Skin Hardness and Epidermal Thickness Affect the Vibration Sensitivity of the Foot Sole
Received: 11-Aug-2017 / Accepted Date: 21-Aug-2017 / Published Date: 28-Aug-2017 DOI: 10.4172/2329-910X.1000245
Abstract
Objective: The cutaneous mechanoreceptors of the foot sole detect the changes in the application of mechanical loads on the plantar surface during gait and standing, and contribute to controlling the standing balance and postural reflexes in healthy subjects. A local thickening of the foot sole skin occurs in response to repetitive load application. We hypothesized that an elevated skin hardness of the foot sole could reduce its mechano sensitivity.
Methods: In healthy subjects, we quantified the sensation produced by different amplitudes of vibratory stimulations at two frequencies (25 and 150 Hz). The vibration threshold was determined on the 1st or 2nd, and 5th metatarsal heads, and the heel at each vibration frequency. The Stevens power function (Ψ=k.Φn) allowed to obtain regression equations between the estimate (Ψ) of the vibratory stimuli and their physical magnitude (Φ). Any increase in the absolute k value (all were negative) indicated a reduced sensitivity to the lowest loads. The n coefficient measured the global perception. The highest skin hardness (Shore) was measured on the 5th metatarsal head and the heel. In some subjects, superficial skin abrasion of the 5th metatarsal head was performed and the vibration sensitivity was tested again.
Results: The vibration threshold was significantly higher at the level of the 5th metatarsal head and the heel. The k value was significantly higher at the 25 and 150 Hz frequencies for the 5th metatarsal head, and only at 25 Hz for the heel. At both vibration frequencies, negative correlations were obtained between the k values and skin hardness. After skin abrasion, the n coefficient was significantly higher at both vibration frequencies.
Conclusion: Skin hardness affects the foot sole mechano sensitivity and could alter the control of posture during standing and walking. This indicates that foot care by podiatrist are relevant to improve posture control.
Keywords: Skin hardness; Foot sole; Sensitivity to vibration; Stevens power law; Healthy subjects
24555Introduction
The cutaneous mechanoreceptors of the foot sole detect the changes in the application of mechanical loads on the plantar surface during gait and standing, and contribute to controlling the standing balance and postural reflexes in healthy subjects [1-3].
The foot sole is subjected to high mechanical pressure and shear forces. A local thickening of the foot sole skin occurs in response to repetitive load application. This results from keratinization and increased number of collagen fibers [4]. We hypothesized that an elevated skin hardness of the foot sole could alter its mechanosensitivity.
The foot sole mechanosensitivity was studied in humans. Ribot- Ciscar et al. [5] Vedel and Roll [6] and Kennedy and Inglis [7] reported the presence of both slow (Merkel and Ruffini corpuscles) and fast (Meissner and Pacinian corpuscles) adapting mechanoreceptors. The skin mechanoreceptors can be classified into four groups based on their afferent firing properties [fast adapting (FA) vs. slow adapting (SA)] and receptive field size [type I (small defined boundaries), vs. type II (large undefined boundaries)]. The sensitivity of the foot sole afferents could depend on their location in the skin and their proximity to the epidermal layer. The Merkel and Meissner corpuscles are located at the dermal-epidermal junction, whereas the Ruffini and Pacinian corpuscles are only present in the deeper dermal layers [8].
Electrophysiological human studies have shown that the cutaneous afferents project on the somatosensory cortex leading to a perceptual representation [9].
The perceptual thresholds for the foot sole mechanosensitivity were expected to correlate with the mechanical property of the skin. In their princeps study, Strzalkowski et al. [10] have explored the vibration threshold of the medial arch of the foot sole and the heel. They only noted a trend of elevated sensory threshold at harder and thicker sites and they did not find any correlation between skin hardness and the vibration perceptual thresholds, concluding that skin hardness and epidermal thickness appeared to have a negligible influence on the vibration sensitivity. However, this interesting study was limited to measurements of perceptual thresholds. In previous studies, we used the Stevens psychological law [11] to obtain estimate of tactile stimuli [12] or mechanical vibrations [13] in a wide range of amplitude and found that this approach brought further information than the sole detection threshold.
