Review Article Open Access
Smoking, Periodontitis and Vascular Disease -Collaboration Study with Dentists and Vascular Surgeons
Takehisa Iwai1* and Makoto Umeda2
1Tsukuba Vascular Center, and Buerger Disease Research Institute, Tatsuzawa, Moriya city, Japan
2Department of Periodontology, Osaka Dental University, Japan
- Corresponding Author:
- Takehisa Iwai
Director and Head Tsukuba Vascular Center
and Buerger Disease Research Institute
Tatsuzawa, Moriya city, Japan
Tel: +81(297)45-47-9955
Fax: +81(297)45-4541
E-mail: iwai@keiyu.or.jp
Received Date: December 26, 2013; Accepted Date: February 05, 2014; Published Date: February 10, 2014
Citation: Iwai T, Umeda M (2014) Smoking, Periodontitis and Vascular Disease -Collaboration Study with Dentists and Vascular Surgeons. J Interdiscipl Med Dent Sci 2:113. doi: 10.4172/2376-032X.1000113
Copyright: © 2014 Iwai T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
Weak oral bacteria such as periodontal bacteria have been observed in various arterial and venous lesions with epidemiological data reported prior to the discovery of bacterial invasion into vessels. Rich lymph vessels easily bring the bacteria from the mouth to the neck and the venous angle, which is directly open to the blood vessels. Periodontal bacteria travel within platelets. Periodontal bacteria, especially P. gingivalis aggregates platelets and forms thrombus. At the same time, secretions such as serotonin, various cytokines, and adhesion factors also appear in the blood. The characteristic of the arterial lesions are dependent on the age of the patient and the condition of the endothelial cells. In young patients, infectious incidents occur due to embolic mechanisms in Buerger disease or adhesion to the superficial veins valves in varicose veins. In aged patients, incidents result in adhesion in the proximal aorta, coronary arteries or large arteries. The hypotheses here unify the evidence or vessel lesion development and explain possible discrepancy between vascular diseases. We were able to emphasize the collaboration study in this study with dentists and vascular surgeons.
Keywords
Dentist; Vascular surgeon; Weak oral bacteria; Buerger disease; Transportation of oral bacteria; Oral care
Introduction
Since 1999, various weak oral bacteria have been identified in atherosclerotic lesions [1]. Among these bacteria, Chlamydia pneumoniae which resides in the mouth, pharynx, or bronchus, has been thoroughly investigated and confirmed to be transported to vessel walls by monocytes [2]. This invasion mechanism appears to be a factor in the development of atherosclerosis. Additionally, cytomegalovirus can be absorbed from the oral cavity resulting in opportunistic infections. Recently, the so called inflammatory abdominal aortic aneurismal walls revealed the presence of cytomegalovirus [3]. Helicobacter pylori is well-known bacteria that resides in the stomach and may also appear in the oral cavity. This one was also identified in vessel walls. Over the past 13 years, the periodontal bacteria group that includes several species has been shown within vessel walls [4-6]. We, dentists and surgeons, started a study about periodontal bacteria invading the vessels in which we clarified the relationship between periodontal bacteria and vascular diseases, especially Buerger disease, which is still major vascular disease in south and western Asia [7].
Details of Buerger Disease Infection Theory
Many investigators, including Leo Buerger, believed that Buerger disease is an infectious disease [8]. They studied actual cases and conducted animal experiments, but nobody could find the pathogen. In 1928, Professor Allen of the Mayo Clinic was also suspicious about oral bacteria as a cause and mentioned that 75% of 87 Buerger disease sufferers showed periodontal infection and 80% showed tonsil enlargement or pus attachment [9]. Allen believed that Buerger disease was an infectious disease until he died in 1967. After his death, however, Buerger disease was classified as an inflammatory autoimmune disease.
Clinical diagnosis of Buerger disease is made using Shionoya’s criteria, namely 1) young heavy smoker under 50 years of age, 2) lower leg arterial occlusion, 3) phlebitis migrans or upper extremities arterial occlusion, 4) no risk factors for atherosclerosis except smoking. There are about 8000 persons registered in Japan. Thus, the total number of patients in Asia is estimated one million.
Periodontal Bacterial Invasion to the Arterial Wall and Thrombus
Because periodontal bacteria are not detectable with the usual cultures, they are extremely difficult to identify. Most periodontal bacteria are anaerobic and were difficult to detect by cultural method in 1960s but after the development of anaerobic culturing, the cultural detection of periodontal bacteria is not difficult. These includes more than 300 species overall. Usually 6-7 species are examined as representative bacteria nowadays. Therefore the established PCR (polymerase chain reaction) method to detect the DNA of the oral bacteria has become popular [10].
