Research Article Open Access
Six Candidate Proneural Factor Messenger RNAs for the Differentiation of Neuroendocrine and Non-Neuroendocrine Carcinomas of the Lung
Masunaga A1,2*, Hayashi S1, Omatsu M3, Kunimura T3, Suzuki K1, Uematsu S1, Kitami A1, Oide T2, Miyagi Y4, Hiroshima K2 and Suzuki T11Respiratory Disease Centre, Northern Yokohama Hospital, Showa University, Japan
2Department of Pathology, Yachiyo Medical Centre, Tokyo Women’s Medical University, Japan
3Department of Clinic-Diagnostic Pathology, Northern Yokohama Hospital, Showa University, Japan
4Molecular Pathology & Genetics Division, Kanagawa Cancer Centre Research Institute, Japan
- *Corresponding Author:
- Atsuko Masunaga
Department of Pathology, Yachiyo Medical Centre
Tokyo Women’s Medical University, Ohwada-Shinden 477-96
Yachiyo, Chiba, 276-8524, Japan
Tel: +81474506000
Fax: +8147587047
E-mail: nonkoamesho0324@yahoo.co.jp
Received date: August 17, 2016; Accepted date: September 06, 2016; Published date: September 08, 2016
Citation: Masunaga A, Hayashi S, Omatsu M, Kunimura T, Suzuki K, et al. (2016) Six Candidate Proneural Factor Messenger RNAs for the Differentiation of Neuroendocrine and Non-Neuroendocrine Carcinomas of the Lung. J Clin Exp Pathol 6:292. doi: 10.4172/2161-0681.1000292
Copyright: © 2016 Masunaga A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Background: Lung neuroendocrine carcinomas, i.e., small cell lung carcinoma (SCLC) and large cell neuroendocrine carcinoma (LCNEC), and non-neuroendocrine carcinomas, e.g. squamous cell carcinoma and adenocarcinoma are both thought to arise from the same endoderm as respiratory epithelium. However, it is not clear how neuroendocrine carcinomas acquire and maintain their neuroendocrine features. To date, 19 proneural factors that function in the development of the fetal neural system and differentiation of neuroendocrine cells of endodermal origin have been identified. In this study, we investigated the specificity of proneural factor expression in SCLC, lung LCNEC, lung squamous cell carcinoma and lung adenocarcinoma.
Methods: RNA was extracted from 3 SCLCs, 3 LCNECs, 10 invasive squamous cell carcinomas and 10 invasive adenocarcinomas. Specific PCR primers were generated for the 19 proneural factors and messenger RNA copy numbers were measured using reverse transcription real time PCR. Differences in expression were then statistically analysed.
Results: Insulinoma-associated protein 1(INSM1) and mammalian achaete-scute homolog (MASH) 1 mRNA was significantly higher in SCLCs than in squamous cell carcinomas. Oligodendrocyte transcription factor (OLIG)1 and mammalian atonal homolog (MATH)2 mRNA levels were significantly lower in SCLCs and squamous cell carcinomas than in adenocarcinomas. OLIG2 mRNA levels were significantly lower in LCNECs than in adenocarcinomas. MATH3 mRNA levels in LCNECs were significantly higher than in both squamous cell carcinomas and adenocarcinomas.
Conclusion: INSM1, MASH1, OLIG1, OLIG2, MATH2 and MATH3 are candidate proneural factors that could potentially differentiate lung neuroendocrine carcinomas from non-neuroendocrine carcinomas. In particular, MATH3 might be an LCNEC-specific factor.
Keywords
Lung; Neuroendocrine carcinoma; Proneural factors; MAT3; Non-neuroendocrine carcinoma
Abbreviations: SCLC: Small Cell Lung Cancer; LCNEC: Large Cell Neuroendocrine Carcinoma; INSM1: Insulinoma-associated Protein 1; NGN: Neurogenin; MASH: Mammalian achaete-scute Homolog; TCF3: Transcription Factor 3; ATOH or MATH: Mammalian Atonal Homolog; NATO3: Nephew of Atonal 3; OLIG: Oligodendrocyte Transcription Factor; NSCL: Neurologic Stem Cell Leukemia
Introduction
The means through which pulmonary neuroendocrine tumors, i.e., carcinoid tumor, small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC) arise and maintain their neuroendocrine features remain undetermined. It is believed that the cells of origin of neuroendocrine carcinomas (SCLC and LCNEC) and non-neuroendocrine carcinomas, e.g. squamous cell carcinoma and adenocarcinoma, both originate from the same endoderm as respiratory epithelium: non-neuroendocrine carcinomas can occasionally be observed in combination with SCLC and/or LCNEC, usually not with carcinoid tumor [1].
