Research Article Open Access
Simultaneous Determination of Asaranin and Sesamin in Piper chaba Fruit by using HPTLC-MS Method: Effect of Different Extraction Methods on the Yield of Marker Compounds
Kothapalli Haribabu1, Makula Ajitha2 and Uppuluri Venkata Mallavadhani1*1Natural Products Chemistry Division, CSIR-Indian Institute of Chemical Technology, Hyderabad, India
2Center for Pharmaceutical Sciences, Jawaharlal Nehru Technological University, Hyderabad, India
- *Corresponding Author:
- Dr. U. V. Mallavadhani
Senior Principal Scientist
Natural Product Chemistry Division
Indian Institute of Chemical Technology [IICT]
Tarnaka, Uppal Road
Hyderabad-607, India
Tel: +91-40-27193167
E-mail: mallavadhani@iict.res.in, uvmavadani@yahoo.com
Received date: July 04, 2014;Accepted date: August 04, 2014; Published date: August 08, 2014
Citation: Haribabu K, Ajitha M, Mallavadhani UV (2014) Simultaneous Determination of Asaranin and Sesamin in Piper chaba Fruit by using HPTLC-MS Method: Effect of Different Extraction Methods on the Yield of Marker Compounds. J Anal Bioanal Tech 5:199 doi: 10.4172/2155-9872.1000199
Copyright: 2014 Haribabu K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
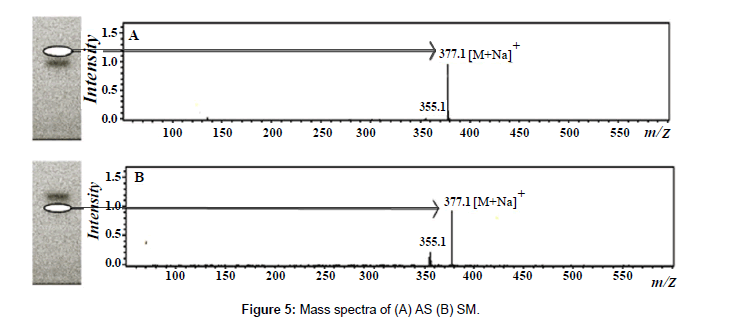
A simple, rapid and accurate HPTLC-MS method was developed for the simultaneous quantification of asaranin (AS) and sesamin (SM), the two epimeric furofuran lignans in Piper chaba (Piperaceae) fruit extracts obtained by cold percolation (PER), Soxhlet extraction (SOX), ultrasound assisted extraction (USAE), accelerated solvent extractor (ASE) and microwave assisted extraction (MAE). Separation of AS and SM was achieved on 20×10 cm pre-coated silica gel 60 F254S HPTLC plates with ethyl acetate: hexane (30:70) as mobile phase. Detection and quantification were performed densitometrically at λmax 295 nm. The peak identity was confirmed by electron spray ionization (ESI) mass spectra, which showed the [M + Na]+ ions for both AS and SM at m/z 377. The method was validated according to the ICH guidelines in terms of limit of detection (LOD) and limit of quantification (LOQ), selectivity, linearity, precision, accuracy, and robustness. The results indicate that AS (2.108%) and SM (0.103%) found to present maximum in ASE extract and minimum in PER extract (AS 0.848%; SM 0.048%). By considering the validation results, the method was found to be reproducible and convenient for quantitative analysis of AS and SM in different P. chaba fruit extracts.
Keywords
Asaranin; Furofuran lignans; HPTLC-MS; Piper chaba fruit; Sesamina
Introduction
Piper chaba HUNTER (syn. P. retrofractum VAHL; Fam: Piperaceae) is a climbing, glabrous shrub widely distributed in Southeast Asia particularly in India and Malay Islands. It is commonly known as ‘Chavya’ (Gajapippali) in Ayurveda [1]. The fruit of this plant is popularly known as ‘Dee Plee’ in Thailand and has been used for several years as a spice and traditional medicine as antiflatulant, expectorant, antitussive, antifungal, uterus-contracting agent, sedative-hypnotic, appetizer, and counter-irritant [2]. The fruits of P.chaba are being used as a substitute for P.longum in the Indian traditional systems of medicine. P. chaba fruits were reportedly used as pungent, aromatic, stimulant, anthelmintic, expectorant and carminative [3]. The aqueous acetone extract of P. chaba fruits reported to exhibit protective effects on gastric lesions in rats, inhibitory effects on liver injury in mice, inhibited TNF-α induced death ofhepatocytesand promoting effects on adipogenesis of 3T3-L1 cells [4-7]. Phytochemical evaluation of P.chaba fruits revealed the presence of various classes of compounds such as lignans, amides, long-chain ester, terpenoids, steroids, pyrones, chalcones and flavonoids [8,9]. Interestingly the major metabolites of P. chaba fruit area asaranin and sesamin, the two biologically active furofuran lignin epimers.