Until now, periodontal bacterial DNA was detected from carotid arterial plaques, coronary arterial plaques, abdominal aortic aneurysmal walls (86% of patients) and intraluminal thrombi (88%), atherosclerotic vessel plaques (52%), occluded arteries of Buerger disease patients (93%), migrating phlebitis samples (2 cases, 100%), and primary varicose veins (48%) [4-6]. In these studies, specimen were obtained from the vascular surgical unit and stored at -80°C. These specimens were then moved to be examined in the periodontal unit laboratory of the same university.
How are the Bacteria Transported to the Vessel Wall?
Our results using rats after continuous intravenous oral bacterial infusion showed newly formed thrombus in the small arteries of the extremities with 50% of the specimens having bacterial DNA [11].
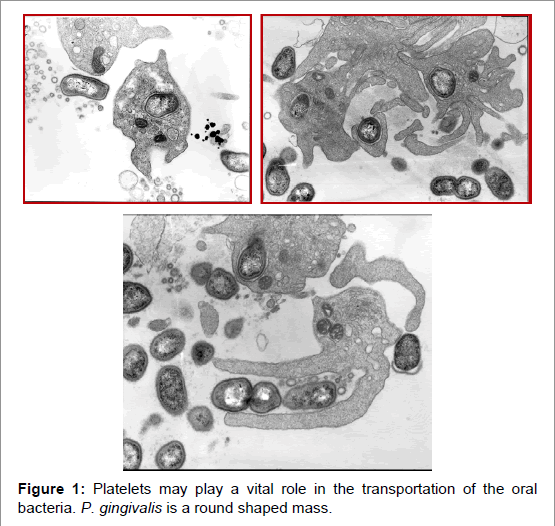
In 2004, we, vascular surgeons, began using the Platelets Rich Plasma (PRP) to stimulate good wound healing. When we accidentally added periodontal bacteria (P. gingivalis) to the sample and saw the mixed fluid through a stereoscopic microscope, we could find active movement. After examining the sample by electron microscopy, we observed that periodontal bacteria (P. gingivalis) were engulfed by platelets and morphologically there were no change observed in the bacteria for one hour. Additionally, platelet aggregation was also observed. These observations confirmed that P. gingivalis bacteria aggregate strongly in the platelets, and they don’t die (Figure 1) [12].
P. gingivalis induced platelet aggregation reached the maximum in a few minutes and the mass became more than 20 microns larger than the size of the small artery in vitro. Additionally, as periodontitis itself expresses inflammatory substances such as IL-6, and TNFα, it should be considered a systemic disorder [13]. A strong relationship with diabetes mellitus control is coming from the abovementioned mechanism. Fortunately, for our healthcare is now associated with good social manners.
Serum Bacterial Antibody Titer Changes in Periodontal Disease and Buerger Disease
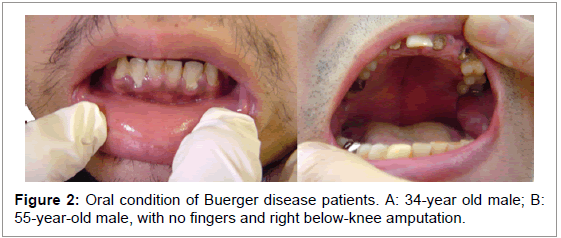
Chen et al. [14] who are dentists, reported that the antibody titer for periodontal bacteria is significantly elevated in Buerger disease patients, reconfirming that Buerger disease patients have very poor periodontal conditions [5] (Figure 2). In Buerger disease the antibody titers may actually be changing related to the severity of periodontitis. Effective antibiotic treatment against the bacteria can decrease the titer level rapidly.
Do All Persons with Serious Periodontitis Develop Buerger Disease or another Vascular Arterial Disorder?
Recent studies on Buerger disease have shown a specific HLA locus and infection susceptibility for basilar bacteria, such as periodontal bacteria [15]. Varicose veins seem to occur in mothers and daughters. Interestingly approximately 50% of varicose veins contain periodontal bacterial DNA by our study suggesting that pregnancy may be linked to varicosity development when the woman suffers from periodontitis during pregnancy. However it is questionable that the periodontal bacteria may transmit from mothers to daughters.
