Smoking Cessation Outcomes among Individuals with Substance Use and/or Psychiatric Disorders
Received: 06-Sep-2011 / Accepted Date: 19-Oct-2011 / Published Date: 24-Oct-2011 DOI: 10.4172/2155-6105.1000115
Abstract
Objectives: The population of individuals with substance use (SUD) and/or psychiatric disorders (PD) has a high prevalence of smoking and a consequent increase in tobacco-related morbidity and mortality when compared to the general population. The aim of this study is to examine the outcomes of a program in a real-life setting which takes a tailored approach to smoking cessation among individuals with SUD and/or PD.
Methods: A retrospective chart review of tailored tobacco dependence treatment was performed on individuals with histories of SUD and/or PD attending a Tobacco Dependence Clinic (TDC) program in Vancouver, British Columbia, Canada. Participants of the TDC received a combination of behavioural counselling and pharmacotherapy for smoking cessation. Data from 540 participants enrolled in the TDC between September 2007 and May 2011 was reviewed. Outcome measures included seven-day point-prevalence abstinence (validated by expired carbon monoxide) and program completion rates.
Results: For individuals who completed the program the abstinence rate was 41.1% (167/406). Significant predictors of successful smoking cessation were: a) a lower expired carbon monoxide level at baseline (OR=.98, 95%CI=.96-1.00), and b) a longer duration of treatment (OR=1.09, 95%CI=1.05-1.12). Significant predictors of program completion were: a) being female (OR=1.86, 95%CI=1.21-2.87).
Discussion: Tailored smoking cessation among individuals with SUD and/or PD yields modest end-of-treatment smoking cessation rates and can be an effective approach to reducing the burden of tobacco use in substance use and mental health treatment settings.
Keywords: Smoking cessation (SC); Substance use disorders (SUD); Psychiatric disorders (PD); Tailored interventions
Introduction
Tobacco use remains the number one preventable cause of morbidity and mortality in Canada [1]. Based on the Canadian Tobacco Use Monitoring Survey (CTUMS), at present smoking rates for Canadians aged 15 years and older is 18%, representing about 4.9 million smokers [2]. Although there has been a steady decline in the overall smoking prevalence in Canada during the last decade [3], some subsets of the population continue to smoke at high rates, particularly individuals with a history of substance use disorders (SUD) and/or psychiatric disorders (PD). Studies suggest that these individuals often have disproportionately higher mortality than the general U.S. population [4] - mortality which is often smoking related [5]. In the U.S., studies have estimated smoking rates of up to four times the national average among clinical populations with SUD and PD [6-8]. Although there are no estimates for smoking among individuals with PD in Canada as a whole, a recent survey among individuals with severe and persistent mental illness accessed through Community Mental Health Centres in Western Canada reported a smoking prevalence of 46.8% [9]. This high prevalence of smoking among individuals with SUD and PD clearly warrants a need for targetted efforts to reduce the disease burden and increased mortality risk among these often disparate sub-populations.
Fortunately, individuals with SUD and PD are often willing to engage in smoking cessation treatment with some measure of success. A survey among participants in a methadone maintenance treatment program found that 80% of smokers expressed an interest (i.e., responded: ‘somewhat’ or ‘very’ interested) in quitting smoking [10]. In a more recent review of nine studies assessing the motivation to quit smoking among individuals with PD, over 50% of smokers in this population contemplated quitting smoking in the next 60 days [11]. Moreover, previous studies have demonstrated that smoking cessation interventions among individuals with co-occurring SUD’s may result in improved health outcomes [12] and drug abstinence [13-16]. A meta-analysis of nineteen studies addressing smoking cessation interventions among individuals in addictions treatment and recovery found that such interventions resulted in increased smoking cessation at 12 week (although non-significant at 6-month follow-up) and a 25% likelihood of long-term abstinence from alcohol and illicit drugs [17]. These findings were corroborated by a recent review of the literature which found that smoking cessation interventions do not impair cessation from other substances of abuse and may actually enhance the success of substance abuse treatment [18]. Thus, with the current state of evidence, smoking cessation is not only contemplated but can result in improved health (and other substance abuse) outcomes among individuals with SUD and/or PD.
The purpose of this study is to report smoking cessation outcomes from a program with an innovative, tailored approach to smoking cessation among individuals with SUD and/or PD histories accessing outpatient addiction services [19]. Since its inception in September 2007, the Tobacco Dependence Clinic (TDC) has provided services to over 700 tobacco dependent individuals with a history of SUD and/or PD. The specific aims of this study are to examine:
a) Smoking cessation outcomes at end-of-treatment
b) Smoking cessation outcomes by histories of SUD, PD, or both
c) Predictors of successful smoking cessation
d) Predictors of successful program completion.