Asaranin has a number of beneficial health effects in humans in lipid and glucose metabolism, hypertension, inflammation and free radical scavenging mechanism [10]. Sesamin has been reported to exhibit anti-oxidative effect [11,12], promotes immunity functions, anti-carcinogen activity, a blood pressure-lowering effect and exerts serum lipid lowering and hepatocyte-protecting effects [13]. It has also been reported that Sesamin could decrease the levels of blood lipid, blood glucose and depress the expression of the VCAM-1 protein in the aorta in rats with metabolic syndrome [14].
In view of interesting biological activities, asaranin and sesamin need to be isolated in large quantities for further developmental work. In this connection, we have carried out extensive chemical and analytical studies on P. chaba fruits and the results are presented herein
Materials and Methods
Plant materials
Fruits of P. chaba were procured through commercial suppliers in Hyderabad, India in December, 2013. Their identity was confirmed by taxonomists.
Chemicals
All the solvents used are either analytical or of HPLC grade and purchased from E. Merck (Mumbai, India). Pre-coated highperformance thin layer chromatography (HPTLC) silica gel 60F254 (E. Merck, Darmstadt, Germany) plates were used.
Apparatus
Automatic TLC sampler (ATS-4), Vario system, Immersion device III, TLC scanner with Win Cats-III software and Reprostar 3 (All CAMAG, Muttenz, Switzerland), TLC–MS interface (Camag), LC-MSD-TRAP-SL ( M/s Agilent technologies-Bruker, Waldbronn, Germany) Dionex Accelerated solvent Extractor (ASE 350), Micro Wave Extractor (BPL-BMC 900 T at 2450 MHz), ultra sonic bath (Bandelin sonorex), centrifuge (model 2-16P) by sigma (Zurich, Switzerland) were used during the study.
Extraction and isolation of markers from P.chaba
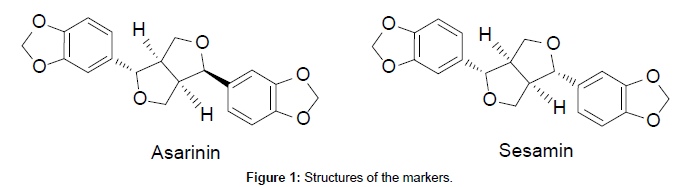
The shade dried fruits of P.chaba (285 g) were powdered in a pulverizer and the powdered material was soaked in methanol (1 L) and extracted under hot condition using a Soxhlet extractor for 8 h. The resultant methanol solubles on concentration under reduced pressure yielded the methanolic extract (33.22 g). Column chromatographic fractionation of the methanol extract (5 g) was carried out on silica gel (100-200 mesh) using the gradient elution of n-hexane and ethyl acetate. Based on TLC profile, the column fractions of n-hexane–ethyl acetate 70:30 were combined and concentrated to afford two major fractions. These two major fractions on repetitive column chromatographic purifications followed by recrystallisation from n-hexane-chloroform yielded pure AS and SM in 43 and 24 mg respectively. The structures of AS and SM (Figure 1) were confirmed by their spectral data (1H-NMR, 13C NMR and Mass) and comparison with the reported values [15,16].
Generation of different P.chaba fruit extracts
The following extraction techniques were used for the extraction of P.chaba fruits.
Percolation (PER)
The fruit powder of P. chaba (5 g) in methanol (100 mL) was kept at room temperature for 3 h with shaking for 5 min after every 30 min.
