Research Article Open Access
Smoking Severity and Functional MRI Results In Schizophrenia: A Case-Series
Zsuzsa Szombathyne Meszaros*, Ynesse Abdul Malak, Daniel J Zaccarini, Tolani O Ajagbe, Ioana Coman and Wendy KatesDepartment of Psychiatry, SUNY Upstate Medical University, New York, USA
- Corresponding Author:
- Zsuzsa Szombathyne Meszaros, MD, PhD
Department of Psychiatry, SUNY Upstate Medical University
750 East Adams Street, IHP 3302, Syracuse, New York, USA
Tel: +13154641705
Fax: +13154641719
E-mail: meszaroz@upstate.edu
Received date: April 14, 2014; Accepted date: August 26, 2014; Published date: August 29, 2014
Citation: Meszaros ZS, Malak YA, Zaccarini DJ, Ajagbe TO, Coman I et al. (2014) Smoking Severity and Functional MRI Results In Schizophrenia: A Case-Series. J Addict Res Ther 5:189. doi:10.4172/2155-6105.1000189
Copyright: © 2014 Szombathyne-Meszaros Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Objective: Although the majority of patients with schizophrenia smoke, assessment of smoking severity is usually ignored in functional magnetic resonance imaging (fMRI) studies. The aim of this study was to identify whether smoking severity was associated with changes in neural activation in patients with schizophrenia and alcohol use disorder.
Methods: Seven smokers with schizophrenia and alcohol use disorder who were enrolled in a smoking cessation pilot study underwent fMRI at baseline. Executive function was assessed with the multi-source interference task (MSIT); working memory was assessed using the N-back task. Smoking severity was measured using serum cotinine and nicotine levels and the Fagerstrom Test for Nicotine Dependence.
Results: During the multi-source interference task (MSIT) task, we observed significant neural activation in left and right precuneus, left and right inferior parietal lobule, left superior frontal gyrus and the right insula. After including serum cotinine level as a covariate, the left precuneus and the left superior frontal gyrus was no longer significantly activated. During the working memory (N-back) task we observed significant neural activation in the right precuneus and superior parietal lobule, the right inferior parietal lobule and the right middle frontal gyrus. After including serum cotinine level as a covariate, the right middle frontal gyrus was no longer significantly activated.
Conclusion: These preliminary results suggest that smoking severity may influence neural activation in the frontal lobe and left precuneus in patients with schizophrenia and alcohol use disorder. Measuring serum cotinine level may improve reliability and diagnostic value of fMRI studies.
Keywords
Schizophrenia; Smoking; fMRI; MSIT; N-back task; Frontal lobe; Precuneus
Objective
Although the majority of patients with schizophrenia smoke, assessment of smoking severity is usually ignored in fMRI studies [1]. There are very few studies published on non-smoking schizophrenic patients [1,2]. In most neuroimaging studies patients and healthy subjects are matched for age, gender, and education [3,4], but not for smoking status or alcohol/substance use severity. The aim of this study was to identify whether smoking severity was associated with changes in the blood-oxygenation level dependent (BOLD) response during fMRI studies.
Smoking, alcohol and illicit substance use are major causes of morbidity and mortality in patients with schizophrenia [5]. The prevalence of cigarette smoking is higher in patients with schizophrenia (80%) compared to the general population (20%) and to mentally ill patients (50%) worldwide [6].
The strong association between nicotine dependence and schizophrenia is not understood well. According to the self-medication hypothesis, patients smoke to overcome their neurocognitive impairments, symptom distress [7] and to counteract side effects of neuroleptics [8].
Negative symptoms of schizophrenia (especially passive withdrawal and social avoidance) have been found to be associated with increased smoking [9]. Smoking temporarily improves negative symptoms and attention in patients with schizophrenia and reduces sensory-gating deficits [10,11]. Higher symptom distress was found to be associated with decreased nicotine use [7]. Smoking abstinence in schizophrenic patients worsens spatial working memory, while abstinence improves working memory in controls [12].
While acute nicotine administration may be beneficial for cognition, chronic smoking is associated with global brain atrophy and increased risk of neurodegenerative diseases [13,14]. Cigarette smoke contains many toxic compounds (e.g., carbon monoxide, free radicals, nitrosamines) that may lead to neurocognitive abnormalities in smokers [15,16]. Furthermore, smoking leads to cerebral hypoperfusion, increased oxidative stress, and cortical atrophy [17]. In addition to direct neurotoxicity, smoking status may affect the blood oxygen level dependent (BOLD) signal in fMRI studies due to atherosclerosis and endothelial damage, which may reduce autoregulation of cerebral blood flow [2].
Chronic cigarette smoking adversely affects auditory-verbal learning [18,19], working memory [20], executive function [21], cognitive flexibility, learning and memory processing speed [15] in the general population. Most of these cognitive deficits (e.g. impaired working memory and executive function) are also present in schizophrenia [22].
Substance use disorders (SUD) are also common among adults with schizophrenia; comorbidity rates have been reported to be as high as 40 to 50 % [23]. Among substance use disorders, alcohol use disorders (AUD) are the most prevalent. The Epidemiologic Catchment Area (ECA) study found that 33.7 % of people with a diagnosis of schizophrenia or schizophreniform disorder also met the criteria for an AUD diagnosis at some time during their lives [24].
Compared to the rest of the population, individuals with schizophrenia are 3.3 times as likely to have an alcohol-related disorder [24]. Alcohol use might exacerbate the cognitive and structural deficits seen in chronic smokers and in patients with schizophrenia. Alcohol consumption during adolescence is especially harmful. In a recently published study [25], subclinical alcohol use during adolescence was found to be associated with decreased cortical thickness in several regions; including the right middle frontal gyrus and anterior cingulate cortex [26], which might lead to impaired inhibitory control and error processing [27].
Alcohol use severity correlates with smoking severity in the general population [28], but not in patients with schizophrenia, who are usually not heavy drinkers [7]. Alcohol consumption is associated with impaired attention, memory and executive functions [29]. Chronic alcohol use accelerates loss of brain gray and white matter volumes [30] and affects the cortex, dorsomedial thalamus, mamillary bodies, striatum, cerebellum, insula, pallidum and corpus callosum [31,32].