In the present study in healthy subjects, we quantified the sensations produced by the vibratory stimulations. Based on the vibration frequencies known to recruit mechanoreceptors in the foot sole [14,15], the frequencies were chosen to target the activation of either the SAI receptors (25 Hz: the Merkel disks and Ruffini corpuscles) or the FAII ones (150 Hz: the Meissner and Pacinian corpuscles). In a limited number of subjects who showed the highest skin hardness at the level of the 5th metatarsal head, the podiatrists performed superficial skin abrasion to search for the benefits of a reduced epidermal layer of skin keratinization on the skin sensitivity.
Methods
Subjects
Fifteen healthy young subjects (13 females) (mean age: 23 ± 2 years; extreme ages: 21–39 years) were studied. All were free of foot pain and had no antecedent of trauma or surgery of the feet and legs. None were involved in an exercise program. This research adheres to the principles of the latest revision of the Declaration of Helsinki. The protocol was submitted to and approved by our institutional committee (CPP Sud Mediterranée 1, reference number 2014-AO1969-38). The procedures were carried out with the adequate understanding and written consent of the subjects.
Measurement of skin hardness
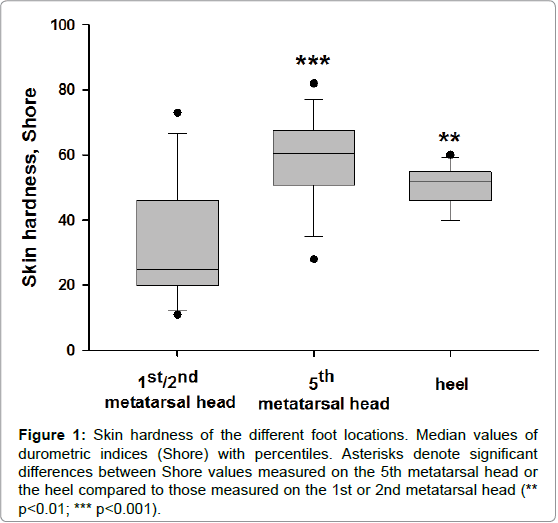
For skin hardness measurements, the participants laid prone with their right knee flexed (90°) and leg supported in a brace. Hardness measurements were taken using a handheld durometer (Type 1600-OO, Rex Gauge, Brampton, ON, Canada) with a 2-mm diameter, columnshaped indenter. The durometer determines hardness by measuring the penetration of an indenter into the skin, which gives a reading of increasing hardness from 1 to 100 (arbitrary units=Shore). We choose three plantar location of different hardness, the fifth metatarsal head having often the highest one (Figure 1). The mean Shore values were for the 1st, 2nd and 5th metatarsal heads and the heel were 30 ± 4, 60 ± 3 and 50 ± 2.
Figure 1: Skin hardness of the different foot locations. Median values of durometric indices (Shore) with percentiles. Asterisks denote significant differences between Shore values measured on the 5th metatarsal head or the heel compared to those measured on the 1st or 2nd metatarsal head (** p< 0.01; *** p< 0.001).
Measurements of vibratory sensations
All subjects had normal detection thresholds to light touch measured with Von Frey monofilaments. The method was largely described in our previous study [13]. The participants sat comfortably with their eyes closed and wore head phones to eliminate auditory cues associated with the onset of the vibration. The skin sensitivity was evaluated using vibration testing at two frequencies (25 and 150 Hz) at each one of three plantar locations (the first or second metatarsal head, the fifth metatarsal head, and the heel). Sinusoidal vibrations were applied to the foot sole via a plastic probe (width: 2 mm; length: 5 mm) attached to a minishaker (model 201, Ling Dynamic Systems, Royston, UK). As recommended by Lowrey and coworkers [15], prior to the onset of each trial the probe of the minishaker was placed in contact with the foot sole and a preload force of 2N was applied, manipulated by a vertical adjustment of the shaker and confirmed with a force transducer (model K13–0.02 kN, Scaime, Annemasse, France). Our vibrator device allowed to deliver different amplitudes of vertical motions of the probe and seven levels were retained (1,2,5,10,15,20 and 25 arbitrary units). The vibration motions expressed in μm were measured using an accelerometer attached to the probe (model EOAS S114 D2500, MAES France, Les Clayes-sous-Bois, France) when applying a force of 2 N on the probe. The vibration magnitude depended on its frequency and varied in a range of 10 to 360 μm at 25 Hz and 10 to 180 μm at 150 Hz. The testing frequencies were randomized at each foot sole location and the testing order of the foot sole location was also randomized across the participants.