Soxhlet extraction (SOX)
The fruit powder of P. chaba (5 g) in methanol (100 mL) was extracted under hot condition (65°C) using a soxhlet extractor for 3 h.
Ultrasound assisted extraction (USAE)
The fruit powder of P. chaba (5 g) in methanol (100 mL) was sonicated at 40°C for 3 h.
Accelerated solvent extractor (ASE)
The fruit powder of P.chaba (5 g) was extracted with methanol (100 mL) in a stainless steel cell using Accelerated Solvent Extraction technique. The temperature was maintained at 40°C and the nitrogen pressure at 50 psi for 3 h.
Microwave assisted extraction (MAE)
The fruit powder of P. chaba (5 g) with methanol (100 mL) was irradiated using a commercial microwave oven operated at a power of 700 W and temperature was maintained at 50°C for 3 h
Concentration of the respective methanol solubles, obtained in the above mentioned methods, under reduced pressure afforded the corresponding methanol extracts.
Standard stock solution and sample preparation
The test sample solutions of the various P. chaba fruit extracts were prepared by dissolving 1.0 mg of extract in 1 mL of HPLC grade MeOH. The standard solutions of markers (AS and SM) were prepared by dissolving accurately weighed 1.0 mg in MeOH as stock solution and stored in a refrigerator at 4°C. These standard solutions were further diluted to obtain a desired concentration for calibration in the range of 50-500 ng/band. The known volumes of mixed standards were applied onto the TLC plate to prepare 6-point calibration curves.
Chromatographic conditions
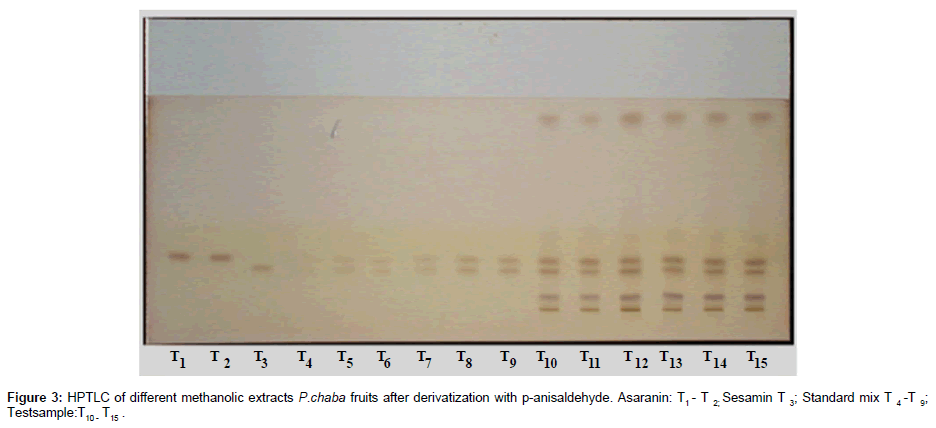
Aluminum backed silica gel 60 F254 E. Merck HPTLC plates (20×10 cm; 0.2 mm layers) were used. HPTLC Plates were prewashed with methanol and activated at 60°C for 5 mins before subjecting to chromatography. Standard and test sample solutions were applied in the form of bands (6 mm) from both the lower and the left edge with (10 mm) space between two bands being 10 mm, with a microliter syringe using an automatic TLC sampler, under continuous nitrogen gas. A constant application rate of 120 nL/s was employed. Linear ascending development was carried out in twin trough glass chambers saturated with the mobile phase. The mobile phase was optimized by varying the compositions of different polar solvents. Finally, a mobile phase consisting of hexane: ethyl acetate (70:30 v/v) was optimized for quantitative chromatography. The saturation time of the TLC chamber with the mobile phase was optimized to 30 min with 10 ml solvent was used for a better resolution of the tested markers. The developed plates up to a distance of about 75 mm were dried at 25°C for 30 min. After the plate was dipped into P-anisaldehyde- sulfuric acid solution (0.5 ml of p-anisaldehyde in 50 ml glacial acetic acid and 1 ml 97% sulfuric acid ) using the immersion device followed by air drying for 5 min. The plates were then heated for 5-10 min at 105-110°C using TLC plate heater and quantified densitometrically at 295 nm.