Methods
Setting and participants
The TDC provides smoking cessation treatment under the auspices of Vancouver Coastal Health (VCH) Mental Health and Addiction Services. Eligibility to participate in the TDC is based upon 4 main criteria:1) 19 years or older, 2) tobacco dependent, 3) history of a substance use disorder and/or a mental illness, and 4) financially disadvantaged (i.e., individuals who had no source of income and/ or were on disability and/or demonstrated that their primary source of income is based on governmental assistance). Intake assessment includes obtaining of a medical, psychiatric, and previous tobacco use and treatment history, and vital signs and expired carbon monoxide (CO) monitoring. Participants also sign a consent form for information from their charts to be used for an ongoing program evaluation of the TDC. Currently, the TDC has seven operating sites at VCH Community Health Centres in Vancouver, BC.
Treatment approach and program completion
Current evidence-based guidelines recommend combining behavioural therapies with pharmacotherapy for optimal tobacco dependence treatment outcomes [20]. In keeping with these guidelines, the TDC program provides behavioural counselling with no-cost pharmacotherapy. The TDC program approach further assumes that smoking cessation is a process and not an event; as such, no set ‘quit date’ is emphasized in the program. Rather, behavioural counselling is provided during 8, weekly, “structured” group sessions of 1.5 hours duration followed immediately by a further optional 18, weekly, “support” group sessions of 1 hour duration. The behavioural counseling sessions aim to enhance motivation and self-efficacy to quit smoking as well as to foster skills for relapse prevention and maintaining abstinence. Group sessions are based on a manual that is standardized between clinics and facilitated by a drug and alcohol counselor (see supplemental materials). Non-attendance at group is followed up by a supportive telephone call by a nurse/counsellor.
Moreover, the novelty of the TDC program approach to tobacco dependence treatment is that pharmacotherapy is tailored to the participants’ need in terms of dose, duration of treatment, and potentially combining multiple smoking cessation medications. Unlike many traditional smoking cessation programs which provide pharmacotherapy for durations of 8 to 12 weeks, the TDC program employs, the principle of “titrating to effect” in which participants are offered nicotine replacement therapy (i.e., nicotine patch, gum, lozenge, and inhaler) and/or oral medications (i.e., bupropion and varenicline) at higher doses and for longer durations (up to 26 weeks) to optimize participants efforts in smoking cessation. This approach to pharmacotherapy is in recognition that traditional smoking cessation programs often use conservative pharmacotherapy approaches which may, in effect, ‘under dose’ participants, particularly individuals with SUD and/or PD which smoke at higher rates and have higher nicotine dependence than the general population. The medications are dispensed weekly after each group session during a brief consultation with the TDC nurse or physician. Each visit is charted, including type of medication dispensed and expired CO monitoring (where appropriate). Program completers are defined as those who complete at least 6 weeks of the 8-week structured program (allowing for two missed sessions). Non-completers are those who engaged for less than 6 weeks before disengaging or dropping out from the program.
Measures
Baseline sociodemographic data included sex (male vs. female) and age (in years). In addition, a smoking history obtained at the participants’ initial visit to the program included age at smoking initiation, number of cigarettes smoked per day (cigs per day), and importance and confidence in quitting smoking (both on a scale of 0 to 10) [21,22]. Moreover, information on substance use (categorized as: none, alcohol, opiates, cocaine, marijuana, methamphetamine and related drugs) and psychiatric disorder (categorized as: none, mood disorder, anxiety disorder, psychotic disorder) histories were obtained. The psychiatric disorder history was assessed primarily by patient history and review of current and/or past medications. Finally, data was obtained from participant charts on the type of pharmacotherapy received during treatment (i.e., monotherapy vs. combination therapy), Fagerstrom Test for Nicotine Dependence (FTND) scores at beginning of treatment [23,24], weekly expired carbon monoxide (CO) levels measured in parts per million (ppm) [25], and total number of weeks in treatment.
