Research Article Open Access
Skin Reactions Associated to Phenytoin Administration: Multifactorial Cause
| Marta Vázquez1*, Pietro Fagiolino1, Silvana Alvariza1,2, Manuel Ibarra1, Cecilia Maldonado1, Raquel González2, Amalia Laborde2, Manuel Uria3,Antonella Carozzi3 and Carlos Azambuja3 | |
| 1Pharmaceutical Sciences Department, Faculty of Chemistry, Universidad de la República, Uruguay | |
| 2Toxicology Department, Hospital de Clínicas, Universidad de la República, Uruguay | |
| 3Genia - Genetics Molecular Laboratory, Uruguay | |
| Corresponding Author : | Prof. Marta Vázquez Head of the Biopharmacy and Therapeutics Area of the Pharmaceutical Sciences Department Faculty of Chemistry, Avenida General Flores 2124 P.O. Box 1157, 11800 Montevideo,Uruguay Tel: 598-2-2097899 (int 215) E-mail: mvazquez@fq.edu.uy |
| Received November 08, 2012; Accepted December 17, 2012; Published December 19, 2012 | |
| Citation: Vázquez M, Fagiolino P, Alvariza S, Ibarra M, Maldonado C, et al. (2014) Skin Reactions Associated to Phenytoin Administration: Multifactorial Cause. Clin Pharmacol Biopharm 3:125. doi:10.4172/2167-065X.1000125 | |
| Copyright: © 2014 Vázquez M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Purpose: Cutaneous reactions can be associated with phenytoin administration. Such reactions can be explained by the formation of reactive species (arene oxide and quinones) capable of interacting covalently with cell macromolecules during phenytoin metabolism. Enzymes involved in reactive species detoxification are polimorphically expressed in humans. A genetic abnormality leading to a defective microsomal epoxidase hydrolase (main detoxification enzyme) activity could be one of the causes leading to this kind of adverse effect, but not the only one. The purpose of this study was to give a deeper insight into the main causes leading to skin reactions.
Methods: Cutaneous reactions experienced by some healthy volunteers enrolled in a pharmacokinetic study of phenytoin were analyzed in depth. The activity of the microsomal epoxidase enzyme was determined.
Results: Six out of twelve healthy volunteers receiving phenytoin in multiple doses exhibited rash. More female subjects or volunteers with a rapid input of the drug and/or a faster phenytoin metabolism or defective microsomal epoxidase hydrolase activity experienced these cutaneous reactions.
Conclusions: Arene oxide metabolite seems to be the responsible entity for cutaneous reactions. The genesis of this adverse effect after phenytoin administration is multifactorial revealing that other risks factors (not only the genetic one) such as being a woman in the fertile life period or under contraceptive therapy, or a rapid drug input and /or a faster phenytoin metabolism could lead to a higher formation rate of the arene oxide.