TLC scanning
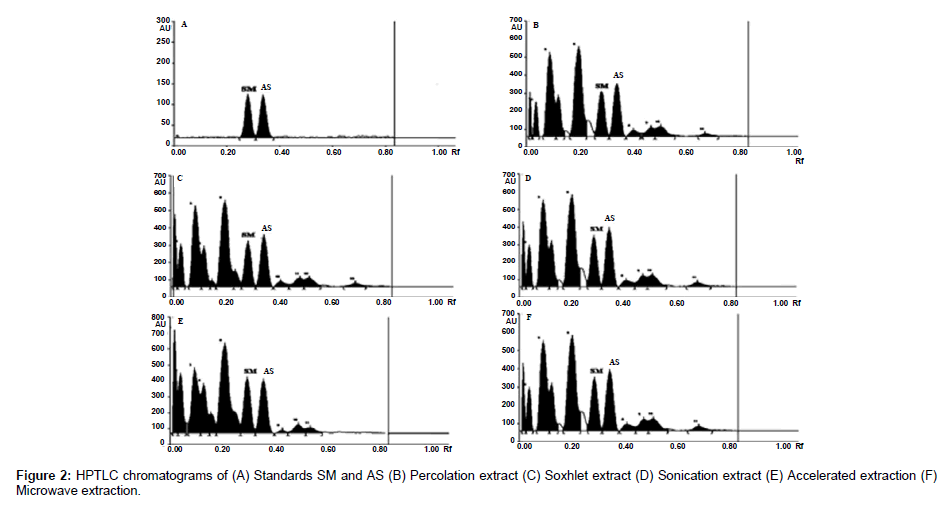
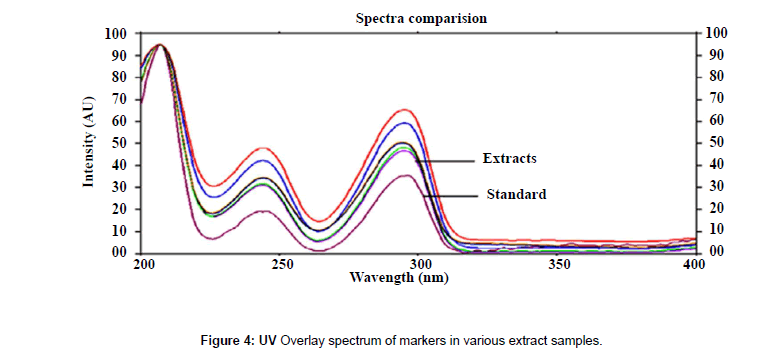
The plates were scanned by a Camag TLC Scanner-III controlled by Win CATS 1.4.2 software. The densitometry scanning was performed in the reflectance/ absorbance mode using the following parameters: slit width 6.00 mm×0.45 mm, scanning speed 20 mm/s and data resolution 100 μm/step were used. The HPTLC chromatograms of the extracts are shown in (Figure 2). For recording of characteristic derivatized spots of compounds and sample tracks in the range of 400–800 nm, tungsten lamp was used. Reprostar 3 with mounted digital camera was used for imaging and archiving the thin layer chromatograms (Figure 3). Quantification was performed using area under peak with linear regression of amount ng/band. Peak profiling was performed in visible region after derivatization. The densitogram were further scanned for their in situ UV spectra from 200 to 700 nm and over laid with the UV spectra of markers and extracts (Figure 4).
TLC–MS coupling and MS conditions
Mass spectrometry was used to confirm the chromatographic profile obtained by HPTLC-UV detection. A TLC–MS interface (Camag) with a 4 mm×2 mm elution head was used for elution of compounds from the HPTLC plates into mass system. Methanol was used as an eluent at a flow rate of 0.5 mL/min. The developed plate was introduced to MS interface in which the compound from each band was extracted by methanol and analyte was transferred online to the mass spectrometer. The mass spectrometer was equipped with ESI source and used with the following optimized conditions: source temperature 325°C, nitrogen was used as nebulizer and its pressure was maintained at 35 psi, dry gas temperature was at 325°C and flow rate was 8.0 L/min. Chemstation 5.3 (Bruker, Waldbronn, Germany) was used for instrument control and acquision of the mass spectrometric data. Molecular ion of AS and SM present in the samples was found to be same with that of the standard markers at same Rf value (Figure 5).