Working memory and executive function deficits are present in schizophrenia, tobacco and alcohol use disorders. We selected validated tasks to assess severity of these deficits. Working memory was assessed using the N-back task [33,34]. Activation of several brain areas is observed during this task, including precuneus, left and right inferior parietal cortex, left and right dorsolateral prefrontal cortex, left premotor cortex, left and right anterior cingulate cortex, cerebellum, fusiform gyrus, middle frontal gyrus, and middle temporal gyrus [35]. In a recent study [36] comparing 10 schizophrenic patients and 10 healthy controls, despite increasingly poor performance, activation increased in the above mentioned areas with increasing load, until activity dropped in DLPFC at 3-back task in patients with schizophrenia. We chose to perform the 1-back and 2-back tasks to avoid the confounding effect of high working memory load and related poor performance.
We selected the Multi Source Interference Task (MSIT) to assess executive function and cognitive control [37,38]. This task has the unique ability to cause significant dACC activation in healthy individuals. In addition to dACC activation, group data of 8 subjects also showed activation of dorsolateral prefrontal, premotor, and parietal cortices [37]. Greater activation of the anterior cingulate, insula, and inferior frontal gyrus during this task predicts favorable smoking cessation treatment outcomes [39] confirmed by reduction in urine cotinine levels.
Objective measurement of smoking severity is of great importance in patients with schizophrenia, because there is increased nicotine intake and/or limited reliability of self-report in this patient population [40]. Selection of serum cotinine as the primary measure of smoking severity instead of self-reported scales (e.g. Fagerstrom Test for Nicotine Dependence - FTND) is an innovative feature of our study.
Measuring serum nicotine concentration is a highly accurate way of determining recent tobacco exposure. However, nicotine’s half-life is very short - two to three hours on average. This causes fluctuations in nicotine concentration during the day. On the other hand, cotinine, a pharmacologically active metabolite of nicotine, has a half-life that is about 18 to 20 hours [41]. Cotinine level shows less daily fluctuation, therefore it is a better measure of smoking severity than serum nicotine or scales based on self-report (craving scales, FTND). The clearance and half-life of cotinine is determined by the polymorphism of cytochrome P450 2A6 enzyme, which is related to the individual’s ethnicity (50% of Japanese people and 43% of Koreans have low enzyme activity, compared to 22% of African Americans and 9% of Caucasians) [42].
Since heavy smoking is associated with mild alcohol use severity in patients with schizophrenia, we decided to focus on the possible relationship between smoking severity and neural activation in our patients, who were enrolled in the parent study: a randomized, double-blind, placebo controlled trial of Varenicline (Chantix®) for the treatment of alcohol and nicotine dependence [43].
Methods
Participants
Seven patients with schizophrenia or schizoaffective disorder and co-occurring tobacco and alcohol use disorder (with current or “life-time” diagnosis of alcohol dependence) who were enrolled in a randomized, double-blind, placebo controlled trial of varenicline were included in the present imaging study. All were outpatients, 18-69 years old, and were receiving antipsychotics at least 4 weeks prior to the study. All participants provided informed consent. The study protocol and informed consent was approved by the SUNY Upstate Medical University Institutional Review Board. Patients who had suicidal ideation, or who were hospitalized for suicidal ideation within a year were excluded, along with patients who had cocaine, opioid, or amphetamine positive urine toxicology screen at baseline.
Not every patient enrolled in the parent study underwent functional MRI. Patients with poor eyesight and no contact lenses, patients with metal implants or devices, and patients with morbid obesity (body weight over 270 lbs.) or claustrophobia were excluded from the present study. One patient underwent fMRI but the imaging data obtained were excluded from analysis, because of an accidental finding of an old stroke. Another subject’s scan had an artifact in the frontal area during the MSIT task, so that scan was not included. Altogether 7 patients completed both fMRI tasks (tests of working memory and executive function) at baseline, before starting varenicline or placebo.
Study design and outcome measures
Three screening visits were conducted over two weeks to determine study eligibility. The Structured Clinical Interview for DSM-IV was conducted to determine psychiatric diagnosis [44].
Primary outcome measures were: number of cigarettes smoked per week – based on the modified Time-Line Follow Back interview [45], and serum nicotine/cotinine level. Blood was drawn from the participants during the second screening visit, after the physical examination. Blood samples were collected into Vacutainer tubes, stored in a refrigerator, and transported in a cooler to Upstate University Hospital Clinical Pathology Laboratory within 4 hours. The processing of samples (separation of serum, cooling, packaging) was done at Upstate University Hospital Clinical Pathology Laboratory. Frozen serum samples were sent to Quest Diagnostics (875 Greentree Road, 4 Parkway Center, Pittsburgh, PA). Liquid Chromatography/Tandem Mass Spectrometry was used to determine serum nicotine and cotinine levels [46,47].
Additional outcome measures included: exhaled CO concentration, and Fagerstrom Test for Nicotine Dependence [48]. Psychiatric symptom severity was assessed using the Positive and Negative Syndrome Scale (PANSS) [49].
Neuroimaging Methods
Eligible subjects performed cognitive tasks to assess working memory and executive function while in the MR scanner. fMRI scans were completed between 10am and 1pm. Patients were allowed to smoke ad libitum on the day of the scan, but were asked not to drink any alcohol that morning. A breathalyzer test was performed before the scan to rule out recent alcohol use.
A laptop computer equipped with E-Prime software (Psychology Software Tools, Pittsburgh, PA) was used to program the paradigms and to control the experimental parameters. Stimuli were visually cued with a mirror attached to the head coil on a back-projection screen in the scanner room.