| Keywords |
| Cutaneous reactions; Phenytoin; p-hydroxy-phenytoin; Arene oxide |
| Introduction |
| Cutaneous reactions are a common adverse effect associated with antiepileptic drugs (AEDs) and a cause of treatment discontinuation. Such reactions range from mild rashes to more serious conditions such as Stevens-Johnson syndrome and toxic epidermal necrolysis [1]. With phenytoin (PHT), for instance, skin rashes occur in up to 16% of patients at the beginning of therapy [2], with a lower percentage (7%) with chronic administration while hypersensitivity syndrome is much less common (1 in 5000 to 10 000 patients) [3]. |
| PHT as well as carbamazepine and phenobarbital treatments are associated with cutaneous eruptions, mainly during the first weeks of treatment. The rash is commonly presented as an exanthematous eruption and is often preceded by a history of fever and lymphadenopathy [4,5]. |
| In all these drugs, the generation of reactive metabolites (arene oxides and quinones) is believed to be an essential component in the development of these hypersensitivity reactions. These metabolites are responsible for interacting covalently with cellular macromolecules [6], because of their higher reactivity in comparison with the parent drug. |
| Although the liver is known to be the most important site for drug metabolism in the body, extrahepatic metabolism (for example in skin) is likely to have an important role in these kinds of reactions. The reactive species formed are so reactive that they are unable to survive long in circulation so the liver will receive a greater exposure but is not the first organ to suffer the injury as it is better protected (high glutathione and N-acetylcysteine levels) and it is an immunologically privileged organ [7]. Thus, the formation of these metabolites in skin and the observable damage in this organ first is a feasible fact as cytochrome enzymes are also present in this organ and it is not as protected as the liver [8-10]. |
| There is evidence that rapid accumulation of AEDs (for example by rapid infusion or rapid drug initiation), or of their metabolites, increases risk for these reactions in susceptible patients [11]. So unbalanced bioactivating and detoxifying pathways can result in increased covalent adduct (hapten) formation. |
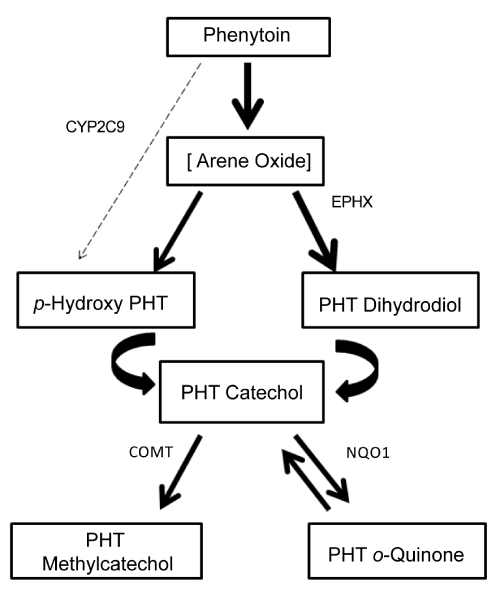
| To clarify this concept, PHT biotransformation pathway is presented in Figure 1. Its main route of biotransformation is para-hydroxilation (via arene oxide intermediate) to form 5-(4-hydroxyphenyl)-5- phenylhydantoin (p-HPPH). Further oxidation of p-HPPH leads to catechol formation (3’-4’-diHPPH), by several P450 enzymes. The catechol spontaneously oxidizes to form a reactive o-quinone that can be metabolized back to the catechol by NADPH-dependent quinone oxidoreductase (NQO1). The catechol can also be metabolized to the methylcatechol by catechol o-methyl transferase (COMT), which is then eliminated in the urine [1]. This enzyme reduces the amount of catechol that can be oxidized to the o-quinone. On the other side, the arene-oxide can also be converted to trans-dihydrodiol phenytoin via microsomal epoxide hydrolase (EPHX), which can also lead to catechol formation. |
| Enzymes involved in the metabolism pathway such as EPHX, NQO1 and COMT are polimorphically expressed in humans [12,13]. In the case of EPHX, two of these polymorphisms 113 Tyr-His in exon 3 (SNP rs 1051740, EPHX1) and 139 His-Arg in exon 4 (SNP rs 2234922, EPHX2) have been associated with a decrease or increase in enzyme activity respectively. Although the reaction catalyzed by EPHX results in detoxification (arene oxide toxicity is increased when epoxide hydrolase is inhibited or defective), some more reactive and mutagenic compounds (o-quinone metabolites) can be formed if its activity is increased. |