Validation parameters
LOD & LOQ: The LOD and the LOQ were calculated for both the markers on the basis of three- and ten-times the noise level, respectively.
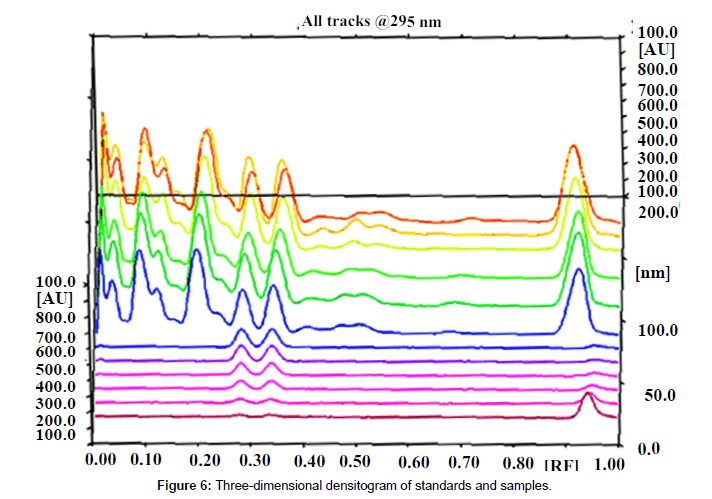
Specificity: The specificity of the method was ascertained by analyzing standards and test samples. The bands for the two marker compounds in test samples were confirmed by comparing the Rf and spectra of the spot with that of standards. The peak purity of individual markers was assessed by comparing the spectra at peak start, peak apex and peak end of the band, respectively. Representative overlay spectra of standards and P.chaba fruit extracts are shown in (Figure 6).
Precision: The repeatability of measurement (n=6) of peak area for compounds was expressed in terms of percent coefficient of variation (% RSD). The intra and inter day variation study in the analysis of was carried out at three different concentration levels.
Accuracy: Accuracy was studied in terms of recovery which was carried out by the standard addition method. Three concentration levels of the standard is spiked in to the sample solution and quantified by the developed method. Percentage recovery is calculated as following.
Recovery (%) = [(Found amount- original amount)/spiked amount] × 100
Results and Discussion
Method validation
Validation of quantitative HPTLC method includes the evaluation of following performance parameters such as linearity, limit of sensitivities, specificity, precision and accuracy, recovery and robustness according to ICH [17] and IUPAC [18] guidelines
Linearity and quantification
The calibration curves were linear in the concentration range of 50- 500 ng/ml band with correlation coefficients (r2) of 0.9996 and 0.9982 for AS and SM respectively. The regression data obtained showed a good linear relationship (Table 1).
| Compound | Rf | Linearity Range (ng/band) | Regression equation | Correlation Coefficient (r2) |
LOD (ng/band) | LOQ (ng/band) |
|---|---|---|---|---|---|---|
| Sesamin | 2.80 ± 0.01 | 50-500 | Y=4.388+3.987X | 0.9988 | 15.21 | 45.63 |
| Asarinin | 0.34 ± 0.01 | 50-500 | Y=6.874+3.888X | 0.9980 | 13.69 | 41.07 |
Table 1: Results of Linearity, LOD and LOQ.
LOD and LOQ
The LOD and the LOQ were calculated for markers on the basis of three- and ten-times the noise level respectively. The LOD values were found to be 15.21 and 13.69 ng/band for compounds AS and SM respectively, whereas LOQ values were 45.63 and 41.07 ng/band respectively (Table 1).
Specificity
Good correlation was also obtained between standards and sample overlay spectra. Representative overlay spectra of standards and P. chaba extracts are shown in Figure 4.