Data analysis
Retrospective data from participants’ charts were extracted by two research assistants for those enrolled in the seven TDC sites from September 2007 to May 2011.The characteristics of the sample were described using frequencies and means (M) with standard deviations (SD). Differences between program completers and non-completers for all study variables were analyzed using chi-square (with degrees of freedom=DF) for categorical and ordered categorical variables and independent sample t-tests (with Levine’s test for equality of variance) for all continuous variables. Of the 739 individuals that engaged with the TDC in the specified time frame, 196 (26.5%) had 2 or less contacts with the program (including intake assessment and orientation to the program) and were deemed insufficiently engaged in the program. We compared baseline characteristics between those who remained in the program and those who had 2 or less contacts and found that there were no differences in the sex (χ2=0.07, DF=1, p=.785) or self-disclosed diagnosis of a psychiatric disorder (χ2=0.67, DF=3, p=.879); however, individuals who did not engage in the program were younger in age (45.1yrs vs. 48.0yrs, p=.003), had lower mean nicotine dependence scores (5.7 vs. 6.1, p=.040) and had on average lower expired CO levels at baseline (18.3ppm vs. 20.7ppm, p=.027). Furthermore, 3 individuals described their gender as ‘other’ than male or female and where excluded from further analysis. Thus the analysis is based on 540 individuals.
The primary outcome measure was smoking status at end-Of treatment. End-of-treatment was defined as completing the 8-week structured group and stopping treatment (in consultation and agreement with program providers) any time within the additional nine to eighteen weeks of support group therapy. Successful smoking cessation was defined using seven-day point-prevalence of smoking abstinence (i.e., not smoking in the past 7 days, based on participant self-report with biochemical validation of expired CO<8ppm when available). If an expired CO level was not recorded on the participant’s chart at the end-of-treatment, we used a next-observation-Carried backward (NOCB) or last-observation-carried-forward (LOCF) method [26], from the weekly expired CO record, validated by participants self-report of no cigarette use in the preceding week to determine abstinence. If there was no record in the chart regarding smoking status or available expired CO, the participant was considered still smoking and a treatment failure (n=60). Counting such missing records as failures is an approach which will conservatively underestimate (but not inflate) the effects of the treatment.
The secondary outcomes were to examine smoking cessation by SUD and PD history, by length of stay in the program, and to assess predictors of successful smoking cessation and program completion. We employed chi-square analyses to examine smoking cessation by histories of SUD, PD, and co-occurring disorders (i.e., none vs. SUD only vs. PD only vs. both SUD and PD) and by length of stay in the program. To assess predictors of smoking cessation and program completion, we employed a two-step model building procedure [27]. In the first step, crude odds ratios (reported in Odds Ratio=OR, and 95% Confidence Intervals=95%CI) were used to determine the associations between smoking cessation and all study variables. In the second step, only variables associated with smoking cessation (alpha <.10) were included in the final multivariate analyses using adjusted odds ratios. In assessing predictors of smoking cessation, the final logistic regression analysis was further stratified by gender to determine disaggregated differences in smoking cessation outcomes among women and men. The Hosmer-Lemeshow test was employed to assess global goodness-of-fit. For analyses, a p-value of .05 or lower was used to determine statistical significance. All analyses were performed using PASW statistics version 18.0.
| Total (N = 540) |
Completersb (n = 406) |
Non-completersc (n = 134) |
Difference** | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | χ2 (df), p | |
| Gender | 9.53 (1), .002 | ||||||
| Female | 210 | 38.9 | 173 | 42.6 | 37 | 27.6 | |
