SIMULTANEOUS ATYPICAL MENINGIOMA AND CEREBRAL METASTASIS FROM BREAST CARCINOMA A CASE REPORT
Received: 01-Feb-2025 / Manuscript No. jhcpn-25-161030 / Editor assigned: 02-Feb-2025 / PreQC No. jhcpn-25-161030 (PQ) / Reviewed: 17-Feb-2025 / QC No. jhcpn-25-161030 / Revised: 24-Feb-2025 / Manuscript No. jhcpn-25-161030 (R) / Published Date: 01-Mar-2025
Abstract
Introduction: Dissemination of breast cancer to the central nervous system (CNS) occurs in 15%-30% of patients with invasive breast cancer. There are three distinct patterns for the occurrence of brain metastases: about 10% of people diagnosed with breast cancer will develop brain metastases during the first year from the diagnosis of the primary tumor; bimodal recurrence of central nervous system (CNS) metastasis occurring within 2 years from the initial breast cancer diagnosis; delayed recurrence of brain metastases, occurring more than 4-5 years after breast cancer diagnosis.
Material and method: We present the case of a female patient, 63 years old, who was admitted in the Neurosurgery Clinic of “Prof. Dr. N. Oblu” Emergency Clinical Hospital from Iasi, Romania, for: headaches, dizziness, aphasia of expression, and right hemiparesis with progressive evolution. Her personal medical history revealed a right breast carcinoma, which was diagnosed and surgically treated in 2017.
Results and discussion: The Magnetic Resonance Imaging (MRI) identified an intraaxial inhomogeneous solid formation, located on the left, at the level of the insula, external capsule, extreme capsule, in putamen and in the corona radiata, with the suspicion of a metastasis from the breast cancer. There were also two other solid extraaxial formations, one in the left temporal region, and the other one in the left posterior parietal region, which, due to patient's personal medical history and tumor locations, were difficult to be diagnosed as two meningiomas or two dural metastases. The surgeons excised the intraaxial tumor, which was pathologically diagnosed as a metastasis from the breast carcinoma, and the extraaxial left temporal tumor, which, from a histopathological point of view, proved to be an atypical meningioma.
Conclusion: The simultaneous occurrence of an intracranial meningioma and a single metastasis in the same patient is an unusual event. The presented case demonstrates that when multiple intracranial tumors are present in a patient with a known extracranial cancer, they should not always be regarded as metastases.
Keywords
Meningioma; Breast carcinoma; MRI; Histogenesis; Pathology
Introduction
Usually, when two or more intracranial tumors are identified on imaging, especially in a patient with a known primary cancer, they are suspected to be metastases. As far as we know, in the English literature, there are only few cases of patients with multiple intracranial tumors and different histogenesis that have been published in the literature up to date. Most of them refer to associations between: meningiomas and diffuse astrocytomas [1], glioblastoma multiforme with pituitary adenoma [2, 3], sphenoidal meningiomas and pituitary adenomas [4], or epidermoid cyst and vestibular schwannoma [5]. Due to advanced oncologic cancer treatments, the general increase in cancer incidence and better diagnostic tools, the incidence of brain metastases is currently increasing. Up to now, very few cases have been published describing the simultaneous association of an intracranial meningioma with a carcinoma metastasis originating from the ovary, lung, kidney or breast [6, 7]. Dissemination of breast cancer to the central nervous system (CNS) occurs in 24% of patients with invasive breast cancer [8]. There are three distinct patterns for the occurrence of brain metastases: about 10% of people diagnosed with breast cancer will develop brain metastases during the first year from the diagnosis of the primary tumor; bimodal recurrence of central nervous system (CNS) metastasis occurring within 2 years from the initial breast cancer diagnosis; delayed recurrence of brain metastases, occurring more than 4-5 years after breast cancer diagnosis. Brain metastases can manifest in two ways. There is the rare situation of the development of a cancer metastasis into another tumor (i.e. collision tumor), which was first described in 1902. This is a well-documented phenomenon in which a host tumor, which is usually more indolent, serves as a source for the growth of a neoplasm more aggressive. This phenomenon often occurs in the case of a meningioma, which allows a metastatic lesion to grow within it. There is also a very rare situation in which synchronous or simultaneous tumors can occur. In this case we are talking about two histological tumors compromising CNS at the same time, but there is no histopathological evidence that one tumor served as a source of growth for the other [7, 9, 10]. In the present paper we will present the situation in which synchronous intracranial tumors developed, but having two different cells of origin.