Recovery
Recovery studies of the analytes in the sample were carried out to assess the accuracy of the method. The average recoveries for AS and SM were found to be 95.80-100.22 respectively, which are within the acceptable % RSD (Table 2).
| Marker compound | Amount added (ng) | Average Recovery (Conc ± SD) |
% RSD | % Recovery |
|---|---|---|---|---|
| (Intra Day) | ||||
| 50 | 49.02 ± 0.35 | 0.71 | 98.04 | |
| Sesamin | 200 | 198.03 ± 0.16 | 0.81 | 99.01 |
| 500 | 498.36 ± 0.56 | 0.01 | 99.67 | |
| 50 | 47.90 ± 0.67 | 1.39 | 95.80 | |
| Asarinin | 200 | 196.29 ± 1.21 | 0.61 | 98.14 |
| 500 | 496.36 ± 0.70 | 0.14 | 99.27 | |
| (Inter Day) | ||||
| 50 | 49.00 ± 0.81 | 1.65 | 98.00 | |
| Sesamin | 200 | 197.37 ± 1.25 | 0.63 | 98.68 |
| 500 | 498.37 ± 0.92 | 0.18 | 99.67 | |
| Asarinin | 50 | 48.78 ± 1.39 | 2.84 | 97.56 |
| 200 | 200.44 ± 1.17 | 0.58 | 100.22 | |
| 500 | 499.56 ± 0.65 | 0.58 | 99.91 | |
Table 2: Accuracy Intra and inter-day precision (%RSD) of methods (n=6).
Precision
The repeatability of measurement (n=6) of peak area for AS and SM was expressed in terms of percent coefficient of variation (% RSD). The intra and inter day variation study in the analysis of AS and SM was carried out at three different concentration levels such as 50, 200 and 500 ng/band. The results are summarized in Table 2, which shows no significant inter and intraday variations were observed in the analysis of the compounds AS and SM.
Robustness
The robustness of the method was determined by introducing small changes in certain chromatographic parameters. Mobile phase having hexane/ethyl acetate (70:30 v/v) was tried with a variation of 0.5% v/v in each solvent. Time gap between spotting to chromatography, from chromatography to scanning and derivatization time (heating time) was varied from 0, 30, 60 min. dipping time was also varied between 2 and 10 s. Robustness was performed at three levels such as 50, 300 and 500 ng/band for AS and SM marker compounds; At a time, only one parameter was varied, whereas the rest were kept constant. The low values of %RSD (Table 3) is indicative of robustness of the method. Separation was not affected by changing the scanning wavelength ± 5 min.
| Parameters | RSD of peak area (%) | |
|---|---|---|
| Sesamin | Asaranin | |
| Mobile phase composition | 1.872 | 2.489 |
| Time gap between spotting and plate development | 2.181 | 2.121 |
| Dipping time | 2.416 | 1.944 |
| Derivatization time (Heating time) |
2.612 | 1.833 |
| Time gap between derivatization and scanning | 2.382 | 1.850 |
Table 3: Robustness testing of HPTLC method
Sample analysis
Based on the developed method the accumulation of AS and SM in P. chaba fruit extracts generated by different extraction methods was evaluated and the results are presented in Table 4. The markers, AS and SM were found to be present in highest quantity (2.108 & 0.103%) in the ASE Extract and lowest (0.848 & 0.048%) in the PER extract. Further, it was observed that though the cold extraction yield is less than the other four methods, its yield of marker was higher in ASE extractor. These results clearly indicate that no temperature is need for the extraction of markers. Hence the extractive methods ASE, MAE, UAE, and SOX are more suitable for high markers content.
| Extraction methods | Sesamin (%) | Asarinin (%) |
|---|---|---|
| Cold Extraction | 0.048 | 0.848 |
| Soxhlet Extraction | 0.051 | 0.924 |
| Sonic Extraction | 0.084 | 1.108 |
| Microwave Extraction | 0.090 | 1.503 |
| Accelerated solvent Extraction | 0.103 | 2.108 |
Table 4: Markers yield vs extraction method.
Conclusion
Comparison of various extraction techniques revealed that the ASE technique is the most efficient one for both the markers. When compared the extraction techniques, ASE system showed high extraction efficiency. The developed HPTLC method for quantitative estimation of AS and SM in P. chaba is rapid, simple, and accurate. Hence, the proposed method can be used for routine quality control purpose. To the best of our knowledge, this is the first densitometric method for the simultaneous determination of AS and SM in P. chaba fruits. In addition, the suggested method offers high sensitivity and simplicity compared with other reported methods developed for determination of makers and additionally peak identification was achieved by using MS-interface.