| Male | 330 | 61.1 | 233 | 57.4 | 97 | 72.4 | |
| Primary Substance Use History | 5.20 (5), .393 | ||||||
| None | 65 | 12.0 | 51 | 12.6 | 14 | 10.4 | |
| Alcohol | 183 | 33.9 | 129 | 31.8 | 54 | 40.3 | |
| Opiates* | 64 | 11.9 | 51 | 12.6 | 13 | 9.7 | |
| Cocaine | 140 | 25.9 | 105 | 25.9 | 35 | 26.1 | |
| Marijuana | 63 | 11.7 | 52 | 12.8 | 11 | 8.2 | |
| Methamphetamine and related drugs | 25 | 4.6 | 18 | 4.4 | 7 | 5.2 | |
| Primary Mental Health Diagnosisa | 3.36 (3), .339 | ||||||
| None | 125 | 23.1 | 88 | 21.7 | 37 | 27.6 | |
| Mood disorder | 301 | 55.7 | 227 | 55.9 | 74 | 55.2 | |
| Anxiety disorder | 77 | 14.3 | 63 | 15.5 | 14 | 10.4 | |
| Psychotic disorder | 37 | 6.9 | 28 | 6.9 | 9 | 6.7 | |
| Pharmacotherapyd | 1.45 (1), .228 | ||||||
| Monotherapy | 141 | 26.9 | 103 | 25.6 | 38 | 31.1 | |
| Combination therapy | 383 | 73.1 | 300 | 74.4 | 84 | 68.9 | |
| Mean | SD | Mean | SD | Mean | SD | t (df), p | |
| Age of participant (years) | 48.1 | 11.0 | 48.3 | 11.0 | 47.4 | 10.9 | .833 (536), .405 |
| Age at smoking initiation | 14.8 | 4.9 | 14.9 | 5.0 | 14.5 | 4.7 | .739 (532), .460 |
| Importance of quitting | 9.0 | 1.3 | 9.0 | 1.3 | 9.0 | 1.4 | .286 (520), .775 |
| Confidence in quitting | 7.3 | 2.2 | 7.3 | 2.3 | 7.4 | 2.1 | .330 (515), .741 |
| Cigarettes smoked per day | 21.0 | 10.3 | 21.1 | 10.6 | 20.6 | 9.5 | .469 (538), .639 |
| FTNDf at baseline | 6.1 | 2.1 | 6.1 | 2.1 | 6.1 | 2.0 | .325 (532), .746 |
| CO level at baseline | 20.7 | 12.5 | 20.6 | 12.1 | 21.2 | 13.5 | .482 (520), .630 |
a. The ‘mood disorder’ category includes primarily individuals with depression and bipolar disorder. The ‘anxiety disorder’ category includes individuals primarily with generalized anxiety disorder, posttraumatic stress disorder, and panic disorders. The ‘psychotic disorder’ category includes individuals with a primary past history of schizophrenia, schizoaffective disorder, psychotic disorder.
b. Program completers are individuals who had at least 6 contacts with the Tobacco Dependence Clinic program. The typical program involves 8 weeks (8 contact points) of a closed group therapy session. Individuals could miss no more than two weeks (two contacts) to still have meaningful participation in the program. Of program completers, 8.4% (n =17) had 6 or 7 contacts with the program.
c. Program non-completers are individuals who had engaged in the program for more than two weeks, but had a total of less than 6 contact points (i.e., less than 6 weeks in the program).
d. Pharmacotherapy represents the form of treatment (i.e., monotherapy vs. combination therapy) in the first month of treatment.
e. mean differences between completers and non-completers is expected as, by definition, non-completers did not have more than 6 weeks (6 contacts) with the program.
f. FTND = Fagerstrom Test for Nicotine Dependence. This is a six-item questionnaire of nicotine dependence, scored on a scale of ‘0’ (low) and ‘10’ (high).
* opiates (i.e., oxycontin, codeine, methadone, heroin)
** differences between groups was based on chi-square analysis for categorical variables and independent sample t-tests for continuous variables.
Table 1: Sample Characteristics.
Results
Sample characteristics
The participants were mostly male (61.1%) with a mean age of 48.1 (SD=11.0) years. The most prevalent SUD and PD were alcohol use disorders (33.9%) and mood disorders (55.7%), respectively, and 68% of the sample had co-occurring SUD and PD. Approximately 75.2% of participants completed the program, with significantly more females completing the program compared to males. On average, program completers (n=406) attended the TDC for 14.6 weeks (SD=6.8) whereas non-completers (n=134) attended for 4 weeks (SD=0.8). There were no other differences in baseline characteristics between program completers and non-completers (Table 1).
Smoking cessation at end-of-treatment
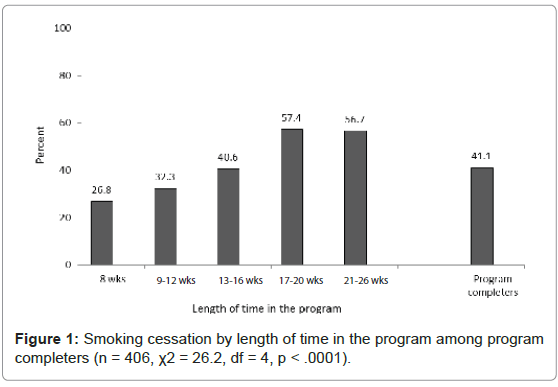
Among program completers, 41.1% (167/406) achieved abstinence by end-of-treatment. There was a significant likelihood of smoking cessation with increased length of stay in treatment (χ2=26.2, DF=4, p< .0001, see Figure 1). A greater proportion of men achieve smoking cessation as compared to women (42.1% vs. 39.9%) albeit this difference was non-significant (p = .660).