Stimuli
Working memory was assessed using the N-back task [33,34]. The subjects were asked to watch a changing screen display that showed black capital letters on a white background. We used a block design, in which blocks of stimuli for N=1, N=2, and N=0 (control) were alternated three times. Each block consisted of 25 stimuli, each stimulus lasting for 2.5 seconds for a total of 62.5 seconds per block. A 7.5 seconds block of instructions preceded each block of stimuli. During the N=1 experiment, the subjects had to respond by pressing a button on a button box each time they saw the same letter displayed in the previous screen. During the N=2 experiment, the subjects had to respond when they saw the same stimuli two screens back. For the control experiment, they had to press when they saw the letter 'X'. The experiment was preceded and followed by 25s resting periods with fixation crosses. The complete paradigm lasted 11 minutes and 20 seconds.
The Multi Source Interference Task (MSIT) is a measure of the effect of interference on performance of a number identification task. This task was performed to assess frontal (anterior cingulate cortex) function [37,38]. The MSIT paradigm is described in detail in a recent publication by Bush et al. [37]. A block design was used, where each 60s block consisted of 24 visual stimuli (black text on gray background) lasting for 2s, followed by intra-stimulus intervals of 0.5s when a white display was presented. The subjects were given a button-press keypad with 3 buttons and were asked to use their index, middle and the ring fingers to respond. The stimuli consisted of sets of three numbers (1 and/or 2 and/or 3) and/or letters (x) appearing on the center of the screen every 2.5s. One number was always different than the other two numbers or letters. The subjects were asked to respond via the button-press keypad to identify the number that was different from the other two characters. In trials with numbers and letters (the control trials), the target number always matched its position (i.e. the number '2' would always appear in the middle position) and it was always larger than the letters. During the interference trials, only numbers were used, and the target number never matched its position. The subjects were informed that the target number could be either larger or smaller than the other numbers, and they were instructed to report the target number, regardless of its position. The paradigm consisted of a 20s rest block with a fixation cross, a 10s instructions block, followed by four 60s alternating MSIT blocks (control-interference-control-interference), then a 5s resting block with a white screen, followed by four 60s alternating MSIT blocks, and ending with a 20s resting period with a text message for a total duration of 8 min and 55s.
Fmri Acquisition
Functional MRI scans were acquired once at baseline, before receiving the first dose of varenicline or placebo. Every participant had a practice session before the fMRI scan. We used notecards and a different sequence of numbers, than the actual task. Patients were scanned after they demonstrated understanding of the tasks, and performed well (without errors) on the practice tests. Every patient performed well (with over 80% accuracy) in the scanner.
All functional MRI scans were acquired on a 1.5T Philips Interra scanner, version release 11 (Philips Medical Systems, Best, The Netherlands), equipped with a Phillips Sense head coil. For each experiment (N-Back and MSIT), 25 slices were acquired every 2.5 s (4 mm thickness, 1 mm gap) using an FFE-EPI sequence (TR/TE=2500 ms/60 ms, voxel size=3.75 × 3.75, acq. matrix=64×64×25 slices). A conventional 3D scan was also obtained for anatomic localization.
Data Analysis
Neuroimaging data were transferred from the MRI machine to IBM compatible PC workstations using an internal network connection. Functional MRI data were analyzed offline using the Statistical Parametric Mapping-SPM5 software package (Department of Cognitive Neurology, London, UK, 2005), running under a Windows version of Matlab 2007b (Mathworks Inc., Natick, MA) on a Centrino Duo based Dell Latitude Laptop.
Images were visually inspected at intermediate stages of preprocessing to ensure the absence of ghosting, significant signal dropout, and image processing artifacts, using the Art Repair toolbox (50). Preprocessing steps included:
1. motion correction (INRIalign) utilizing an algorithm unbiased by local signal changes [51];
2. spatial normalization of motion-corrected images into the standard Montreal Neurological Institute space [52], using a hybrid algorithm of affine transformation and nonlinear warping, where the voxels were resampled at a resolution of 3×3×3 mm3 using trilinear interpolation; and
3. Gaussian spatial filtering with a full-width, half maximum (FWHM) of 6 mm.
Art Repair was utilized for slice outlier detection and repair, motion correction, and band-pass filtering.
SPM5 software was also used to carry out first-level parametric analyses individually for every subject utilizing the general linear model (GLM). For each subject, the stimulus was modeled as a set of regressors in the GLM analysis. The stimulus was a block design, and boxcar functions were used to define regressors, which modeled the onsets and the durations of the appearances of each stimulus. Regressors were convolved with the canonical Hemodynamic Response Function (HRF) and estimated using classical restricted maximum likelihood (ReML). At every voxel, parameter estimates for each regressor were compared using t-tests to establish the significance of differences in neuronal activation between conditions. In the N-back experiment the main effects were 1Back vs. Control (1Back-Control), 2Back vs. Control (2Back-Control), and 1-Back vs. 2-Back (1Back-2Back). In the MSIT experiment, the main effect was Interference vs. Control condition (Interference-Control). The parameter estimates from the first-level analyses were entered into a second level, 1-sample t-test inference about the mean neuronal activation during the 2Back-Control effect in the N-back task and the Interference-Control effect in the MSIT task. D-prime score during the N-back task (for the 2Back-Control effect) and cotinine levels (for both the 2Back-Control effect as well as the Interference-Control effect) were entered as additional regressors in the design matrix (as covariates) to specify subject specific task performance as well as smoking severity. We report significant results at the cluster corrected level of p<0.001 for the second level group analysis. Coordinates are reported for statistical maxima of neural activation in the MNI(SPM) coordinate system. For anatomical label localization, statistical maxima of activation were converted from the MNI(SPM) coordinates to conform to the standard Talairach space [53] using BrainMapGingerALE (www.brainmap.org) and Talairach Client (www.talairach.org).
