-81.
- Vignatelli L, D'Alessandro R, Mosconi P, Ferini-Strambi L, Guidolin L, et al. (2004) Health-related quality of life in Italian patients with narcolepsy: the SF-36 health survey. Sleep Med 5: 467-475.
- Kind P, Hardman G, Macran S (1999) UK population norms for EQ-5D. York: Centre for Health Economics, University of York.
Citation: Lanting L, Chris R, Lain L, Ken S (2014) Sodium Oxybate for Narcolepsy with Cataplexy–A Cost-Effective Analysis. J Neuroinfect Dis 5:161.
Copyright: © 2014 Lanting L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 14992
- [From(publication date): 6-2014 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 10362
- PDF downloads: 4630