To date, 19 proneural factors, which play important roles in fetal neural development, have been discovered in humans: Insulinomaassociated protein 1 (INSM1); neurogenin (NGN1); NGN2; NGN3; NEUROD/BETA2; NEUROD2; mammalian achaete-scute homolog (MASH1); MASH2, transcription factor 3 (TCF3); mammalian atonal homolog (ATOH1); ATOH7; nephew of atonal 3 (NATO3); oligodendrocyte transcription factor (OLIG1); OLIG2; OLIG3; mammalian atonal homolog (MATH2); MATH3; neurologic stem cell leukemia (NSCL1): and NSCL2 [2]. In addition to their in vivo roles, NGN1 and/or NGN2 have also been shown to induce neurogenesis in human fibroblasts and human stem cells in vitro [3,4]. Some of these proneural factors also play important roles in the development of neuroendocrine cells that originate in the endoderm. For example, INSM1, NEUROD/BETA2 and NGN3 are essential for the differentiation of pancreatic neuroendocrine cells from pancreatic duct cells, which have an endodermal origin [5,6]. The development of gastric endocrine cells also requires MASH1 and NGN3 in mice [7,8]. With regard to lung neuroendocrine carcinomas, some studies have shown that MASH1 and/or INSM1 are related to the development of SCLC [9-12].
At present, there is some controversy as to whether LCNEC is biologically similar to SCLC, and should therefore be treated in a similar fashion to SCLC. A recent study by Rekhtman et al., which employed next-generation sequencing of somatic DNA, demonstrated that lung LCNEC composed of SCLC-like and non-SCLC-like subsets and that LCNEC was thought to be composed of biologically heterogeneous groups [13].
In this study, we investigated whether proneural factors, which might be assumed to be related to the tumorigenesis and maintenance of lung neuroendocrine carcinomas, can be used to differentiate neuroendocrine from non-neuroendocrine lung carcinomas, and possibly SCLC from LCNEC.
Although the somatic DNA genetic profile could provide some key characteristics through which SCLC and LCNEC, and neuroendocrine and non-neuroendocrine carcinomas can be differentiated, protein expression is the key determinant of cell morphology and function. However, it can be difficult to accurately quantify protein expression. For this reason, we have chosen to measure messenger RNA (mRNA) using reverse transcription real-time polymerase chain reaction (PCR) in this study. We have measured and statistically analysed the mRNA expression of all 19 human proneural factors in SCLC, LCNEC invasive squamous cell carcinoma, and invasive adenocarcinoma.
Materials and Methods
We obtained freshly frozen tissue from six pulmonary neuroendocrine carcinomas (3 SCLCs and 3 LCNECs), 10 pulmonary invasive adenocarcinoma and 10 pulmonary invasive squamous cell carcinomas. We choose neuroendocrine carcinomas that were not combined with another histological type. All of the tumors had developed at the periphery of the lung. All of the patients underwent lobectomies with regional lymph node resection and did not receive any preoperative chemotherapy. The tumors were snap-frozen at the time of resection and stored at -80°C until use. The tumor sizes, and patents’ age and gender are listed (Table 1).