Results
We analyzed baseline smoking and neuroimaging data from 7 eligible patients. (Table 1). All of our patients were heavy smokers (lowest serum cotinine level was 75 ng/mL). Two subjects were on typical antipsychotics (haloperidol and trifluoperazine respectively), all others were on atypical neuroleptics (risperidone, paliperidone, aripiprazole or quetiapine).
| N=7 | Number or Average ± S.D | Range |
|---|---|---|
| Gender (Male/Female) | 5/2 | |
| Race (Caucasian/African-American) | 4/3 | |
| Age (years) | 44.7 ± 5.8 | 33-52 |
| Cigarettes/day | 11.5 ± 13.0 | 2-40 |
| Nicotine level (ng/mL) | 0 ± 0 | 0 |
| Cotinine level (ng/mL) | 272.5 ± 258.0 | 75-760 |
| FTND total | 5.8 ± 2.9 | 1-10 |
| Breath CO level (ppm) | 10.1 ± 13.2 | 0-39 |
| Craving for nicotine (%) | 77.9 ± 16.3 | 60-100 |
| Standard drinks/week | 14.6 ± 7.8 | 0-22 |
| Number of drinks/drinking day | 2.1 ± 1.1 | 0-4 |
| Total PANSS score | 71.1 ± 10 | 58-82 |
| PANSS positive score | 17 ± 3.5 | 11-22 |
| PANSS negative score | 15.3 ± 2.4 | 12-19 |
| PANSS general score | 34.4 ± 6.5 | 25-43 |
Table 1: Patient demographics, smoking and drinking severity, and psychosis severity (PANSS).
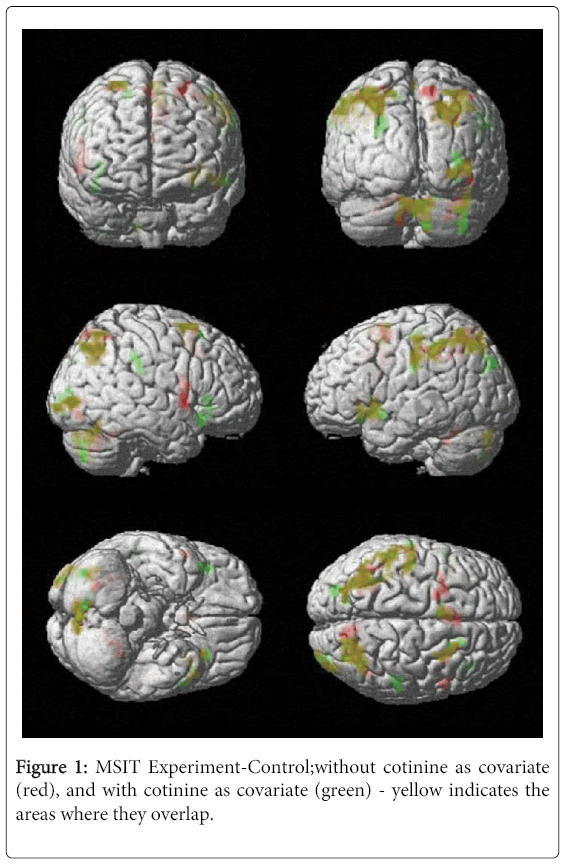
During the multi-source interference task (MSIT) task, we observed significant neural activation in left and right precuneus (Brodmann Areas [BA] 7 and 19), left and right inferior parietal lobule (BA 40), left superior frontal gyrus (BA 6) and the right insula. After including serum cotinine level as a covariate, the left precuneus (BA 7) and the left superior frontal gyrus (BA 6) were no longer significantly activated (Table 2). These results suggest that smoking severity may have influenced activation in the left (BA 7)precuneus and the left superior frontal gyrus (BA 6) during the MSIT task (Figure 1).
| MSIT Experiment-Control | |||||||
|---|---|---|---|---|---|---|---|
| cluster | cluster | voxel | MNI Coordinates | ||||
| p(cor) | size | T | x,y,z {mm} | Side | Region | BA | |
| <0.001 | 499 | 22.1 | 21 -69 42 | Right | Parietal Lobe | Precuneus | |
| 11.61 | 33 -75 45 | Right | Parietal Lobe | Precuneus | 19 | ||
| 6.29 | 9 -72 57 | Right | Parietal Lobe | Precuneus | 7 | ||
| 6.04 | 27 -66 57 | Right | Parietal Lobe | Precuneus | 7 | ||
| 4.15 | 9 -63 60 | Right | Parietal Lobe | Precuneus | 7 | ||
| 4.03 | 45 -54 54 | Right | Parietal Lobe | Superior Parietal Lobule | 7 | ||
| 2.98 | 33 -42 66 | Right | Parietal Lobe | Superior Parietal Lobule | 7 | ||
| 2.48 | 9 -72 45 | Right | Parietal Lobe | Precuneus | 7 | ||
| <0.001 | 1051 | 15.33 | 0 -81 -30 | Left | Posterior Lobe | Pyramis of Vermis | |
| 7.21 | -132 | Left | Posterior Lobe | Inferior Semi-Lunar Lobule | |||
| 7.12 | -6 -81 -27 | Left | Posterior Lobe | Pyramis | |||
| 11.36 | -6 -60 -9 | Left | Anterior Lobe | Culmen | |||
| 4.79 | -90 | Left | Anterior Lobe | * | Dentate | ||
| 6.88 | 24 -66 -39 | Right | Posterior Lobe | Cerebellar Tonsil | |||
| 6.77 | 42 -63 -18 | Right | Posterior Lobe | Declive | |||
| 6.42 | 39 -60 -45 | Right | Posterior Lobe | Cerebellar Tonsil | |||
| 5.13 | 33 -84 -33 | Right | Posterior Lobe | Pyramis | |||
| 5 | 39 -72 -30 | Right | Posterior Lobe | Tuber | |||
| 4.98 | 39 -66 -21 | Right | Posterior Lobe | Declive | |||
| 4.67 | 33 -69 -33 | Right | Posterior Lobe | Pyramis | |||
| 4.11 | 36 -72 -42 | Right | Posterior Lobe | Inferior Semi-Lunar Lobule | |||
| 3.76 | 6 -72 -18 | Right | Posterior Lobe | Declive | |||