| The level of COMT enzyme activity is genetically polymorphic in human tissues with a trimodal distribution: low, intermediate and high activities [13]. Val/Val genotype has the highest activity, Val/ Met intermediate and Met/Met the lowest. This latter genotype can decrease the detoxification pathway and more quinone metabolite can be formed. |
| Increased arene-oxide or quinone formation due to decreased epoxide hydrolase or decreased COMT activity respectively can exert toxic effect. |
| Previous work has suggested that the predisposition to such reactions is based on a genetic abnormality in the detoxification of reactive metabolites of the drugs [14]. However, perhaps this could not be the only cause for such adverse effect. |
| In the current study, we examined data obtained from a pharmacokinetic study in healthy volunteers comparing two different dosage regimes, using two brands of phenytoin, by means of PHT and p-HPPH plasma concentrations. Cutaneous reactions were detected in some subjects who immediately discontinued the study and the activity of EPHX and COMT was determined. The aim of this work was to have a deeper insight of the genesis of the skin reactions observed. |
| Materials and Methods |
| Clinical design |
| Pharmacokinetic data was taken from a PHT study involving two different treatments A and B with the same administration rate but with different dosing interval (A: 600 mg of PHT every 3 days, during 10 days and B: 100 mg of PHT every 12 hours during 10 days). This clinical trial was performed in 12 healthy Caucasian subjects (6 men and 6 women) according to an open-label, randomized, multipledose, two-period (I and II) crossover design with a wash-out period of 5 days. Two brands of PHT were used: Epanutin® capsules (Pfizer Laboratories) for 6 volunteers and tablets commercially available in our country (Comitoina®, Roemmers Laboratories) for the other six. Subject characteristics are summarized in Table 1. Four volunteers under Epanutin treatment had familial bonds (3 siblings and 1 cousin). |
| The study protocol was approved by the Institutional Ethics Review Committee of the Faculty of Chemistry – Universidad de la República, Uruguay. Written consent was obtained from all subjects prior their participation in the study. |
| Sample collection and analytical methodology for drug determination |
| On day 10, blood samples were withdrawn at morning predose, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 15, 16, 24, 36, 48, 72 and 96 hours after dose, and placed in heparinized tubes. Plasma was separated by centrifugation and stored at -25°C until analysis. |
| PHT and p-HPPH plasma concentrations were determined by a high performance liquid chromatography method based on a procedure previously developed [15] with minor modifications. |
| Fifty microliters of internal standard solution (nitrazepam, 16 μg/mL in methanol) were added to 500 μL of plasma. The extraction of analytes was performed by adding 3 mL of ethyl acetate and then vortexed for 1 minute. After centrifugation, the supernatant was separated and dried under nitrogen stream at 37-40°C. Dry residue was dissolved with 100 μL of mobile phase and 20 μL injected into a Dionex Ultimate 3000 series chromatograph. A Phenomenex® Luna C18 (5 μm, 150 mm × 4.6 mm) column was used as a reversed stationary phase. The mobile phase was a mixture of water/methanol/acetonitrile (43:47:10) pumped with a flow rate of 1.0 mL/min. The column compartment was kept at 40°C and the wavelength detection was 220 nm. Under these conditions, the retention times of analytes were 3.5, 4.5 and 5.8 for p-HPPH, PHT and nitrazepam respectively. |
| The HPLC method was linear between 0.5 (the lower limit of quantification, LLOQ) and 25.0 mg/L for PHT, and between 0.05 and 3.0 mg/L for p-HPPH. Inter and intraday coefficients of variation (CVs) were below 15% and the accuracy of the method was between 85-115% for both analytes. |
| Sample collection and analytical methodology for genetic determination |