Acknowledgements
The authors wish to thank the Director, CSIR-IICT for support and constant encouragement.
Conflict of Interest
The authors have declared no conflict of interest
References
- Kirtikar KR, Basu BD (1980) Indian Medicinal Plants. 2nd edition, Volume I, Dehradun, India.
- Chauhan K, Solanki R, Patel A, Macwan C, Patel M (2011) Phytochemical and therapeutic potential of Piper longum Linn-A Review. Int J Res Ayurveda Pharm 12: 157-161.
- Kirtiker KR, Basu BD (2003) Indian Medicinal Plants. 2nd edition, Volume III, Dehradun, India.
- Morikawa T, Matsuda H, Yamaguchi I, Pongpiriyadacha Y, Yoshikawa M (2004) New amides and gastroprotective constituents from the fruit of Piper chaba. Planta Med 70: 152-159.
- Matsuda H, Ninomiya K, Morikawa T, Yasuda D, Yamaguchi I, et al. (2008) Protective effects of amide constituents from the fruit of Piper chaba on D-galactosamine/TNF-alpha-induced cell death in mouse hepatocytes. Bioorg Med Chem Lett 18: 2038-2042.
- Zhang H, Matsuda H, Nakamura S, Yoshikawa M (2008) Effects of amide constituents from pepper on adipogenesis in 3T3-L1 cells. Bioorg Med Chem Lett 18: 3272-3277.
- Matsuda H, Ninomiya K, Morikawa T, Yasuda D, Yamaguchi I, et al. (2009) Hepatoprotective amide constituents from the fruit of Piper chaba: Structural requirements, mode of action, and new amides. Bioorg Med Chem 17: 7313-7323.
- Parmar VS, Jain SC, Gupta S, Talwar S, Rajwanshi VK, et al. (1998) Polyphenols and alkaloids from piper species. Phytochemistry 49: 1069-1078.
- Scott IM, Jensen HR, Philogene BJR, Arnason JTA (2008) A review of piper spp. (Piperaceae) Phytochemistry, insecticidal activity and mode of action. Phytochem 7: 65-75.
- Anilakumar KR, Ajay Pal K, Farhath K, Amarinder SB (2010) Nutritional, Medicinal and Industrial Uses of Sesame (Sesamum indicum L.) Seeds. Conspect Sci 75: 159-168.
- Fukuda Y, Osawa T, Namiki M, Ozaki T (1985) Studies on antioxidative substances in sesame seed. Agric Biol Chem 49: 301–306.
- Dong Y, Gao Y, Xu B (2007) Extraction of antioxidant from sesame cake and its activity study. Food Sci 28: 44–47.
- Wusan W, Jianguo S (2006) The protective effects of sesamin against liver damage in rodents. Pharmacol Clin Chin Mater Med 22: 27–32.
- Zhou Y, Yang JR (2008) Effects of sesamin on blood glucose, blood lipids and vascular cell adhesion molecule-1 protein expression of aorta in rats with metabolic syndrome. Chin J Clin Pharmacol Ther 13: 195–200.
- Endo J, Nakamura T (1978) [Studies in Asiasarum (Aristolochiaceae). I. Three new compounds in Asiasarum heteropoides (author's transl)]. Yakugaku Zasshi 98: 789-793.
- Ishii H, Hosoya K, Ishikawa T, Haginiwa J (1974) [Studies on the chemical constituents of rutaceous plants. XX. The chemical constituents of Xanthoxylum arnottianum Maxim. (1). Isolation of the chemical constituents of the xylem of roots (author's transl)]. Yakugaku Zasshi 94: 309-321.
- ICH (1996) Q2B Validation of Analytical Procedure-Methodology. Consensus Guideline, ICH Harmonized Tripartite Guidelines. Geneva.
- IUPAC (2002) Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl Chem 74: 835–855.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14982
- [From(publication date):
September-2014 - Apr 29, 2025] - Breakdown by view type
- HTML page views : 10354
- PDF downloads : 4628