Smoking cessation by history of SUD and/or PD
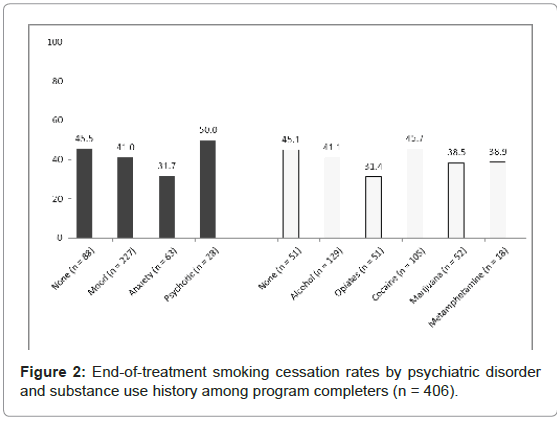
There were no significant differences in smoking cessation between different diagnostic groups for those with histories of a SUD (χ2=3.44, DF=5, p=.633) or a PD (χ2=3.88, DF=3, p=.274, see Figure 2). When the participants were categorized by history of co-occurring psychiatric and substance use disorder, we found these individuals were less likely to achieve smoking cessation than those with no disorder, a SUD only, or a PD only (no disorder=43.8% vs. SUD only=46.7% vs. PD only=45.7% vs. co-occurring=38.9%), however, the differences between groups were non-significant.
Predictors of smoking cessation
Among the total sample of program completers, in the unadjusted logistic regression analyses of the first-step in the model building process, psychiatric disorder history (particularly anxiety disorder), use of monotherapy in the first 4 weeks of treatment, lower nicotine dependence scores, lower expired CO levels at baseline, and higher total number of visits to the TDC were associated with smoking cessation at alpha < 1.0. However, in the adjusted multivariate analyses, having an anxiety disorder (as compared to no psychiatric disorder) lower FTND scores and expired CO levels at baseline, and having longer treatment duration were significant predictors of smoking cessation at the end-of-treatment (Table 2). However, when the data was disaggregated by sex, in the adjusted multivariate analysis among both women and men only having lower CO levels at baseline and longer treatment duration significantly predicted smoking cessation at the end-of-treatment.
Predictors of successful program completion
In the first-step of the model building process, being female (OR=1.95, 95%CI=1.27-2.98) and having an anxiety disorder (relative to no psychiatric disorder) (OR=1.03, 95%CI=1.00-1.05) remained predictive of program completion at alpha < 1.0. However in the adjusted model only being female remained predictive of program completion. [n=540, Hosmer-Lemeshow goodness-of-fit: χ2=2.87 (DF=4), p=.579].
Conclusions
This study presents the smoking cessation and program completion outcomes of individuals with a history of SUD and/or PD in an outpatient treatment setting. Moreover, these findings are an update of the findings from a pilot study of outcomes from the TDC [19]. Similar to the earlier pilot study, end-of-treatment smoking cessation outcomes were 41.1% among program completers. These end-of-treatment findings are comparable to those reported by similar tailored approaches to smoking cessation among individuals with SUD and/or PD. For example, among 231 male cigarette smokers enrolled in a Veterans Affairs Mental Health Clinic Smoking Cessation Program, Gershon Grand et al. [28] reported end-of-treatment outcomes of 36.4%. Also, in a recent examination of a tailored smoking cessation program among individuals with psychiatric disorders, Selby et al. [26] reported seven-day point-prevalence quit rates of 20.6% at end-of-treatment. Moreover, the pooled results of 18 studies of smoking cessation interventions among individuals in addictions treatment and recovery reported smoking cessation rates of 20.5% among participants in the treatment groups vs. 8.6% among participants in the control groups at end-of-treatment (RR = 1.82, 95% CI = 1.45-2.29); [17] however, these studies did not specifically provide tailored smoking cessation interventions. This study presents the smoking cessation and program completion outcomes of individuals with a history of SUD and/or PD in an outpatient treatment setting. Moreover, these findings are an update of the findings from a pilot study of outcomes from the TDC [19]. Similar to the earlier pilot study, end-of-treatment smoking cessation outcomes were 41.1% among program completers. These end-of-treatment findings are comparable to those reported by similar tailored approaches to smoking cessation among individuals with SUD and/or PD. For example, among 231 male cigarette smokers enrolled in a Veterans Affairs Mental Health Clinic Smoking Cessation Program, Gershon Grand et al. [28] reported end-of-treatment outcomes of 36.4%. Also, in a recent examination of a tailored smoking cessation program among individuals with psychiatric disorders, Selby et al. [26] reported seven-day point-prevalence quit rates of 20.6% at end-of-treatment. Moreover, the pooled results of 18 studies of smoking cessation interventions among individuals in addictions treatment and recovery reported smoking cessation rates of 20.5% among participants in the treatment groups vs. 8.6% among participants in the control groups at end-of-treatment (RR = 1.82, 95% CI = 1.45-2.29); [17] however, these studies did not specifically provide tailored smoking cessation interventions.