| Blood sample (5 mL) was collected by venipuncture in sterile, siliconised, ethylenediaminetetra-acetic acid (EDTA) tubes. Immediately after collection, blood was stored in a refrigerator (4-8°C) until analysis. Genomic DNA was isolated from whole blood using the Wizard® genomic DNA purification kit, according to the manufacturer´s instructions (Promega). Then, it was quantified by spectrophotometry (260/280 nm) in NanoQuant-Tecan and, according to this method of quantification, a dilution was performed in order to put 3 to 20 ng in the reaction mix, for the EPHX1 and EPHX2 genotyping protocol, and between 50 and 100 ng, for the COMT genotyping protocol. To determine the genotype of EPHX1 and EPHX2 a real time-PCR (Polymerase chain reaction) protocol was used, TaqMan Drug Metabolism Genotyping Assay (Applied Biosystems kits), for rs1051740 and rs2234922 performed in StepOne (Applied Biosystems). To determine COMT activity, a conventional PCR was performed, the PCR product was verified by gel electrophoresis in polyacrylamide 6%, 5 μL of the PCR product was purified and sequenced with specially designed primers and BigDye® Terminator v3.1 Cycle Sequencing Kit, then the product was purified with Big Dye X-Terminator Purification kit and a capillary electrophoresis in ABI3500 sequencer was performed. |
| Pharmacokinetic analysis |
| The area under the steady-state plasma concentration versus time curve (AUCss 0-T) was calculated using the trapezoidal rule until the end of the interval of administration (T). Beta (β), the first order elimination rate constant, was calculated from the slope of the terminal log-linear concentration-time regression in Treatment A. In Treatment B, β was estimated once the drug was discontinued (from day 11th). Steady state average concentration (Cmean ss) was determined as AUCss0-T /T and elimination half- life (t1/2) as 0.693/β. |
| In vitro dissolution study |
| Six units of each product were tested in Distek® dissolution system 2100C equipment. The conditions were: USP Apparatus 2 (paddle); 75 rpm stirring speed; volume 900 mL of water; temperature 37 ± 0.5°C. Samples were automatically withdrawn by the use of an Agilent 89092EO pump at: 5, 10, 15, 20 and 30 minutes for capsules; and 10, 15, 20, 40, 60, 80, 100 and 120 minutes for tablets. The drug release at different time intervals was measured by UV-visible spectrophotometer (Agilent 8453 and ChemStation® software). |
| Results and Discussion |
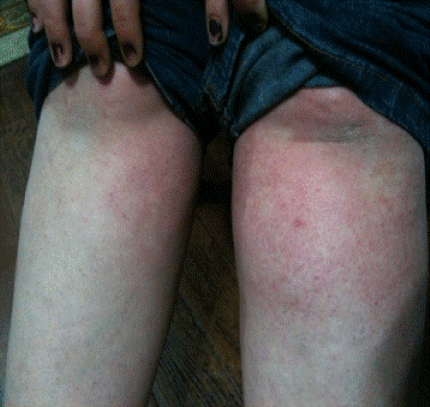
| Six subjects (1 male and 5 female) showed mild skin reactions (four subjects in Epanutin group and two subjects in Comitoina group). The exanthem observed consisted of widespread pink-to-red flat spots as shown in Figure 2. The rash was confined to trunk and legs in all of them. In two subjects the reaction started with fever and over the next days, cutaneous reactions appeared. Laboratory analysis showed no elevations in transaminases or abnormalities in liver function tests in the six subjects. Blood abnormalities were no present either. Treatment was discontinued in these subjects and a H1 antihistamine was administered. All recovered completely after discontinuation of treatment. |
| Tables 2 and 3 show the main pharmacokinetic values, rash occurrence and the activity of the main detoxification enzyme (EPHX) in all volunteers. COMT activity was only investigated in those subjects with rash. Four of the subjects experienced cutaneous reactions at the 10th day of treatment. Volunteer C5 presented rash the third day of exposure after changing treatment and volunteer C4 the third day of her first exposure to PHT. |
| All individuals with cutaneous rash presented mutations in the EPHX (increased or decreased activities). Subjects with low activity were homozygous for His 113 or heterozygous for His 113 in combination with homozygosity for His 139. Subjects with intermediate activity were homozygous for both Tyr 113 and His 139 or heterozygous for both Tyr 113 and His 139. Subjects with high activity were homozygous for Arg 139 or heterozygous for Arg in combination with homozygosity for Tyr 113 [16]. |