| 5.87 | 33 -51 -27 | Right | Anterior Lobe | Culmen | |||
| 5.08 | 6 -63 -18 | Right | Anterior Lobe | Culmen | |||
| 4.99 | 3 -69 -24 | Right | Anterior Lobe | Pyramis | |||
| 4.46 | 42 -48 -33 | Right | Anterior Lobe | Culmen | |||
| 4.03 | 18 -57 -24 | Right | Anterior Lobe | * | Dentate | ||
| 4.51 | 33 -90 9 | Right | Occipital Lobe | Middle Occipital Gyrus | 18 | ||
| 0.002 | 245 | 14.19 | -36 9 -6 | Left | Sub-lobar | Claustrum | |
| 6.3 | -51 12 -9 | Left | Temporal Lobe | Superior Temporal Gyrus | 22 | ||
| <0.001 | 2018 | 10.84 | -24 -75 48 | Left | Parietal Lobe | Precuneus | 7 |
| 9.91 | -45 -39 45 | Left | Parietal Lobe | Inferior Parietal Lobule | 40 | ||
| 8.6 | -36 -63 51 | Left | Parietal Lobe | Superior Parietal Lobule | 7 | ||
| 6.93 | -42 -21 51 | Left | Parietal Lobe | PostcentralGyrus | 2 | ||
| 5.93 | -45 -60 51 | Left | Parietal Lobe | Inferior Parietal Lobule | 40 | ||
| 5.68 | -24 -75 54 | Left | Parietal Lobe | Precuneus | 7 | ||
| 7.78 | -18 0 57 | Left | Frontal Lobe | Sub-Gyral | 6 | ||
| 5.89 | -6 6 66 | Left | Frontal Lobe | Superior Frontal Gyrus | 6 | ||
| 5.69 | -24 6 60 | Left | Frontal Lobe | Sub-Gyral | 6 | ||
| 6.98 | -6 12 48 | Left | Limbic Lobe | Cingulate Gyrus | 24 | ||
| 5.74 | -9 9 39 | Left | Limbic Lobe | Cingulate Gyrus | 24 | ||
| 0.002 | 237 | 11.59 | -126 | Left | Temporal Lobe | Fusiform Gyrus | 37 |
| 5.21 | -126 | Left | Temporal Lobe | Middle Temporal Gyrus | 37 | ||
| 4.7 | -54 -63 0 | Left | Temporal Lobe | Inferior Temporal Gyrus | 19 | ||
| 4.5 | -57 -66 3 | Left | Temporal Lobe | Middle Temporal Gyrus | 37 | ||
| 2.72 | -60 -54 15 | Left | Temporal Lobe | Middle Temporal Gyrus | 39 | ||
| 3.58 | -42 -84 12 | Left | Occipital Lobe | Middle Occipital Gyrus | 19 | ||
| 3.12 | -30 -99 0 | Left | Occipital Lobe | Inferior Occipital Gyrus | 18 | ||
| 3.49 | -129 | Left | Posterior Lobe | Tuber | |||
| 0.002 | 233 | 6.28 | 60 18 27 | Right | Frontal Lobe | Inferior Frontal Gyrus | 9 |
| 0.007 | 202 | 5.88 | 54 -30 51 | Right | Parietal Lobe | Inferior Parietal Lobule | 40 |
| 4.05 | 54 -30 36 | Right | Parietal Lobe | Inferior Parietal Lobule | 40 | ||
| 3.8 | 60 -36 42 | Right | Parietal Lobe | Inferior Parietal Lobule | 40 | ||
| 5.58 | 54 -21 15 | Right | Sub-lobar | Insula | 40 | ||
| MSIT Experiment-Control with Cotinine Levels as Covariate | |||||||
| cluster | cluster | voxel | MNI Coordinates | ||||
| p(cor) | size | T | x,y,z {mm} | Side | Region | BA | |
| <0.001 | 1026 | 44.99 | 9 -75 -45 | Right | Posterior Lobe | Inferior Semi-Lunar Lobule | |
| 14.88 | 42 -48 -36 | Right | Posterior Lobe | Cerebellar Tonsil | |||
| 29.98 | -6 -60 -9 | Left | Anterior Lobe | Culmen | |||
| <0.001 | 1329 | 15.74 | -54 -36 45 | Left | Parietal Lobe | Inferior Parietal Lobule | 40 |
| <0.001 | 440 | 14.15 | 33 -75 45 | Right | Parietal Lobe | Precuneus | 19 |
| <0.001 | 193 | 17.7 | -36 9 -6 | Left | Sub-lobar | Claustrum | |
| <0.001 | 239 | 16.49 | 39 27 3 | Right | Frontal Lobe | Inferior Frontal Gyrus | 13 |
| 0.004 | 124 | 13.65 | -126 | Left | Temporal Lobe | Fusiform Gyrus | 37 |
| 5.68 | -120 | Left | Occipital Lobe | Middle Occipital Gyrus | 19 | ||
| <0.001 | 181 | 7.22 | 51 -30 51 | Right | Parietal Lobe | Inferior Parietal Lobule | 40 |
| 0.039 | 92 | 9.49 | -150 | Left | Posterior Lobe | Inferior Semi-Lunar Lobule | |
| 5.47 | -144 | Left | Posterior Lobe | Cerebellar Tonsil | |||
Table 2: MSIT Interference vs. Control task results without and with cotinine level as covariate.