| Total Sampled | Womend | Mend | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95%CI | Odds Ratio | 95%CI | Odds Ratio | 95%CI | |
| Primary Mental Health Diagnosisa | ||||||
| None (reference) | 1.00 | - | 1.00 | - | 1.00 | - |
| Mood disorder | .78 | .45-1.35 | 1.75 | .61-5.03 | .54 | .27-1.07 |
| Anxiety disorder | .45* | .22-.94 | .86 | .25-2.93 | .37 | .13-1.05 |
| Psychotic disorder | .89 | .35-2.25 | 2.54 | .56-11.60 | .62 | .17-2.25 |
| Pharmacotherapy | ||||||
| Monotherapy | 1.00 | - | - | - | - | - |
| Combination Therapy | .66 | .40-.109 | - | - | - | - |
| Confidence in quitting | - | - | - | - | 1.13 | .99-1.29 |
| FTNDc at baseline | .88* | .70-1.00 | - | - | .93 | .80-1.09 |
| COc level at baseline (ppm) | .98** | .96-1.00 | .97* | .94-1.00 | .97* | .94-1.00 |
| Total number of visitsc to the TDC | 1.09*** | 1.05-1.12 | 1.07** | 1.02-1.13 | 1.08*** | 1.04-1.13 |
aThe ‘mood disorder’ category includes primarily individuals with depression and bipolar disorder. The ‘anxiety disorder’ category includes individuals primarily with generalized anxiety disorder, posttraumatic stress disorder, and panic disorders. The ‘psychotic disorder’ category includes individuals with a primary past history of schizophrenia, schizoaffective disorder, psychotic disorder.
bProgram completers are individuals who had at least 6 contacts with the Tobacco Dependence Clinic program. The typical program involves 8 weeks (8 contact points) of a closed group therapy session. Individuals could miss no more than two weeks (two contacts) to still have meaningful participation in the program.
cFTND = Fagerstrom Test for Nicotine Dependence. This is a six-item questionnaire of nicotine dependence, scored on a scale of ‘0’ (low) and ‘10’ (high). CO = Expired Carbon monoxide. This is measured in parts per millimeter (ppm). For FTND, CO level at baseline, and Total number of visits, the odds ratios represent the odds of smoking cessation per each unit increase in the variable.
dHosmer-Lemeshow goodness-of-fit results for Total sample (n= 388): χ2= 8.02 (DF=8), p=.432; for Women (n = 167): χ2= 7.30 (DF=8), p=.505; and for Men (n = 218):
χ2= 14.00 (DF=8), p=.082
*p <.05, **= p<.01, *** = p <.001.
Table 2: Adjusted predictors of smoking cessation for 26 weeks for program completersb by gender.
In our current study, although individuals with histories of alcohol, opiates, and/or marijuana use and a history of co-occurring SUD and PD were less likely to achieve smoking cessation compared to those without such histories, the difference was not statistically significant. In a pilot study we had found that individuals with alcohol, opiate and marijuana use were significantly less likely to achieve cessation as compared to those without an SUD [19]. The non-significant findings in our current study may be a reflection of the more rigorous tailoring of medications, in terms of using combination therapy with participants of the TDC. At least in theory, tailored approaches, when appropriately delivered should not produce differences in outcomes between diagnostic groups as has been observed in a previous study [26]. However, our assumptions can only be speculative at best given the observational nature of our current study.
However, we found that lower expired CO levels at baseline and a longer duration of treatment remained significant predictors of successful smoking cessation. This finding is also supported by previous studies using such tailored approaches [26]. Moreover, our present findings further suggest that, on average, the benefit of length of treatment peaks at between 4 to 5 months (i.e., 16 to 20 weeks) duration. Therefore, when considering the optimal duration of tailored smoking cessation programming among individuals with SUD and/ or PD, our findings would suggest longer-term pharmacotherapy and counselling support beyond the traditional 8-12 week treatment models.