Psychometrical evaluation of sensations
The measurement task for each participant was to judge the magnitude of seven vibration amplitudes at each frequency (25 and 150 Hz) which were randomly applied to the metatarsal heads or the heel. Participant specific standards for 0 and 10 were established in pilot tests in which the lowest and highest stimuli were presented twice in order to acquaint the subjects with the full range of loads. Then, the experimenter remained silent during further tests and participants indicated their estimate immediately after the test.
First, the vibration detection threshold was determined in each plantar location by considering the lowest detectable load at each vibration frequency.
The Stevens power function (Ψ=k × Φn) [11] allowed to obtain regression equations between the estimate (Ψ) of the vibratory stimuli and their physical magnitude (Φ). The exponent n in the power law was determined by a linear regression analysis between Napierian logarithmic (Ln) transformed stimuli and estimation data. Regressions were obtained for each test performed in each individual and the significance against zero of the R coefficient was tested. The scattering of pair values collected for each run was estimated by the standard errors of both Ln k and n coefficients. All k values were negative and thus any increase in absolute value of k indicated a reduced sensitivity to the lowest loads. The n coefficient measures the changes in perception between the extreme values of loads.
Superficial skin abrasion
In 6 subjects where the highest skin hardness was measured at the 5th metatarsal head the podiatrists performed a superficial skin abrasion of epidermal layer. The vibration sensitivity at the two frequencies was tested immediately after.
Statistical Analyses
All data are given by their mean ± standard error (SEM). Regressions between the estimate (Ψ) of the vibratory stimuli and their physical magnitude (Φ). were obtained for each test performed in each individual and the significance against zero of the R coefficient was tested. The scattering of pair values collected for each run was estimated by the standard errors of both k and n coefficients. The normal distribution of variables was verified with the Kolmogorov-Smirnov test. Differences between regression lines obtained at each foot location were assessed by a Student’s t test comparing mean and SEM of n and k coefficients. We also searched for correlations between the skin hardness and the values of the n and k coefficients determined for tactile and vibratory stimulations. A paired t test was used to determine significant changes in n and k coefficients in the 6 subjects after skin abrasion of the 5th metatarsal head. The level of the statistical significance was set at a twotailed p value of 0.05.
Results
Relationship between the skin hardness and vibration sensitivity
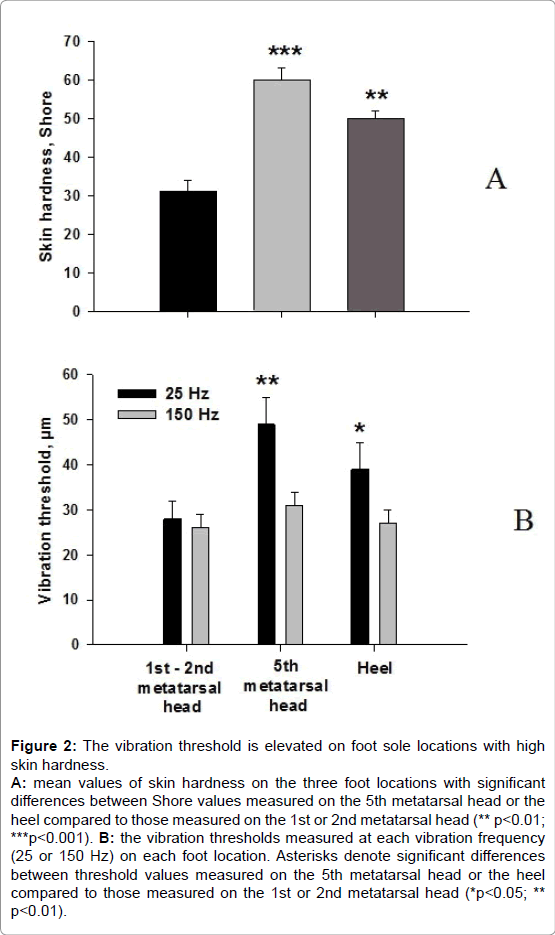
The vibration threshold was significantly higher at the level of the 5th metatarsal head and the heel but only for the 25 Hz vibration frequency (Figure 2).