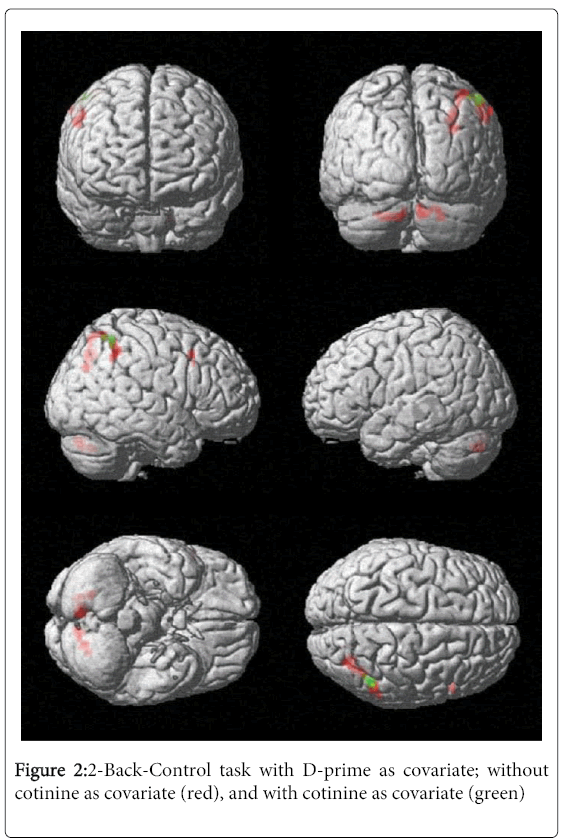
During the working memory (N-back) task we observed significant neural activation in the right precuneus and superior parietal lobule (BA 7), the right inferior parietal lobule (BA 40) and the right middle frontal gyrus (BA 6 and BA 8). After including serum cotinine level as a covariate, the right middle frontal gyrus (BA 6 and BA 8) was no longer significantly activated (Table 3). These results suggest that smoking severity may have influenced activation in the right middle frontal gyrus (BA 6 and BA 8) during the 2-back task (Figure 2).
| 2Back-Control Dprime Covariate | |||||||
|---|---|---|---|---|---|---|---|
| cluster | cluster | voxel | MNI Coordinates | ||||
| p(cor) | size | T | x,y,z{mm} | Side | Region | BA | |
| <0.001 | 16 | 34.59 | 9 -78 -33 | Right | Posterior Lobe | Uvula | |
| <0.001 | 30 | 22.49 | 33 -72 33 | Right | Parietal Lobe | Precuneus | 31 |
| 12.86 | 30 -66 45 | Right | Parietal Lobe | Precuneus | 7 | ||
| 8.48 | 42 -60 60 | Right | Parietal Lobe | Superior Parietal Lobule | 7 | ||
| 8.31 | 36 -66 60 | Right | Parietal Lobe | Superior Parietal Lobule | 7 | ||
| <0.001 | 11 | 21 | 24 -66 -36 | Right | Posterior Lobe | Cerebellar Tonsil | |
| 16.31 | 21 -78 -30 | Right | Posterior Lobe | Pyramis | |||
| <0.001 | 20 | 15.78 | 60 -45 48 | Right | Parietal Lobe | Inferior Parietal Lobule | 40 |
| 12.3 | 51 -51 51 | Right | Parietal Lobe | Inferior Parietal Lobule | 40 | ||
| 10.91 | 51 -48 45 | Right | Parietal Lobe | Inferior Parietal Lobule | 40 | ||
| 7.59 | 51 -48 57 | Right | Parietal Lobe | Inferior Parietal Lobule | 40 | ||
| <0.001 | 23 | 12.91 | -126 | Left | Posterior Lobe | Inferior Semi-Lunar Lobule | |
| 12.5 | -126 | Left | Posterior Lobe | Cerebellar Tonsil | |||
| 9.09 | -6 -81 -30 | Left | Posterior Lobe | Pyramis | |||
| <0.001 | 12 | 11.92 | 57 15 42 | Right | Frontal Lobe | Middle Frontal Gyrus | 6 |
| 10.74 | 54 18 36 | Right | Frontal Lobe | Middle Frontal Gyrus | 8 | ||
| 2Back-Control Dprime and Cotinine Levels Covariates | |||||||
| cluster | cluster | voxel | MNI Coordinates | ||||
| p(cor) | size | T | x,y,z {mm} | Side | Region | BA | |
| <0.001 | 10 | 31.91 | 51 -51 54 | Right | Parietal Lobe | Inferior Parietal Lobule | 40 |
| 28.51 | 48 -54 57 | Right | Parietal Lobe | Superior Parietal Lobule | 7 | ||
| 19.75 | 45 -57 60 | Right | Parietal Lobe | Superior Parietal Lobule | 7 | ||
Table 3: 2Back vs. Control task results without (first table) and with cotinine level as covariate (second table).
Conclusion
Our preliminary results suggest that smoking and associated alcohol and drug use might be important confounding variables in neuroimaging studies of schizophrenia. Using serum cotinine level as an objective measure of smoking severity we were able to observe the effect of smoking (or a factor associated with smoking severity) on neuronal activation. Activation of frontal lobe and left precuneus (BA7) during a cognitive processing task (MSIT) was related to smoking severity in alcohol dependent patients with schizophrenia. Precuneus (BA7) is an area responsible for visual orientation, eye movements, judgment of size and distance, and storage of motor sequences in spatial working memory [54].
During a working memory task (N-back) we found significant activation of the right middle frontal gyrus, right precuneus and right superior and inferior parietal lobule. Smoking was associated with activation of the middle frontal gyrus. Activation of BA7 and several other regions (e.g. left and right inferior parietal cortex, left and right DLPFC, left premotor cortex, left and right ACC) is observed in patients with schizophrenia during the N-back task (36). The lack of DLPFC activation in our patients might be related to the effect of alcohol or antipsychotic medications. Our patient’s psychosis severity (PANSS positive and total score) was relatively high. This might have also contributed to the lack of DLFPC activation. Decreased dorsolateral prefrontal cortex activation was observed in schizophrenic patients compared to control subjects in prior studies [55].
Limitations of the present study include its observational nature, small sample size, lack of non-schizophrenic, non-smoking and non-drinking control groups, and lack of repeated observations. Disease duration, duration of smoking and alcohol dependence, medication type and dose, and psychiatric, medical and neurological co-morbidities are important confounding factors, which we could not include in the statistical analyses due to the small sample size. Further, larger scale, naturalistic, longitudinal studies are needed to confirm our findings and to clarify the clinical significance of our observations, preferably without the confounding effect of alcohol use.
At present, it is unclear, whether changes in the left BA 7 activation in heavy smoking schizophrenic patients are related to nicotine, carbon monoxide or other chemicals in cigarette smoke, or other factors; e.g. alcohol use, psychosis severity, medications. Lack of activation in the right middle frontal gyrus [25] and anterior cingulate cortex [26] in heavy smokers may be a result of associated alcohol use.