In addition, we found that although there are no significant differences between men and women in terms of smoking cessation outcomes, women are twice as likely to complete the program when compared to men. Few studies have examined gender differences in program completion for smoking cessation programs in addictions treatment settings. A previous study found sex-disaggregated differences in predictors of program completion among men and women in a Health Management Organization (HMO) -based substance abuse program [29]. In the study, predictors of program completion among women included higher income and legal/agency referral, whereas older age predicted completion among men [29]. Although our current study did not obtain demographic measures other than sex and age of participants, we did find that older age was a predictor of program completion, and specifically among men.
We had anticipated that using combinations of pharmacotherapy would have been an important significant predictor of abstinence due to recent evidence-based recommendations [30]. Surprisingly, when we compared those who were given some form of combination therapy (i.e., combination NRT and/or NRT with oral medications) with those who were given only a monotherapy (i.e., either a single NRT product or a single oral medication), we found that those given the combination therapy were less likely to achieve cessation compared to those given monotherapy, albeit, the difference was not significant in the multivariate analysis. As this study is not a controlled trial, it is challenging to know the reasons why we obtained such findings. However, future controlled trials may be required to assess the effectiveness of combination therapy over monotherapy among individuals with SUD and/or PD.
One limitation of this study relates to the verification of smoking abstinence based on expired CO monitoring. Counted as treatment failures were 11% (i.e. 60/540) of participants without available expired CO levels at the end-of-treatment. Hence, the smoking cessation rates at end-of-treatment may be an underestimate. A second limitation is the retrospective design of the study which may introduce incomplete study groups and interpretive bias during the chart review. Moreover, because it is an observational study, no random assignment of participants to treatment groups was performed. A third limitation is that due to the transient nature of the population accessing the TDC services, follow-up beyond end-of-treatment was challenging, although several attempts were made. Without such follow-up data, we cannot determine the long-term effectiveness of these tailored smoking cessation outcomes. A fourth limitation is that beyond gender and age, no other socio-demographic variables (i.e., income, education) were obtained from the participants. However, all participants had to meet the criteria of being financially disadvantaged in order to participate in the TDC program. Despite the inherent limitations in the design of the study, we believe this study is an important addition to the literature examining the effectiveness of tailored approaches to smoking cessation among individuals with SUD and/or PD in real life treatment settings.
In summary, the findings of our study provide some evidence to suggest that an intensive, tailored approach to smoking cessation among individuals with SUD and/or PD’s can yield modest end-of treatment quit rates. Such an approach can yield high retention rates among participants, partly because it reduces barriers to smoking cessation by providing pharmacotherapy and counselling services at no-cost. Future studies may determine the benefits of combination therapy over monotherapy in this population, in addition to determining the long-term effect of such interventions. Such studies will be important to provide practice and policy directions in terms of the provision of smoking cessation in addictions and mental health treatment settings.
Acknowledgements
This study was made possible through a financial contribution from Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.
The authors would like to thank Lindsay Killam who synthesized the intervention manuals. The authors would also like to acknowledge the dedication of the many clinicians in the Tobacco Dependence Clinics and the clients who made this work possible.
Financial Disclosure
Dr. Milan Khara has received unrestricted research funding, speaker’s honoraria, consultation fees or product from the following organisations/ companies in the previous 12 months: Health Canada, Interior Health Authority, Pfizer, TEACH, Quit Now Services, Ottawa Heart Institute, Johnson and Johnson, Provincial Health Services Authority, College of Physicians and Surgeons of British Columbia.
Dr. Chizimuzo Okoli has received consultation fees from Vancouver Coastal Health Authority and Pfizer in the past 12 months.
References
- Makomaski-Illing EM, Kaiserman MJ (2004) Mortality attributable to tobacco use in Canada and its regions, 1998. Can J Public Health 95: 38-44.
- Canadian Tobacco Use Monitoring Survey (2010) CTUMS 2010 Wave 1 Survey Results.
- Canadian Tobacco Use Monitoring Survey (2009) Overview of Historical Data, 1999-2009.
- Colton CW, Manderscheid RW (2006) Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev of Chronic Dis 3: A42.
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, et al. (1996) Mortality following Inpatient addictions treatment: Role of tobacco use in a community-based cohort. JAMA 275: 1097-1103.
- Kalman D, Morissette SB, George TP (2005) Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict 14: 106 - 123.