| Although the hydrolysis of arene oxide catalyzed by the microsomal epoxide hydrolase generally leads to detoxification, some derivatives formed undergo additional metabolism resulting in much more reactive compounds such as o-quinone metabolites that can bind to DNA. NQO1 activity was not investigated in this study since none of the volunteers with rash had low activity of COMT, and only a combination of low COMT and low NQO1 activities could result in increased amounts of o-quinone metabolites. Hence, an increased production and/or decreased detoxification of arene oxide metabolites seemed to be the cause in triggering the immune response. Genetic polymorphisms of EPHX in some individuals increase susceptibility to these reactions but awareness that it could not be the only factor involved should be kept in mind. |
| Volunteers in Epanutin group |
| The four subjects with rash were family members (3 siblings and 1 cousin) and all of them had a decreased activity in EPHX confirming the inheritance of a predisposition to these reactions. So, a family history of a drug hypersensitivity reaction, as it was observed, should alert physicians to the probability of a markedly increased risk of an adverse reaction in family members. The other two volunteers did not evidence any reactions although one of them (E1) showed a decreased activity of EPHX. As it can be observed in Table 2, he presented a lower β value for PHT, so probably a lower formation rate of the reactive metabolite could be the cause for not having the adverse reaction. |
| Volunteers in Comitoina group |
| Two subjects (C4 and C5, female) had cutaneous reactions. C4 had a decreased activity of EPHX. The rest of the volunteers did not experience rash and they had intermediate or increased EPHX activity. Besides, C4 exhibited a high β for PHT (Table 3), similar to the affected individuals of Epanutin group, explaining the sudden appearance of rash in this subject. Subject C5 was the only female under contraceptive therapy. It is reported in the literature [17] that exogenous estrogens represent a potential factor for the development of neoplasms at certain organs where such hormones are metabolized to reactive metabolites. One possible explanation for rash appearance in subject C5 could be the generation, during estrogens metabolism, of reactive intermediates such as arene oxides. So, in this subject, the reactive metabolite coming from two different xenobiotics could be the cause of the observed reaction. |
| Influence of drug delivery from formulations and drug elimination rate in the individuals |
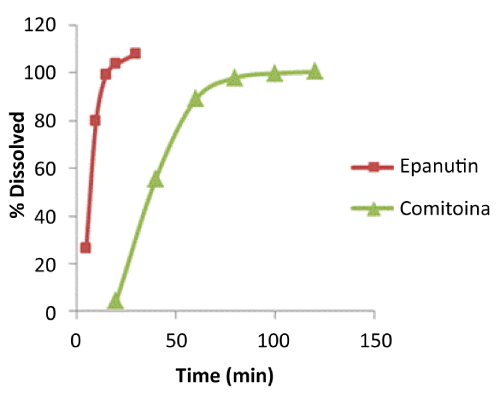
| Not only the detoxification pathway but also the rate of formation of this toxic metabolite must be taken into account. Mean dissolution profiles of both formulations are shown in Figure 3. As it can be seen in the figure, the two patterns of dissolution behavior are completely different and they clearly show the two products releasing drug at different rates. At 15 minutes, Comitoina did not start to dissolve whereas the percentage dissolved of Epanuntin at 10 minutes was 80%. Comitoina behaves as a slow-release tablet. A more rapid dissolution of Epanutin, and hence a more rapid absorption could impact notoriously in the clinical setting. |
| More volunteers under Epanutin treatment experienced skin reactions in comparison to subjects under Comitoina treatment as our study evidenced. As it was mentioned above, subject E1 did not experience a cutaneous reaction even though he received a fast drug release formulation and he had low activity of EPHX. So, in the group of affected volunteers, regardless the decreased EPHX activity, an increased rate of formation of the arene oxide, either because of a rapid drug delivery or a rapid drug metabolism (Tables 2 and 3), could be inferred. Drug formulations that dissolve PHT quickly and short PHT half-lives of patients could exacerbate arene-oxide formation. |