Material and method
We present the case of female patient, 63 years old, who was admitted in January 2019 in the Neurosurgery Clinic of “Prof. Dr. N. Oblu” Emergency Clinical Hospital, from Iasi, Romania, accusing headaches and dizziness. Neurological exam revealed aphasia of expression and right hemiparesis with progressive evolution. Her personal medical history revealed a right breast carcinoma, which was diagnosed and surgically treated in 2017
Results and discussion
Results
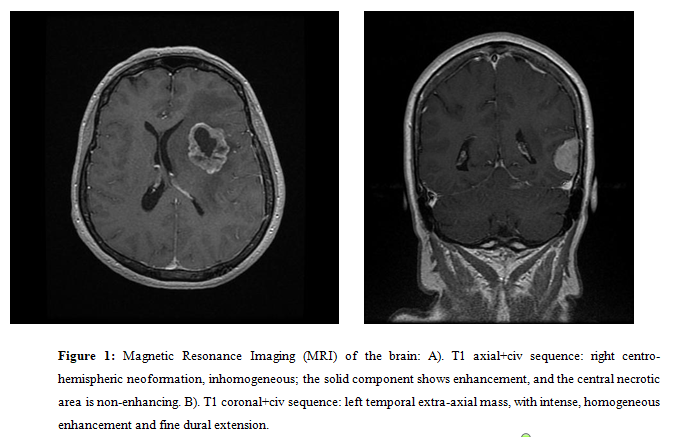
The cranio-cerebral Magnetic Resonance Imaging (MRI) (Figure1) with intravenous contrast identified an intraaxial inhomogeneous solid formation, located on the left, at the level of the insula, external capsule, extreme capsule, in putamen and in the corona radiata, measuring 4.2/3.1/3.8 cm. The imaging suspicion was that of a metastasis from the breast cancer. There were also two other solid extraaxial formations, one in the left temporal region, having the dimensions: 2.3/2.3/2.6 cm, and the other one in the left posterior parietal region, having the dimensions: 0.9/0.5/0.8 cm. The radiologist considered that, due to patient's personal medical history and tumor locations, it was difficult for him to differentiate between two meningiomas or two dural metastases.
Figure 1: Magnetic Resonance Imaging (MRI) of the brain: A). T1 axial+civ sequence: right centro-hemispheric neoformation, inhomogeneous; the solid component shows enhancement, and the central necrotic area is non-enhancing. B). T1 coronal+civ sequence: left temporal extra-axial mass, with intense, homogeneous enhancement and fine dural extension.
The laboratory analysis revealed the following pathological values within the hematology: red blood cells (RBCs) = 3,9 x106/µL, leucocytes = 5,9x 103/µL; Hemoglobin =11,2 g/dl, Hematocrit=-33,4 %. The biochemistry tests revealed: ASAT = 126 U/L, ALAT = 260 U/L, seric glucose = 127 mg/dl, K+ =3,2 mmol/l, thus concluding that the patient presented anemia and leucopenia, and hepatic cytolysis due to previous oncological treatment for breast cancer. The chest X-ray did not revealed any secondary pulmonary lesions.
The neurosurgeons realized a total microsurgical ablation of left intraaxial putaminal tumor and left extraaxial temporal tumor.
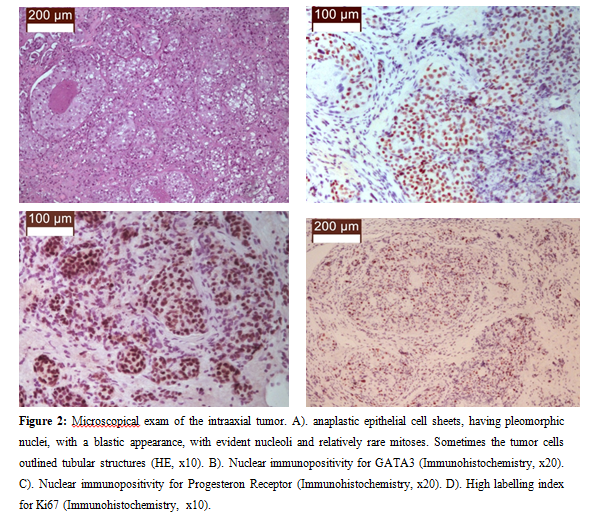
The microscopic examination of the left intraaxial putaminal formation revealed a tumor that was relatively well demarcated from the adjacent gliotic nervous tissue and was made up of anaplastic epithelial cell sheets, having pleomorphic nuclei, with a blastic appearance, with evident nucleoli and relatively rare mitoses. Sometimes the tumor cells outlined tubular structures. Immunohistochemical stains revealed cytoplasmic immunohistochemical positivity for CK7, and nuclear immnopositivity for GATA3, Progesteron Receptor, Her2neu, but cytoplasmic immunonegativity for CK20, and nuclear immunonegativity for TTF1 and Estrogen Receptor. Also, a high labelling index for Ki67 was noted (Figure 2).
Figure 2: Microscopical exam of the intraaxial tumor. A). anaplastic epithelial cell sheets, having pleomorphic nuclei, with a blastic appearance, with evident nucleoli and relatively rare mitoses. Sometimes the tumor cells outlined tubular structures (HE, x10). B). Nuclear immunopositivity for GATA3 (Immunohistochemistry, x20). C). Nuclear immunopositivity for Progesteron Receptor (Immunohistochemistry, x20). D). High labelling index for Ki67 (Immunohistochemistry, x10).