| Case No. | Sex | Age (yrs.) | Histological Type | Max diameter of tumor |
|---|---|---|---|---|
| 1 | Male | 56 | Small Cell Carcinoma | 20mm |
| 2 | Male | 64 | Small Cell Carcinoma | 10mm |
| 3 | Male | 68 | Small Cell Carcinoma | 20mm |
| 4 | Male | 76 | Large Cell Neuroendocrine Carcinoma | 40mm |
| 5 | Male | 69 | Large Cell Neuroendocrine Carcinoma | 40mm |
| 6 | Male | 83 | Large Cell Neuroendocrine Carcinoma | 20mm |
| 7 | Male | 83 | Squamous Cell Carcinoma | 40mm |
| 8 | Female | 70 | Squamous Cell Carcinoma | 50mm |
| 9 | Male | 77 | Squamous Cell Carcinoma | 70mm |
| 10 | Male | 76 | Squamous Cell Carcinoma | 60mm |
| 11 | Male | 78 | Squamous Cell Carcinoma | 60mm |
| 12 | Male | 66 | Squamous Cell Carcinoma | 40mm |
| 13 | Male | 59 | Squamous Cell Carcinoma | 40mm |
| 14 | Male | 63 | Squamous Cell Carcinoma | 40mm |
| 15 | Male | 78 | Squamous Cell Carcinoma | 28mm |
| 16 | Male | 82 | Squamous Cell Carcinoma | 32 mm |
| 17 | Female | 68 | Adenocarcinoma, Acinar Predominance | 35mm |
| 18 | Female | 44 | Adenocarcinoma, Acinar Predominance | 20mm |
| 19 | Female | 57 | Adenocarcinoma, Acinar Predominance | 30mm |
| 20 | Male | 70 | Adenocarcinoma, Acinar Predominance | 60mm |
| 21 | Female | 74 | Adenocarcinoma, Acinar Predominance | 35mm |
| 22 | Male | 52 | Adenocarcinoma, Acinar Predominance | 25mm |
| 23 | Female | 82 | Adenocarcinoma, Acinar Predominance | 30mm |
| 24 | Male | 82 | Adenocarcinoma, Acinar Predominance | 40mm |
| 25 | Male | 57 | Adenocarcinoma, Acinar Predominance | 23mm |
| 26 | Female | 47 | Adenocarcinoma, Acinar Predominance | 40mm |
Table 1: Histological types and patient’s profiles.
Total RNA was extracted from snap-frozen samples using the RNeasy Microkit (Qiagen GmbH, Hilden, Germany), RNA was reverse-transcribed to generate complementary DNA using the Primescript II first strand cDNA synthesis kit (Takara Bio Inc, Kusatsu, Shiga, Japan) and intercalating dye-based real-time PCR was performed using Realtime PCR Master Mix (Toyobo co., Ltd., Osaka, Japan) and an Illumina Eco (Illumina Inc., San Diego, CA, USA). Primers that were specific for each proneural factor were developed using the National Centre for Biotechnology Information gene database; the primer sequences and their annealing temperatures are listed (Table 2). Standard copy numbers for measurement of the mRNA expression were obtained using PCR of complementary DNA through reverse-transcribed RNA of human fetal brain aborted due to intrauterine death at early gestation. The TATA-box binding protein mRNA primer pair was used to normalize for copy number as previously described [14].
| Proneural factor | Primer sequence | Amplicon size | Annealing temperature (°C) | |
|---|---|---|---|---|
| INSM1 | Forward | 5'-TCTACGAGTGCCATCACTGT-3' | 140bp | 60 |