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA, et al. (2004) Nicotine dependence and psychiatric disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 61: 1107-1115.
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, et al. (2000) Smoking and mental illness: A population-based prevalence study. JAMA 284: 2606-2610.
- Johnson J, Ratner P, Malchy L, Okoli CT, Procyshyn RM, et al. (2010) Gender-specific profiles of tobacco use among non-institutionalized people with serious mental illness. BMC Psychiatry 10: 101.
- Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH (2001) Tobacco use and quit attempts among methadone maintenance clients. Am J Public Health 91: 296-299.
- Siru R, Hulse GK, Tait RJ (2009) Assessing motivation to quit smoking in people with mental illness: a review. Addiction 104: 719-733.
- McCarthy WJ, Zhou Y, Hser YI, Collins C (2002) To smoke or not to smoke: Impact on disability, quality of life, and illicit drug use in baseline polydrug users. J Addict Dis 21: 35-54.
- Lemon SC, Friedmann PD, Stein MD (2003) The impact of smoking cessation on drug abuse treatment outcome. Addict Behav 28: 1323-1331.
- Mc Carthy WJ, Collins C, Hser YI (2002) Does cigarette smoking affect drug abuse treatment? Journal of Drug Issues 32: 61-80.
- Friend KB, Pagano ME (2005) Changes in cigarette consumption and drinking outcomes: Findings from Project MATCH. J Subst Abuse Treat 29: 221-229.
- Kalman D, Kahler CW, Garvey AJ, Monti PM (2006) High-dose nicotine patch therapy for smokers with a history of alcohol dependence: 36-week outcomes. J Subst Abuse Treat 30: 213-217.
- Prochaska JJ, Delucchi K, Hall SM (2004) A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol 72: 1144-1156.
- Baca CT, Yahne CE (2009) Smoking cessation during substance abuse treatment: What you need to know. J Subst Abuse Treat 36: 205-219.
- Khara M, Okoli CTC (2011) The Tobacco-Dependence Clinic: Intensive tobacco-dependence treatment in an addiction services outpatient setting. Am J Addict 20: 45-55.
- Fiore M, Jaén C, Baker T, (2008) Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service.
- Kahler CW, LaChance HR, Strong DR, Ramsey SE, Monti PM, et al. (2007) The commitment to quitting smoking scale: Initial validation in a smoking cessation trial for heavy social drinkers. Addict Behav 32: 2420-2424.
- Burke MV, Ebbert JO, Hays JT (2008) Treatment of Tobacco Dependence. Mayo Clin Proc 83: 479-484.
- Etter J-F, Duc TV, Perneger TV (1999) Validity of the Fagerstrom test for nicotine dependence and of the Heaviness of Smoking Index among relatively light smokers. Addiction 94: 269-281.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86: 1119-1127.
- Middleton ET, Morice AH (2000) Breath carbon monoxide as an indication of smoking Habit. Chest 117: 758-763.
- Selby P, Voci SC, Zawertailo LA, George TP, Brands B (2010) Individualized smoking cessation treatment in an outpatient setting: Predictors of outcome in a sample with psychiatric and addictions co-morbidity. Addict Behav 35: 811-817.
- Hosmer D, Lemeshow S (2000) Applied logistic regression. 2nd ed. New York: Wiley.
- Gershon Grand RB, Hwang S, Han J, George T Brody AL (2007) Short-term naturalistic treatment outcomes in cigarette smokers with substance abuse and/or mental illness. J Clin Psychiatry 68: 892-898.
- Green CA, Polen MR, Dickinson DM, Lynch FL Bennett MD (2002) Gender differences in predictors of initiation, retention, and completion in an HMObased substance abuse treatment program. J Subst Abuse Treat 23: 285- 295.
- Clinical Practice Guideline Treating TobaccoUse and Dependence 2008 Update Panel, Liaisons, and Staff (2008) A Clinical Practice Guideline for Treating Tobacco Use and Dependence: 2008 Update: A U.S. Public Health Service Report. Am J Prev Med 35: 158-176.
Citation: Khara M, Okoli CTC (2011) Smoking Cessation Outcomes among Individuals with Substance Use and/or Psychiatric Disorders. J Addict Res Ther 2:115. DOI: 10.4172/2155-6105.1000115
Copyright: © 2011 Khara M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15487
- [From(publication date): 10-2011 - Dec 22, 2024]
- Breakdown by view type
- HTML page views: 11052
- PDF downloads: 4435