Surprisingly, according to a recent study, executive function deficits are relatively stable in non-schizophrenic subjects; moderate to severe nicotine, alcohol, cannabis, and illicit drug use did not impair working memory in a 3-year follow-up fMRI study [56]. In contrast to these findings, our patients with schizophrenia developed nicotine dose dependent impairments in neural activation - this might be due to their unique genetic vulnerability to nicotine or chemicals in cigarette smoke or other confounding factors which correlate with smoking severity (e.g. alcohol, cannabis, cocaine use or high dose of antipsychotic medications).
In summary, our preliminary data suggest that smoking and associated alcohol and drug use might be important confounding variables in neuroimaging studies of schizophrenia. In order to draw firm conclusions, confirmatory studies are needed on non-alcohol dependent subjects. If these larger-scale studies confirm our findings, then routine measurement of serum cotinine level in patients with schizophrenia may improve reliability and diagnostic value of fMRI studies.
Acknowledgements
This study was funded by the Brain and Behavior Research Foundation (2008 Young Investigator Award PI: Dr. Meszaros). The sponsor was not involved in the study design, interpretation of data or writing of the report.
References
- Friedman L, Turner JA, Stern H, Mathalon DH, Trondsen LC, et al. (2008) Chronic smoking and the BOLD response to a visual activation task and a breath hold task in patients with schizophrenia and healthy controls. See comment in PubMed Commons below Neuroimage 40: 1181-1194.
- Leyba L, Mayer AR, Gollub RL, Andreasen NC, Clark VP (2008) Smoking status as a potential confound in the BOLD response of patients with schizophrenia. See comment in PubMed Commons below Schizophr Res 104: 79-84.
- Harrison BJ, Yücel M, Fornito A, Wood SJ, Seal ML, et al. (2007) Characterizing anterior cingulate activation in chronic schizophrenia: a group and single-subject fMRI study. See comment in PubMed Commons below ActaPsychiatrScand 116: 271-279.
- Carter CS, MacDonald AW 3rd, Ross LL, Stenger VA (2001) Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. See comment in PubMed Commons below Am J Psychiatry 158: 1423-1428.
- de Leon J, Diaz FJ (2005) A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. See comment in PubMed Commons below Schizophr Res 76: 135-157.
- Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW (1998) Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. See comment in PubMed Commons below Schizophr Res 33: 113-118.
- Hamera E, Schneider JK, Deviney S (1995) Alcohol, cannabis, nicotine, and caffeine use and symptom distress in schizophrenia. See comment in PubMed Commons below J NervMent Dis 183: 559-565.
- McEvoy JP, Freudenreich O, Levin ED, Rose JE (1995) Haloperidol increases smoking in patients with schizophrenia. See comment in PubMed Commons below Psychopharmacology (Berl) 119: 124-126.
- Strand JE, Nybäck H (2005) Tobacco use in schizophrenia: a study of cotinine concentrations in the saliva of patients and controls. See comment in PubMed Commons below Eur Psychiatry 20: 50-54.
- Kumari V, Soni W, Sharma T (2001) Influence of cigarette smoking on prepulse inhibition of the acoustic startle response in schizophrenia. See comment in PubMed Commons below Hum Psychopharmacol 16: 321-326.
- Evans DE, Drobes DJ (2009) Nicotine self-medication of cognitive-attentional processing. See comment in PubMed Commons below Addict Biol 14: 32-42.
- George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, et al. (2002) Effects of smoking abstinence on visuospatial working memory function in schizophrenia. See comment in PubMed Commons below Neuropsychopharmacology 26: 75-85.
- Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, et al. Rates and risk factors for dementia and alzheimer's disease: Results from EURODEM pooled analyses. EURODEM incidence research group and work groups. european studies of dementia. Neurology.52: 78-84.
- Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, et al. (1998) Smoking and risk of dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam Study. See comment in PubMed Commons below Lancet 351: 1840-1843.
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ (2006) A comparison of neurocognitive function in nonsmoking and chronically smoking short-term abstinent alcoholics. See comment in PubMed Commons below Alcohol 39: 1-11.
- Durazzo TC, Meyerhoff DJ, Nixon SJ (2010) Chronic cigarette smoking: implications for neurocognition and brain neurobiology. See comment in PubMed Commons below Int J Environ Res Public Health 7: 3760-3791.
- Meyerhoff DJ, Tizabi Y, Staley JK, Durazzo TC, Glass JM, et al. (2006) Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. See comment in PubMed Commons below Alcohol ClinExp Res 30: 253-264.
- Hill RD, Nilsson LG, Nyberg L, Bäckman L (2003) Cigarette smoking and cognitive performance in healthy Swedish adults. See comment in PubMed Commons below Age Ageing 32: 548-550.
- Schinka JA, Belanger H, Mortimer JA, Borenstein Graves A (2003) Effects of the use of alcohol and cigarettes on cognition in elderly African American adults. See comment in PubMed Commons below J IntNeuropsycholSoc 9: 690-697.
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, et al. (2001) Effect of nicotine on brain activation during performance of a working memory task. See comment in PubMed Commons below ProcNatlAcadSci U S A 98: 4728-4733.
- Richards M, Jarvis MJ, Thompson N, Wadsworth ME (2003) Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. See comment in PubMed Commons below Am J Public Health 93: 994-998.
- Brown GG, Thompson WK (2010) Functional brain imaging in schizophrenia: selected results and methods. See comment in PubMed Commons below Curr Top BehavNeurosci 4: 181-214.
- Blanchard JJ, Brown SA, Horan WP, Sherwood AR (2000) Substance use disorders in schizophrenia: review, integration, and a proposed model. See comment in PubMed Commons below ClinPsychol Rev 20: 207-234.
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, et al. (1990) Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. See comment in PubMed Commons below JAMA 264: 2511-2518.
- Luciana M, Collins PF, Muetzel RL, Lim KO (2013) Effects of alcohol use initiation on brain structure in typically developing adolescents. See comment in PubMed Commons below Am J Drug Alcohol Abuse 39: 345-355.