Figure 2: The vibration threshold is elevated on foot sole locations with high skin hardness. A: mean values of skin hardness on the three foot locations with significant differences between Shore values measured on the 5th metatarsal head or the heel compared to those measured on the 1st or 2nd metatarsal head (** p< 0.01; ***p< 0.001). B: the vibration thresholds measured at each vibration frequency (25 or 150 Hz) on each foot location. Asterisks denote significant differences between threshold values measured on the 5th metatarsal head or the heel compared to those measured on the 1st or 2nd metatarsal head (*p< 0.05; ** p<0.01).
The mean k value was significantly higher at the 25 and 150 Hz frequencies for the 5th metatarsal head, and only at 25 Hz for the heel (Table 1). This indicates a tendency for a reduced sensitivity for the lowest vibration amplitude. No significant difference between the n coefficients was noted.
| Metatarsal heads | Heel | ||
|---|---|---|---|
| 1st–2nd | 5th | ||
| Intercept (k) | -1.60 ± 0.29* | -2.94 ± 0.46* | -2.50 ± 0.33 |
| 25 Hz | |||
| Slope (n) | 0.61 ± 0.05 | 0.77 ± 0.09 | 0.80 ± 0.06 |
| Intercept (k) | -1.84 ± 0.39* | -2.92 ± 0.36 | -1.70 ± 0.46 |
| 150 Hz | |||
| Slope (n) | 0.85 ± 0.08 | 0.93 ± 0.08 | 0.82 ± 0.09 |
Table 1: Values of the k and n coefficients of the Stevens power law obtained between the perception and amplitude of two vibration frequencies (25 and 150 Hz) measured in the 15 participants.
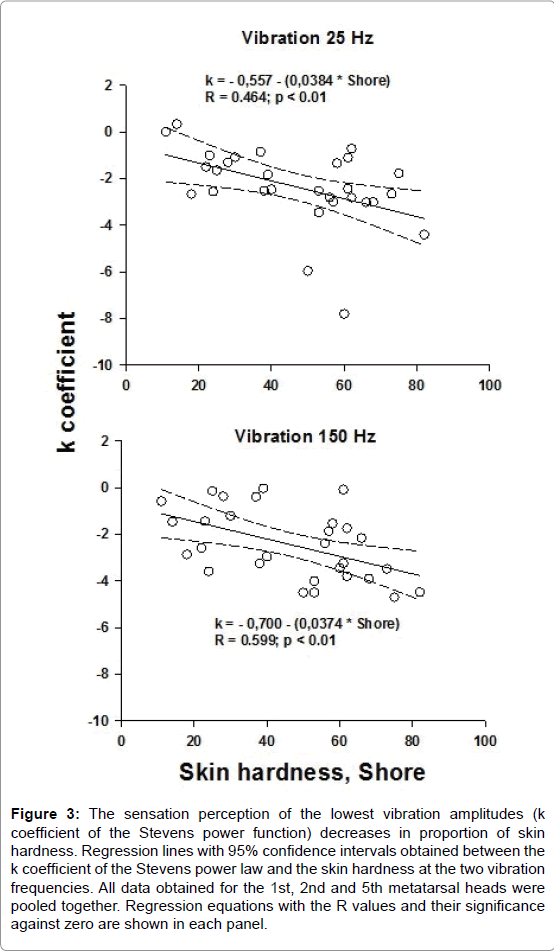
Negative correlations were obtained between the k values and skin hardness at the 25 and 150 Hz vibration frequencies when all data obtained for the 1st, 2nd and 5th metatarsal heads were pooled together (Figure 3). No correlation was found between the n coefficient and skin hardness. For the heel, the k and n coefficients were not correlated to the skin hardness.
Figure 3: The sensation perception of the lowest vibration amplitudes (k coefficient of the Stevens power function) decreases in proportion of skin hardness. Regression lines with 95% confidence intervals obtained between the k coefficient of the Stevens power law and the skin hardness at the two vibration frequencies. All data obtained for the 1st, 2nd and 5th metatarsal heads were pooled together. Regression equations with the R values and their significance against zero are shown in each panel.