| Influence of the sex of individuals |
| As it is observed in this study, more women than men developed rash. According to some authors, women are more prone than men to develop skin reactions to AEDS [18]. They claimed that female sex steroids enhance immune responses, mainly in the reproductive life period compared to male in the same age group. Another study [19] reported no gender differences in skin lesions rates provoked by AEDS considering patients above the age of 50. Other authors [20] reported a higher incidence of rash with nevirapine, a non-nucleoside reverse transcriptase inhibitor, in the second or third trimester of pregnancy. During pregnancy, the production of steroid hormones greatly increases [21]. Once again, it can be said that a higher level of estradiol can be the cause of these findings and therefore, of a higher production of arene oxide intermediate. |
| Self-attenuation of rash by inducer drugs |
| Oral chronic administration of PHT induces microsomal epoxide hydrolase [22]. This could explain why the percentage of patients with toxicity, as it was mentioned in the introduction, is higher at the early stage of the treatment than after a chronic one. A lesser exposure of reactive metabolite during chronic administration could be due to enzyme-induction. So in some cases, the chronic administration of the drug itself could be the solution to the problem rather than drug withdrawal. |
| PHT has inductive properties (concentration-dependant) not only on enzymes but also on efflux transporters [23-25]. This efflux transporter overexpression in the liver (biliary canaliculi) deviates PHT metabolism from a region (hepatocytes) with high content of CYP2C9/CYP2C19 (enzymes responsible for PHT metabolism) to a region (enterocytes) with low content of these enzymes [26], resulting in a lower PHT metabolism and thus a lower arene-oxide production. |
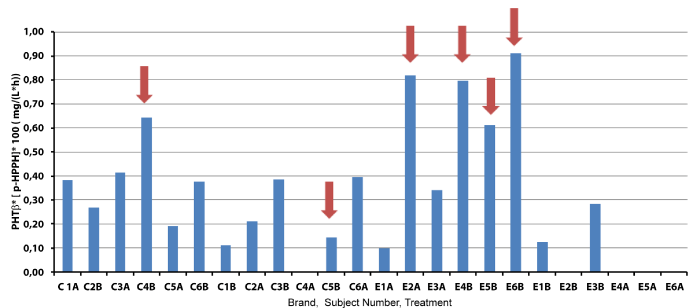
| Figure 4 shows PHT elimination rate (β) multiplied by the plasma concentration of the main metabolite produced during PHT metabolism. As the unstable arene oxide metabolite cannot be measured, its formation could be inferred by p-HPPH concentration [p-HPPH]. When EPXH activity is defective more arene oxide is accumulated that can, in turn, be available to interact with macromolecules or to form more p-HPPH. A rapid PHT elimination (higher β) can also result in rapid formation of arene oxide, regardless EPHX activity. So, both factors considered together take into account the net imbalance between formation and elimination of the toxic metabolite. Therefore, high β and/or a high p-HPPH concentration could reflect a more rapid and/or a lesser reactive arene oxide formation/elimination. As is can be seen in Figure 4, five of the six volunteers with rash have a β*[p- HPPH] product higher than 0.005 mg.L-1.h-1. Only volunteer C5, the only one with contraceptive therapy, did not follow that tendency, since reactivity came from two different entities: arene oxides of PHT and estrogens. |
| Influence of the PHT dosage regime |
| There were more cutaneous reactions after treatment B (100 mg every 12 hours, subjects: E4, E5, E6 and C4) than treatment A (600 mg every 72 hours, E2 and C5). With treatment A, higher mean PHT concentrations were obtained in comparison to treatment B (Tables 2 and 3) due to a lower drug clearance. This lower PHT metabolic clearance, as explained above, might respond to a higher efflux transporter overexpression in the liver [26]. Paradoxically, these higher PHT concentrations were not associated with more cutaneous reactions, as these reactions are not about parent drug concentration but about reactive metabolite concentration. |