- Mashhoon Y, Czerkawski C, Crowley DJ, Cohen-Gilbert JE, Sneider JT, et al. (2014) Binge alcohol consumption in emerging adults: anterior cingulate cortical "thinness" is associated with alcohol use patterns. See comment in PubMed Commons below Alcohol ClinExp Res 38: 1955-1964.
- Luijten M, Machielsen MW, Veltman DJ, Hester R, de Haan L, et al (2014) Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci. 39:149-69.
- Batel P, Pessione F, Maître C, Rueff B (1995) Relationship between alcohol and tobacco dependencies among alcoholics who smoke. See comment in PubMed Commons below Addiction 90: 977-980.
- Pfefferbaum A, Adalsteinsson E, Sullivan EV (2006) Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. See comment in PubMed Commons below Neurobiol Aging 27: 994-1009.
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, et al. (1992) Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. See comment in PubMed Commons below Alcohol ClinExp Res 16: 1078-1089.
- Oscar-Berman M, Marinković K (2007) Alcohol: effects on neurobehavioral functions and the brain. See comment in PubMed Commons below Neuropsychol Rev 17: 239-257.
- Liu IC, Chiu CH, Chen CJ, Kuo LW, Lo YC, et al (2010). The microstructural integrity of the corpus callosum and associated impulsivity in alcohol dependence: A tractography-based segmentation study using diffusion spectrum imaging. Psychiatry Res184:128-34.
- Casey BJ, Cohen JD, O'Craven K, Davidson RJ, Irwin W, et al. (1998) Reproducibility of fMRI results across four institutions using a spatial working memory task. See comment in PubMed Commons below Neuroimage 8: 249-261.
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, et al. (2002) Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. See comment in PubMed Commons below Neuropsychology 16: 370-379.
- Blokland GA, McMahon KL, Hoffman J, Zhu G, Meredith M, et al. (2008) Quantifying the heritability of task-related brain activation and performance during the N-back working memory task: a twin fMRI study. See comment in PubMed Commons below BiolPsychol 79: 70-79.
- Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS (2004) Working memory capacity in schizophrenia: a parametric fMRI study. See comment in PubMed Commons below Schizophr Res 68: 159-171.
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA (2003) The Multi-Source Interference Task: validation study with fMRI in individual subjects. See comment in PubMed Commons below Mol Psychiatry 8: 60-70.
- Bush G, Shin LM (2006) The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. See comment in PubMed Commons below Nat Protoc 1: 308-313.
- Krishnan-Sarin S, Balodis IM, Kober H, Worhunsky PD, Liss T, et al. (2013) An exploratory pilot study of the relationship between neural correlates of cognitive control and reduction in cigarette use among treatment-seeking adolescent smokers. Psychol Addict Behav. 27:526-32.
- Meszaros ZS, Dimmock JA, Ploutz-Snyder RJ, Abdul-Malak Y, Leontieva L, et al. (2011) Predictors of smoking severity in patients with schizophrenia and alcohol use disorders. See comment in PubMed Commons below Am J Addict 20: 462-467.
- Benowitz NL, Jacob P 3rd (1994) Metabolism of nicotine to cotinine studied by a dual stable isotope method. See comment in PubMed Commons below ClinPharmacolTher 56: 483-493.
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J (2005) Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the united states between 1999 and 2004. Am J Epidemiol.169:236-48.
- Meszaros ZS, Abdul-Malak Y, Dimmock JA, Wang D, Ajagbe TO, et al. (2013) Varenicline treatment of concurrent alcohol and nicotine dependence in schizophrenia: a randomized, placebo-controlled pilot trial. See comment in PubMed Commons below J ClinPsychopharmacol 33: 243-247.
- McDermut W, Mattia J, Zimmerman M (2001) Comorbidity burden and its impact on psychosocial morbidity in depressed outpatients. See comment in PubMed Commons below J Affect Disord 65: 289-295.
- Sobell LC, Sobell MB, Maisto SA, Cooper AM (1985)Time-line follow-back assessment method. NIAAA treatment handbook series
- Yuan C1, Kosewick J, Wang S (2013) A simple, fast, and sensitive method for the measurement of serum nicotine, cotinine, and nornicotine by LC-MS/MS. See comment in PubMed Commons below J Sep Sci 36: 2394-2400.
- Gabr RQ1, Elsherbiny ME, Somayaji V, Pollak PT, Brocks DR (2011) A liquid chromatography-mass spectrometry method for nicotine and cotinine; utility in screening tobacco exposure in patients taking amiodarone. See comment in PubMed Commons below Biomed Chromatogr 25: 1124-1131.
- Heatherton TF1, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. See comment in PubMed Commons below Br J Addict 86: 1119-1127.
- Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. See comment in PubMed Commons below Schizophr Bull 13: 261-276.
- Mazaika P, Hoeft F, Glover G, Reiss A (2009) editors. Methods and software for fMRI analysis for clinical subjects.human brain mapping.
- Freire L, Mangin JF (2001) Motion correction algorithms may create spurious brain activations in the absence of subject motion. See comment in PubMed Commons below Neuroimage 14: 709-722.
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD (1995) Spatial registration and normalization of images. Human brain mapping 2:165-89.
- Talairach J, Tournoux P (1995) editors. Co-planar stereotaxic atlas of the human brain: Three-dimensional proportional system. New York, NY: Thieme Medical.
- Sadato N, Campbell G, Ibáñez V, Deiber M, Hallett M (1996) Complexity affects regional cerebral blood flow change during sequential finger movements. See comment in PubMed Commons below J Neurosci 16: 2691-2700.
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, et al. (1998) Functional hypofrontality and working memory dysfunction in schizophrenia. See comment in PubMed Commons below Am J Psychiatry 155: 1285-1287.
- Cousijn J, Vingerhoets WA, Koenders L, de Haan L, van den Brink W, et al. (2014) Relationship between working-memory network function and substance use: a 3-year longitudinal fMRI study in heavy cannabis users and controls. See comment in PubMed Commons below Addict Biol 19: 282-293.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 14772
- [From(publication date):
October-2014 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 10173
- PDF downloads : 4599