| Reverse | 5'-TCTACGAGTGCCATCACTGT-3' | |||
| NEUROGENIN1 | Forward | 5'-AGACCTGCATCTCCGACCT-3' | 102bp | 63 |
| Reverse | 5'-AGGCTGCCTGTTGGAGTCT-3' | |||
| NEUROGENIN2 | Forward | 5'-AGGCTGCCTGTTGGAGTCT-3' | 115bp | 63 |
| Reverse | 5'-GGCCTTCAGTCTACGGGTCT-3' | |||
| NEUROGENIN3 | Forward | 5'-CGCAATCGAATGCACAACCT-3' | 131bp | 64 |
| Reverse | 5'-GTCAGCGCCCAGCTGTAGTT-3' | |||
| NEUROD/BETA2 | Forward | 5'-GTTCTCAGGACGAGGAGCAC-3' | 164bp | 63 |
| Reverse | 5'-CTTGGGCTTTTGATCGTCAT-3' | |||
| NEUROD2 | Forward | 5'-CCTCTTGGCTTTAGCTGGTG-3' | 128bp | 63 |
| Reverse | 5'-TCGTGTATTTGGATGCCTGA-3' | |||
| MASH1 | Forward | 5'-ACTGGGACCTGAGTCAATGC-3' | 115bp | 64 |
| Reverse | 5'-GCTGTGCGTGTTAGAGGTGA-3' | |||
| MASH2 | Forward | 5'-GCCCCCACTATCTGGAGTTT-3' | 148bp | 63 |
| Reverse | 5'-ACACAGGCTTCTCCCTAGCA-3' | |||
| TCF3 | Forward | 5'-TAAGCTGCTCTCCCTTGGAA-3' | 139bp | 63 |
| Reverse | 5'-GGCAAAGGAGTGAAGGACAG-3' | |||
| ATOH1 | Forward | 5'-TGAAGGAGTTGGGAGACCAC-3' | 110bp | 63 |
| Reverse | 5'-GTAGACGGGATGCTCTCTCG-3' | |||
| ATOH7 | Forward | 5'-TTCCCCTTTTCTGGGCTACT-3' | 121bp | 63 |
| Reverse | 5'-CCGAACAGGACAAACTCACA-3' | |||
| NATO3 | Forward | 5'-GCCTGGCCATCGTCTATATC-3' | 119bp | 63 |
| Reverse | 5'-ACACCCCAGCACTACCAGAC-3' | |||
| OLIG1 | Forward | 5'-TCCAGTGTTTTGTCGCAGAG-3' | 149bp | 63 |
| Reverse | 5'-GCGGTTGGTTTTCGTTTTTA-3' | |||
| OLIG2 | Forward | 5'-GAAACTACCCCACCGACTCA-3' | 113bp | 63 |
| Reverse | 5'-ACCCAAACTGTTTCCACAGC-3' | |||
| OLIG3 | Forward | 5'-CTTGCGAAGGGACTTTTGAG-3' | 141bp | 63 |
| Reverse | 5'-CTGTGGCAAGGACAGAGACA-3' | |||
| MATH2 | Forward | 5'-AACGACGCTCTGGACAACTT-3' | 140bp | 63 |
| Reverse | 5'-TCTGGTCTCTTGCCGATTCT-3' | |||
| MATH3 | Forward | 5'-AGCTTCATGCCACATTACCC-3' | 130bp | 63 |
| Reverse | 5'-TACCATGATGGGGGTAGGAA-3' | |||
| NSCL1 | Forward | 5'-ATTCCGGATTAGGGGATGAC-3' | 106bp | 63 |
| Reverse | 5'-AGAGTGGGCTGAGATGAGGA-3' | |||
| NSCL2 | Forward | 5'-AGGAGAACCTCGTGGAGACA-3' | 104bp | 63 |
| Reverse | 5'-GGCGGATGAATGTAGGAGAA-3' |
Table 2: Primer sequences and annealing temperatures for proneural factor amplification.
The Mann-Whitney u-test was used to determine whether there was a statistically significant difference in mRNA levels between SCLC and squamous cell carcinoma; SCLC and adenocarcinoma; LCNEC and squamous cell carcinoma; LCNEC and adenocarcinoma; and squamous cell carcinoma and adenocarcinoma. We were not able to compare SCLC with LCNEC because there were only 3 samples in each group.
This study was approved by the Human Genome and Genetic Analysis Ethics Committee of Showa University (Certification No.232).
Results
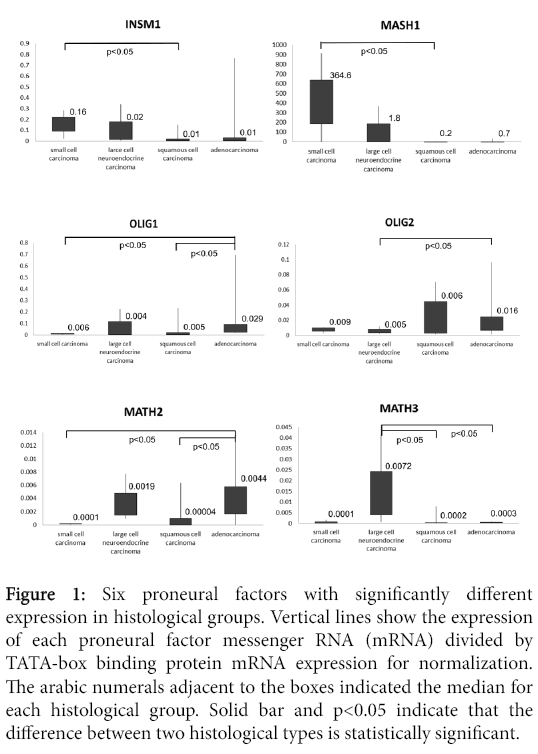
Of the 19 proneural factors, the INSM1, MASH1, OLIG1, OLIG2, MATH2 and MATH3 mRNA copy number was statistically different between histological groups (Figure 1).