Effects of superficial skin abrasion on the vibration sensitivity
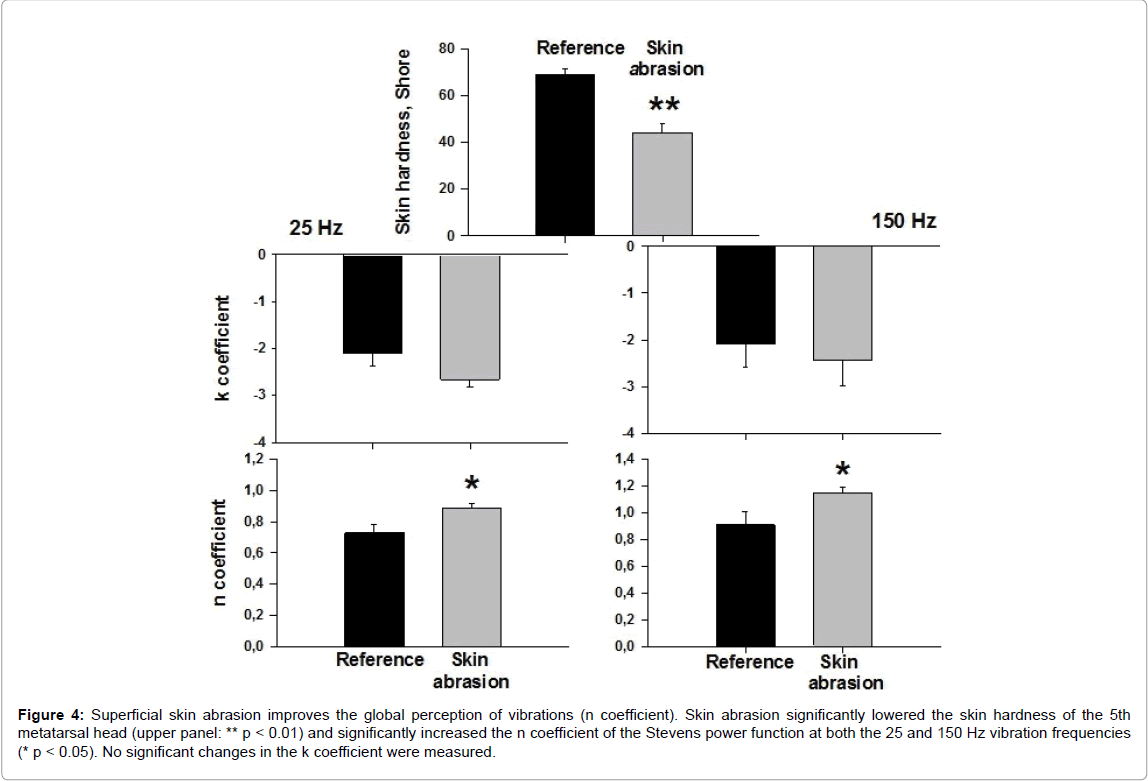
This was examined in 6 subjects who had the highest values of skin hardness on the 5th metatarsal head (61 to 75 Shore). Figure 4 shows that after skin abrasion the n coefficient was significantly higher at both the 25 and 150 Hz vibration frequencies. No significant changes in the k coefficient were measured.
Figure 4: Superficial skin abrasion improves the global perception of vibrations (n coefficient). Skin abrasion significantly lowered the skin hardness of the 5th metatarsal head (upper panel: ** p < 0.01) and significantly increased the n coefficient of the Stevens power function at both the 25 and 150 Hz vibration frequencies (* p < 0.05). No significant changes in the k coefficient were measured.
Discussion
The present study indicates that the vibration sensitivity was affected by the skin hardness. Thus, we measured an elevated vibration threshold for the 25 Hz vibration frequency on the 5th metatarsal head and the heel, i.e. on the locations showing the highest hardness values. The Stevens power law confirmed these data and brought more information. Indeed, a greater value of the k coefficient, i.e. a reduced sensitivity for the lowest amplitudes of vibration, was measured on both the 5th metatarsal head and the heel at the 25 Hz vibration frequency, and also when the 150 Hz frequency was applied on the 5th metatarsal head. The skin hardness of metatarsal heads was also inversely correlated to the k coefficient. No correlation was obtained between skin hardness of the heel and the k or n coefficients but the scattering of heel skin hardness was less (40 to 52 Shore) than that measured for the metatarsal heads (11 to 82 Shore). Superficial skin abrasion of the 5th metatarsal head significantly increased the n coefficient, which measured the global sensitivity to vibration.