Hypothesis and Future Views of Buerger Disease Development
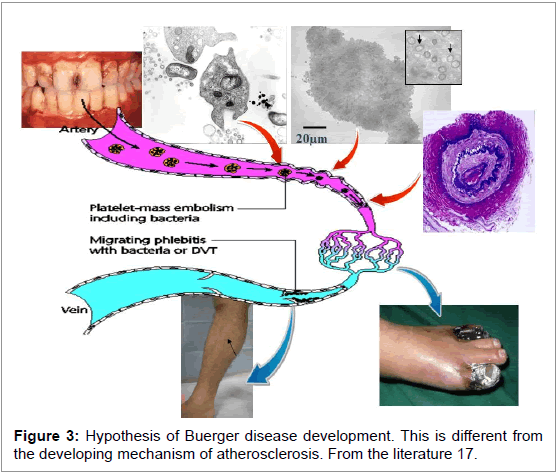
Pathogens in Buerger disease are likely to be mainly periodontal bacteria. Among periodontal bacteria, T. denticola and P. gingivalis are main member of red complex, bacterial group characteristic of periodontitis patients. P. gingivalsis is inevitably moved as an initiator of platelet aggregation, and from the venous angle of the neck, the bacteria group can enter the blood stream and stimulate platelet aggregation after uptake into platelets. It is suggested that aggregation reaches a maximum level when the platelet thrombi passes through the lung, after which the thrombi starts to move in the arterial blood stream. When the arterial wall is young but spastic from cigarette smoking, the platelet thrombus containing the oral bacteria do not adhere to the arterial wall but form a small arterial embolism. It is suggested that the digital arterial obstruction in Buerger disease patient angiography may be initial findings. This change will grow to the proximal arterial regions due to packing. Microorganisms that pass through capillaries can be caught at the venous valves, resulting in phlebitis migrans or deep vein thrombosis formation in the extremities. The literature shows small arterial changes are very common all over the body, but are symptomatic only in the extremities (Figure 3). This mechanism is specific in Buerger disease and considerably different from atherosclerotic disease including aneurysms [16,17].
References
- Chiu B (1999) Multiple infections in carotid atherosclerotic plaques. Am Heart J 138: S534-536.
- Epstein SE, Zhou YF, Zhu J (1999) Infection and atherosclerosis: emerging mechanistic paradigms. Circulation 100: e20-28.
- Yonemitsu Y (1998) Viruses and vascular disease. Nat Med 4: 253-254.
- Kurihara N, Inoue Y, Iwai T, Umeda M, Huang Y, et al. (2004) Detection and localization of periodontopathic bacteria in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 28: 553-558.
- Iwai T, Inoue Y, Umeda M, Huang Y, Kurihara N, et al. (2005) Oral bacteria in the occluded arteries of patients with Buerger disease. J Vasc Surg 42: 107-115.
- Kurihara N, Inoue Y, Iwai T, Sugano N, Umeda M, et al. (2007) Oral bacteria are a possible risk factor for valvular incompetence in primary varicose veins. Eur J Vasc Endovasc Surg 34: 102-106.
- Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, et al. (2007) Treatment of periodontitis and endothelial function. N Engl J Med 356: 911-920.
- Buerger L(1914) Isthromboangiitis an infectious disease? Surg Gynecol Obstet 19:582-588.
- Allen EV, Brown GE(1928) Thromboangiitisobliterans. A clinical study of 200 cases. Ann Intern Med 1:535-549.
- Ashimoto A, Chen C, Bakker I, Slots J (1996) Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol 11: 266-273.
- Kubota T, Inoue Y, Iwai T, Kurihara N, Huang Y, et al. (2008) Arterial thrombosis after intravenous infusion of oral bacterium in a rat model. Ann Vasc Surg 22: 412-416.
- Li X, Iwai T, Nakamura H, Inoue Y, Chen Y, et al. (2008) An ultrastructural study of Porphyromonas gingivalis-induced platelet aggregation. Thromb Res 122: 810-819.
- Chen YW, Umeda M, Nagasawa T, Takeuchi Y, Huang Y, et al. (2008) Periodontitis may increase the risk of peripheral arterial disease. Eur J VascEndovascSurg 35: 153-158.
- Chen YW, Iwai T, Umeda M, Nagasawa T, Huang Y, et al. (2007) Elevated IgG titers to periodontal pathogens related to Buerger disease. Int J Cardiol 122: 79-81.
- Chen Z, Takahashi M, Naruse T, Nakajima T, Chen YW, et al. (2007) Synergistic contribution of CD14 and HLA loci in the susceptibility to Buerger disease. Hum Genet 122: 367-372.
- Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, et al. (2012) Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association?: A scientific statement from the American heart association. Circulation 125:2520-2544.
- Iwai T, Umeda M, Inoue Y (2012) Are There Any Objections against Our Hypothesis That Buerger Disease Is an Infectious Disease? Ann Vasc Dis 5: 300-309.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 17204
- [From(publication date):
April-2014 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 12487
- PDF downloads : 4717