Figure 1: Six proneural factors with significantly different expression in histological groups. Vertical lines show the expression of each proneural factor messenger RNA (mRNA) divided by TATA-box binding protein mRNA expression for normalization. The arabic numerals adjacent to the boxes indicated the median for each histological group. Solid bar and p<0.05 indicate that the difference between two histological types is statistically significant.
INSM1 and MASH1 mRNA levels tended to be significantly higher in SCLC than in squamous cell carcinoma (p<0.05). The level of OLIG1 mRNA tended to be significantly lower in SCLC and squamous cell carcinoma than in adenocarcinoma (p<0.05). In LCNEC, the level of OLIG2 mRNA tended to be significantly lower than in adenocarcinoma (p<0.05). The level of MATH2 mRNA in SCLC and squamous cell carcinoma tended to be significantly lower than in adenocarcinoma (p<0.05). The MATH3 mRNA levels in LCNECs tended to be significantly higher than in both squamous cell carcinoma and adenocarcinoma (p<0.05).
Discussion
To date, 19 proneural factors have been discovered, and these are known to play important roles in fetal human neural development. The expression of these proneural factors is controlled in order to regulate neural development [2]. In addition to their roles in neural development, some of the 19 proneural factors also regulate the development of neuroendocrine cells with an endodermal origin [5-9]. For example, transient expression of NGN3 and subsequent expression of INSM1 and NEUROD/BETA2 initiate the transformation of pancreatic duct cells into neuroendocrine cells [5].
Lung neuroendocrine carcinomas are subdivided into two groups: SCLC and LCNEC. These two carcinomas are thought to be derived from the endoderm, as are the non-neuroendocrine carcinomas, e.g. squamous cell carcinoma and adenocarcinoma; lung neuroendocrine carcinomas that are combined with non-neuroendocrine carcinomas are often observed [1]. Prior to performing this study, we speculated that some proneural factors may play a role in the tumorigenesis and maintenance of lung neuroendocrine carcinomas, and that these factors might be able to differentiate neuroendocrine carcinomas from non-neuroendocrine carcinomas of the lung. However, in this study, we could use a few materials of SCLC and LCNEC because of low incidence of completely removable SCLC that are located peripherally in the lung and low incidence of LCNEC [1,15].
Our results have provided six candidates proneural factors that could be used to differentiate lung neuroendocrine carcinomas from non-neuroendocrine carcinomas: INSM1; MASH1; OLIG1; OLIG2; MATH2; and MATH3. These factors could play an important role in cases of combined neuroendocrine carcinoma with nonneuroendocrine carcinoma. In combined neuroendocrine and nonneuroendocrine carcinoma, INSM1 and MASH1 may be involved in SCLC that develops from squamous cell carcinoma, and vice versa; increased INSM1 and/or MASH1 expression might induce SCLC formation out of squamous cell carcinoma. Previous reports have shown that INSM1 and MASH1 are concerned to SCLC [9-12]. However, in our study, the two proneural factors might merely present the difference between SCLC and squamous cell carcinoma. SCLC that is derived from adenocarcinoma, and vice versa, may be related to OLIG1 and MATH2; decreased OLIG1 and/or MATH2 expression could induce SCLC development from adenocarcinoma. Similarly, LCNEC derived from adenocarcinoma, and vice versa, may be related to OLIG2 expression. Furthermore, MATH3 expression may be associated with the differentiation of LCNEC from squamous cell carcinoma or adenocarcinoma, and vice versa.
It is noteworthy that MATH3 mRNA levels in LCNEC were significantly higher than in either squamous cell carcinoma or adenocarcinoma. Although we were not able to statistically analyze the difference in MATH3 expression in SCLC and LCNEC, the levels of MATH3 mRNA seemed to be lower in SCLC than in LCNEC. As such, MATH3 might be a factor that specifically induces LCNEC tumorigenesis and/or maintenance. However, a recent study by Rekhtman et al. proposed that LCNEC is composed of heterogeneous groups of cells [13]. In this study, we were able to perform our analysis on small number of LCNECs because we used completely resected and fresh-frozen tumor samples to see accurate mRNA copy numbers [16]. To confirm whether MATH3 expression is a defining characteristic of lung LCNEC, MATH3 mRNA expression would need to be determined in a larger number of LCNEC cases. It would also be necessary to determine whether the overexpression of MATH3 mRNA can stimulate LCNEC development in non-tumorous respiratory epithelium or non-neuroendocrine tumor cells in vivo and in vitro .