As in our previous study [13], we found that the sole determination of the vibration threshold gave less information than the Stevens power function did. Indeed, an increased skin hardness was only associated to an elevated vibration threshold at the 25 Hz vibration frequency while the k coefficient of the Stevens power function was affected at both the 25 and 150 Hz frequencies. Examining the data reported by Strzalkowski et al. [10], who reported a discrete relationship between the foot sole skin hardness and the vibration sensitivity, revealed first, that the skin hardness of the five foot sole locations tested, mostly that of the 5th metatarsal head, was lower than that measured in our subjects and also that the difference between the skin hardness was minor, varying only from 33 to 46 Shore between the locations. This could explain the absence of any significant correlation between the perceptual vibration threshold and the skin hardness. Second, the authors only measured the vibration threshold and the present study, as our previous one [13], clearly showed a poor discriminating power of the sensitivity threshold.
The 25 Hz frequency selectively activates the SAI receptors while the 150 Hz frequency activates the FAI skin receptors, which are both present in the metatarsal heads [7]. This could explain that the perceptual representation of both the 25 and 150 Hz vibrations applied on the metatarsal heads was affected by the skin hardness. On the other hand, the k coefficient measured on the heel at 150 Hz was low (-1.70 ± 0.50) compared to that measured on the metatarsal heads, despite the Shore indices of the heel were always elevated and very narrow (40 to 56) compared to those measured on the metatarsal heads (28 to 75). In order to compare the k values measured with the same skin hardness on the different foot locations, we selected subjects who had near the same Shore value on the 5th metatarsal head (n=6:53 ± 3) and the heel (50 ± 2). Despite that, the 5th metatarsal head showed higher absolute k values measured at 150 Hz (-3.13 ± 0.40), indicating an elevated threshold of vibratory sensation. This incites to suppose that the density of FAI (Meissner corpuscles) is basically higher in the skin of the heel than in the 5th metatarsal head, explaining that the perception of high frequency vibration by the heel was less affected by its skin hardness than the metatarsal heads did. These data are supported by the human observations by Kennedy and Inglis [7] who have shown that the density of FAI receptors was higher in the skin of the toes, foot arch and heel than in the 5th metatarsal head.
Surprisingly, the difference in the skin hardness was not associated with any change in the n coefficient which measures the global perceptual representation of mechanical loads. One may suppose that a progressive and prolonged increase in the epidermal thickness could induce an adaptive perceptual response to vibrations, with no change in the slope (n) of the Stevens power function, and solely increased the threshold of perception. After superficial epidermal abrasion which acutely modified the environment of skin receptors, we measured a significant increase in n values at both the 25 and 150 Hz vibration frequencies, and surprisingly no significant change in the k coefficient. We have no satisfactory explanation for the absence of k changes after skin abrasion.
The lowered perception of loads applied on the foot sole should affect the control of both posture and gait. However, opposite results are reported on the role of foot sole mechanoreceptors on posture control. Meyer et al. [2] examined the properties of the center-of-foot-pressure (COP) trajectories and the ground reaction shear forces. They noted that the effects of foot-sole anesthesia were generally small and mostly manifested as an increase in COP velocity whereas the magnitude of COP displacement was unaffected. Forefoot anesthesia mainly influenced mediolateral posture control, whereas complete foot-sole anesthesia had an impact on anteroposterior control. Zhang and Li [16] examining the effects of chronic sensory loss due to peripheral neuropathy, did not find any consequence on plantar pressure distribution in walking and standing. Höhne et al. [17] found that plantar pressure distribution in gait is not affected by targeted reduced plantar cutaneous sensation. Beside, other studies [18-21] reported that reducing the plantar cutaneous sensation altered the walking pattern, modifying the pressure distribution and inducing greater postural sway. Also, Matthew et al. [22] found a relationship between plantar sensitivity and peak pressures at the hallux, and a relationship between sensitivity to higher frequency vibrations and peak force during running. These authors suggest that “neurological feedback should be incorporated in to any model that attempts to explain gait patterns”.
Conclusion
These data incite to suspect that a reduction of mechanosensitive sensory pathways from the foot sole due to a skin hardness could alter the control of posture during standing and walking.