| One limitation of the current research could be the small study group but the family relationship of four volunteers strengthens the genetic effect on the cutaneous adverse reaction. Moreover, unlike patients, healthy volunteers were strictly controlled and under phenytoin monotherapy so the assessment of causality, an inherent problem in pharmacovigilance, was completed elucidated. The use of pharmacokinetic data of these subjects and the study of the two brands, used under two different dosage regimes, enable us to conclude about the multifactorial cause of skin reactions in individuals with phenytoin treatment. |
| Another limitation was that CYP2C9/19 polymorphisms were not studied in the present work. |
| Several investigations informed about the effect of genetic polymorphisms of CYP2C9/19 on the pharmacokinetics of PHT [27,28]. The metabolism capacity of PHT is clearly impaired in subjects with mutations in CYP2C9/19 genes (poor metabolizers) and so could be the detoxification of the arene oxide and the metabolism of p-HPPH. However, if genetic variants were considered, this fact could result in a compensation of the amount of arene oxide and p-HPPH formed so once again a defective EPHX activity seems to be one of the main genetic causes responsible for the cutaneous adverse event. In addition, the metabolite p-HPPH can follow two routes: glucuronidation and PHT catechol formation. The latter involves CYP2C enzymes and arene oxide formation as well. Five out of the six volunteers that experienced rash had a higher p-HPPH concentration (mean ± SD, 0.121 mg/L ± 0.018) and a decreased EPHX activity. Subject E1, despite a decreased EPHX activity, did not experience the cutaneous reaction. This subject had a lower p-HPPH concentration (0.064 mg/L), and thereafter the subsequent arene oxide of pHPPH might also be reduced. |
| Conclusion |
| According to our observations, arene oxide metabolite seems to be the responsible for the immune response observed. In our study population, the genesis of the cutaneous response after PHT administration appears to be multifactorial: to have genetic disposition leading to a defective EPHX activity; to be a woman in the fertile life period, or with contraceptive therapy; to have a higher formation rate of the arene oxide due to a rapid input of the drug (rapid infusion or the formulation itself), or due to a faster PHT metabolism. |
| Conflict of Interests |
| There is no conflict of interests regarding the publication of this paper. |
| Acknowledgements |
| The study received fundings from Comisión Sectorial de Investigación Científica de la Universidad de la República after its approval by the Institutional Ethics Review Committee of the Faculty of Chemistry, Montevideo, Uruguay. |
| References |
References
- Leeder JS (1998) Mechanisms of idiosyncratic hypersensitivity reactions to antiepileptic drugs. Epilepsia 39: S8-S16.
- Chadwick D, Shaw MD, Foy P, Rawlins MD, Turnbull DM (1984) Serum anticonvulsant concentrations and the risk of drug induced skin eruptions. J Neurol Neurosurg Psychiatry 47: 642-644.
- Pirmohamed M (2006) Genetic factors in the predisposition to drug-induced hypersensitivity reactions. AAPS J 8: E20-26.
- Ghaffarpour M, Hejazie SS, Harirchian MH, Pourmahmoodian H (2005) Phenytoin, carbamazepine, sodium valproate and lamotrigine induced cutaneous reactions. Acta Med Iran 4: 37-42.
- Bessmertny O, Pham T (2002) Antiepileptic hypersensitivity syndrome: clinicians beware and be aware. Curr Allergy Asthma Rep 2: 34-39.
- Naisbitt DJ (2004) Drug hypersensitivity reactions in skin: understanding mechanisms and the development of diagnostic and predictive tests. Toxicology 194: 179-196.
- Sanderson JP, Naisbitt DJ, Park BK (2006) Role of bioactivation in drug-induced hypersensitivity reactions. AAPS J 8: E55-E64.
- Roychowdhury S, Svensson CK (2005) Mechanisms of drug-induced delayed-type hypersensitivity reactions in the skin. AAPS J 7: E834-E846.
- Janmohamed A, Dolphin CT, Phillips IR, Shephard EA (2001) Quantification and cellular localization of expression in human skin of genes encoding flavin-containing monooxygenases and cytochromes P450. Biochem Pharmacol 62: 777-786.