The results of our study have also shown that no proneural factors are common to SCLC and LCNEC. This indicates that SCLC and LCNEC differ in their use of proneural factors. Although SCLC and LCNEC both express some common neuroendocrine proteins/ antigens, for example CD56, chromogranin A, and synaptophysin [1], some of the proneural factors are most probably specific to either SCLC or LCNEC. Although most advanced stage LCNEC patients are now treated with SCLC chemotherapy regimens, chemotherapy for advanced stage LCNEC is controversial [13,17]. We hope that proneural factors, in particular MATH3, might provide a basis for new optional treatments for LCNEC.
References
- Brambilia E, Burke AP, Marx A, Nicholson AG (2015) WHO classification of tumours of lung, pleura, thymus and heart, (4th edn). IARC, Lyon, France.
- Bertrand N, Diogo SC, Guillemot F (2002) Proneural genes and the specification of neural cell types. Nat Rev 3: 517-530.
- Liu ML, Zang T, Zou Y, Chang J, Gibson J, et al. (2013) Small molecules enable neurogenin 2 to efficiently co nvert human fibroblasts to cholinergic neurons. Nat Commun 4: 2183.
- Busskamp V, Lewis NE, Guye P, Ng AHM, Shipman SL, et al. (2014) Rapid neurogenesis through transcriptional activation in human stem cells. MolSystBiol 10: 760-780.
- Mellitzer G, Bonne S, Luco RF, De Casteele MV, Lenne-Samuel N, et al. (2006) IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO 25:1344-1352.
- Lan MS, Breslin MB (2009) Structure, expression, and biological function of INSM1 transcription factor in neuroendocrine differentiation. FASEB J 23: 2024-2033.
- Lee CS, Perreault N, Brestelli JE, Kaestner KH (2002) Neurogenin 3 is essential for the proper specification of gastric entroendocrine cells and the maintenance of gastric epithelial cell identity. Gen Dev 16: 1488-1497.
- Kokubu H, Ohtsuka T, Kageyama R (2008) Mash1 is required for neuroendocrine cell development in the glandular stomach. Genes to Cells 13: 41-51.
- Ito T, Udaka N, Okudela K, Yazawa T, Kitamura H (2003) Mechanisms of neuroendocrine differentiation in pulmonary neuroendocrine cells and small cell carcinoma. Endo Pahtol 14: 133-139.
- Pedersen N, Pederson MW, Lan MS, Breslin MB, Poulsen HS (2006) The insulinoma-associated 1: a novel promoter for trgetted cancer gene therapy for small-cell lung cancer. Can Gen Ther 13:375-384.
- Taniwaki M, Daigo Y, Ishikawa N, Takano A, Tsunoda T, et al. (2006) Gene expression profiles of small-cell lung cancers: Molecular signatures of lung cancer. Int J Oncol 29: 567-575.
- Amelung JT, Buhrens R, Beshay M, Reymond MA (2010) Key genes in lung cancer translational research: a meta-analysis. Pathobiol 77: 53-63.
- Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, et al. (2016) Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res 22: 3618-3629.
- Masunaga A, Inoue K, Mizukami H, Hayashi T, Mitsuya T (2014) Neuroendocrine carcinoma arising in a hepatitis C virus-infected liver: Mechanism of the tumor development may be similar to that of development of pancreatic neuroendocrine cells. PatholInt 64: 81-85.
- Fasano M, Corte CMD, Papaccio F, Ciardiello F, Morgillo F (2015) Pulmonary large-cell neuroendocrine carcinoma from epidermology to therapy. J ThoracOncol 10: 1133-1141.
- Castiglione F, RossiDel’Innocenti D, Taddei A, Garbini F, Buccoliero AM, et al. (2007) Real-time PCR analysis of RNA extracted from formalin-fixed and paraffin-embedded issues: effects of the fixation on outcome reliability. ApplImmunohistochemMolMorphol 15: 338-342.
- Makino T, Mikami T, Hata Y, Otsuka H, Koezuka S, et al . (2016) Comprehensive biomarkers for personalized treatment in pulmonary large cell neuroendocrine carcinoma: A comparative analysis with adenocarcinoma. Ann Thora Surg.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11499
- [From(publication date):
October-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10618
- PDF downloads : 881