Conflict of Interest Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Maurer C, Mergner T, Bolha B, Hlavacka F (2001) Human balance control during cutaneous stimulation of the plantar soles. Neurosci Lett 302: 45–48.
- Meyer PF, Oddsson LI, De Luca CJ (2004) The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res 156: 505–512.
- Wu G, Chiang JH (1997) The significance of somatosensory stimulations to the human foot in the control of postural reflexes. Exp Brain Res 114: 163–169.
- Wang YN, Sanders JE (2003) How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses 61: 29-35.
- Ribot-Ciscar E, Vedel JP, Roll JP (1989) Vibration sensitivity of slowly and rapidly adapting cutaneous mechanoreceptors in the human foot and leg. Neurosci Lett 104: 130-135.
- Vedel JP, Roll JP (1982) Response to pressure and vibration of slowly adapting cutaneous mechanoreceptors in the human foot. Neurosci Lett 34: 289-294.
- Kennedy PM, Inglis JT (2002) Distribution and behaviour of glabrous cutaneous receptors in the human foot sole. J Physiol 538: 995–1002.
- Abraira VE, Ginty DD (2016) The sensory neurons of touch. Neuron 79: 618-639.
- Kandel E, Schwartz J, Jessell T (2012) Principles of Neural Science (4th edn). McGraw-Hill Medical, New York.
- Strzalkowski ND, Triano JJ, Lam CK, Templeton CA, Bent LR (2015) Thresholds of skin sensitivity are partially influenced by mechanical properties of the skin on the foot sole. Physiol Rep 3: 1-14.
- Stevens SS (1957) On the psychological law. Psychol Rev 64: 153–181.
- Vie B, Nester CJ, Porte LM, Behr M, Jammes Y (2015) Pilot study demonstrating that sole mechanosensitivity can be affected by insole use. Gait Posture 41:263-268.
- Jammes Y, Guimbaud J, Faure R, Griffon P, Weber JP et al (2016) Psychophysical estimate of plantar vibration sensitivity brings additional information to the detection threshold in young and elderly subjects. Clin Neurophysiol Prac 1: 26-34.
- Johansson RS, Landstrom U, Lundstrom R (1982) Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res 244: 17-25.
- Lowrey CR, Perry SD, Strzalkowski ND, Williams DR, Wood SJ, et al (2014) Selective skin sensitivity changes and sensory reweighting following short-duration space flight. J Appl Physiol 116: 683-692.
- Zhang S, Li L (2013) The differential effects of foot sole sensory on plantar pressure distribution between balance and gait. Gait Posture 37: 532-535.
- Höhne A, Stark C, Brüggemann GP (2009) Plantar pressure distribution in gait is not affected by targeted reduced plantar cutaneous sensation. Clin Biomech 24: 308-313.
- Eils E, Behrens S, Mers O, Thorwesten L, Völker K et al (2004) Reduced plantar sensation causes a cautions walking pattern. Gait Posture 20: 54-60.
- Eils E, Nolte S, Tewes M, Thorwesten L, Völker K, et al. (2002) Modified pressure distribution patterns in walking following reduction of plantar sensation. J Biomech 35: 1307–1313.
- Höhne A, Ali S, Stark C, Brüggemann GP (2012) Reduced plantar cutaneous sensation modifies gait dynamics, lower-limb kinematics and muscle activity during walking. Eur J Appl Physiol 112:3829–3838.
- Wang Ting-Yun, Lin SangI (2008) Sensitivity of plantar cutaneous sensation and postural stability. Clin Biomech 23Â : 493-499.
- Matthew AN, MA, Nigg BM (1999) Quantifying a relationship between tactile and vibration sensitivity of the human foot with plantar pressure distributions during gait. Clin Biomech 14: 667-672.
Citation: Jammes Y, Viala M, Dutto W, Weber JP, Guieu R (2017) Skin Hardness and Epidermal Thickness Affect the Vibration Sensitivity of the Foot Sole. Clin Res Foot Ankle 5: 245. DOI: 10.4172/2329-910X.1000245
Copyright: © 2017 Jammes Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5426
- [From(publication date): 0-2017 - Jul 03, 2025]
- Breakdown by view type
- HTML page views: 4551
- PDF downloads: 875