- Krishna DR, Klotz U (1994) Extrahepatic metabolism of drugs in humans. Clin Pharmacokinet 26: 144-160.
- Krauss G (2006) Current understanding of delayed anticonvulsant hypersensitivity reactions. Epilepsy Curr 6: 33-37.
- Wormhoudt LW, Commandeur JN, Vermeulen NP (1999) Genetic Polymorphisms of Human N-Acetyltransferase, Cytochrome P450, Glutathione-S-Transferase, and Epoxide Hydrolase Enzymes: Relevance to Xenobiotic Metabolism and Toxicity. Crit Rev Toxicol 29: 59-124.
- Männistö PT, Kaakkola S (1999) Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev 51: 593-628.
- Gennis MA, Vemuri R, Burns EA, Hill JV, Miller MA, et al. (1991) Familial occurrence of hypersensitivity to phenytoin. Am J Med 91: 631-634.
- Savio E, Fagiolino P, Solana G, Parente E, León A (1991) Development of water/oil emulsion. Bioavailability in rats. STP Pharma Sciences 1: 379-385.
- Zusterzeel PL, Peters WH, Visser W, Hermsen KJ, Roelofs HM, et al. (2001) A polymorphism in the gene for microsomal epoxide hydrolase is associated with pre-eclampsia. J Med Genet 38: 234-237.
- Le Quesne PW, Durga AV, Subramanyam V, Soloway AH, Hart RW, et al. (1980) Biomimetic synthesis of catechol estrogens: potentially mutagenic arene oxide intermediates in estrogen metabolism. J Med Chem 23: 239-240.
- Alvestad S, Lydersen S, Brodtkorb E (2007) Rash from antiepileptic drugs: influence by gender, age, and learning disability. Epilepsia 48: 1360-1365.
- Blaszczyk B, Szpringer M, Czuczwar SJ, Lason W (2013) Single centre 20 year survey of antiepileptic drug-induced hypersensitivity reactions. Pharmacol Rep 65: 399-409.
- Wong KH, Chan KC, Lee SS (2001) Sex differences in nevirapine rash. Clin Infect Dis 33: 2096-2098.
- Siiteri PK, MacDonald PC (1966) Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab 26: 751-761.
- Hartsfield JK Jr, Benford SA, Hilbelink DR (1995) Induction of microsomal epoxide hydrolase activity in inbred mice by chronic phenytoin exposure. Biochem Mol Med 56: 144-151.
- Alvariza A, Fagiolino P, Vázquez M, Feria-Romero I, Orozco-Suárez S (2014) Chronic administration of phenytoin induces efflux transporter overexpression in rats. Pharmacol Rep 66: 946-951.
- Anderson GD (2004) Pharmacogenetics and enzyme induction/inhibition properties of antiepileptic drugs. Neurology 63: S3-8.
- Nation RL, Evans AM, Milne RW (1990) Pharmacokinetic drug interactions with phenytoin (Part I). Clin Pharmacokinet 18: 37-60.
- Fagiolino P, Vázquez M, Eiraldi R, Maldonado C, Scaramelli A (2011) Influence of efflux transporters on drug metabolism: theoretical approach for bioavailability and clearance prediction. Clin Pharmacokinet 50: 75-80.
- Mamiya K, Ieiri I, Shimamoto J, Yukawa E, Imai J, et al. (1998) The Effects of Genetic Polymorphisms of CYP2C9 and CYP2C 19 on Phenytoin Metabolism in Japanese Adult Patients with Epilepsy: Studies in Stereoselective Hydroxylation and Population Pharmacokinetics. Epilepsia 39: 1317-1323.
- Chung WH, Chang WC, Lee YS, Wu YY, Yang CH, et al. (2014) Genetic Variants Associated With Phenytoin-Related Severe Cutaneous Adverse Reactions. JAMA 312: 525-534.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 19431
- [From(publication date):
November-2014 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 14724
- PDF downloads : 4707