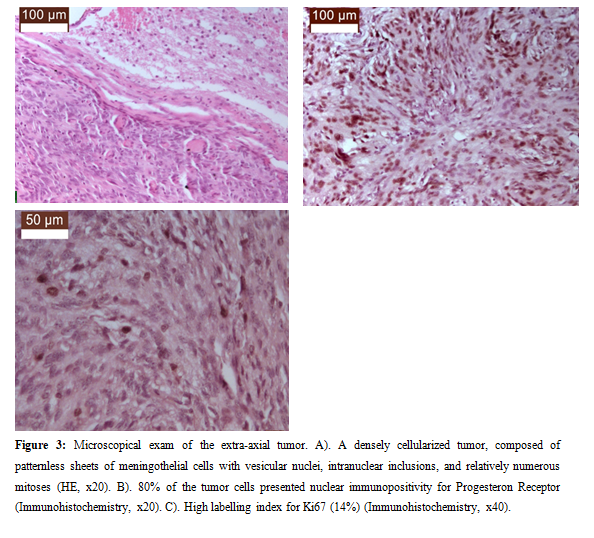
The microscopic examination of the second formation, the left extraaxial temporal one, identified: a densely cellularized tumor, composed of patternless sheets of meningothelial cells with vesicular nuclei, intranuclear inclusions, and relatively numerous mitoses (5 mitoses/10 high power fields). Immunohistochemical stains revealed nuclear immunopositivity for almost 80% of tumor cells and a high labelling index for Ki67 (14%) (Figure 3).
Figure 3: Microscopical exam of the extra-axial tumor. A). A densely cellularized tumor, composed of patternless sheets of meningothelial cells with vesicular nuclei, intranuclear inclusions, and relatively numerous mitoses (HE, x20). B). 80% of the tumor cells presented nuclear immunopositivity for Progesteron Receptor (Immunohistochemistry, x20). C). High labelling index for Ki67 (14%) (Immunohistochemistry, x40).
The final pathological diagnosis was: intracerebral metastasis of invasive breast carcinoma associated with grade 2 atypical meningioma.
The postoperative evolution was slowly favorable, being initially influenced by the onset of a coma as a result of an intracerebral hematoma in the tumor bed for which surgical intervention was performed; later, deep thrombosis of the left lower limb occurred, for which she required vascular surgery and internal medicine consultations. A treatment with heparin administered as a subcutaneous injection into the abdomen. After one month, the patient was discharged in a palliative unit.
Discussion
In the USA, over 70,000 primary central nervous system tumors are diagnosed annually, of which more than a third (36%) are meningiomas, making it the most common primary brain tumor [11]. Also in the USA, between 2004 and 2017, the overall incidence of grade 1 meningioma increased, then stabilized, especially in young people, and the same trend will continue in the coming years [12]. According to the Fifth Edition of the World Health Organisation Classification of the CNS tumors, meningiomas can be assigned with 3 grades of malignity, grade 1, 2 and 3. The grade 1 meningiomas have as morphological characteristics the followings: absence of atypical, anaplastic or rhabdoid features at the histological examination. Grade 2 meningiomas present brain invasion, necrosis and more than 2.5 mitoses/1mm2. Grade 3 meningiomas have more than 20 mitotic divisions in 10 consecutive microscope high power fields [13]. As our case presented a meningioma with 5 mitoses/mm2, it was considered to be an atypical meningioma, grade 2 (WHO, 2021).
In women who are in their fifth to seventh decades of life, meningiomas and breast cancers usually appear at a higher rate than in the general population. The presented case respects this trend, as our patient was a woman aged 63. Moreover, it is also documented that the risk of developing breast cancer is higher in postmenopausal women who have metabolic syndrome, namely the association of a low level of HDL and the presence of abdominal obesity [14, 15].
However, the association between metastasis from breast cancer and intracranial meningiomas is documented as rare because, until now, in the literature, there are only seven reports of intracranial meningioma associated with brain metastasis from breast carcinoma as case presentation [7, 16-19].
In a recent article published in British Journal of Neurosurgery, the authors presented a case report that presented two neighboring, but different tumors that invaded each other. The tumor-to-tumor metastasis revealed a growth within the host tumor. This was not associated with an adjacent growth, collision or embolization, but with the idea of a metastasis that was parasitizing within the meningioma [9]. Another case report published in the Japanese Journal of Clinical Oncology in 2021 presents the association between breast cancer metastasis and an intracranial meningioma in a 53-year-old female patient who underwent radical mastectomy for stage IIA breast carcinoma (histologically discovered as intraductal carcinoma). Immunohistochemical positivity for Progesterone Receptor in the breast carcinoma was identified. After a four year follow-up, on a routine follow-up bone scintigram it was revealed a bone and/or brain lesion that was suspected to be a metastasis. The MRI and angiography investigations showed the existence of a right para-sagittal mass that was suspected to be a meningioma. After surgical resection, the histological exam confirmed the diagnosis of a grade 1 meningioma, which also showed immunopositivity for Progesteron Receptor [17]. However, there are published articles that highlighted the fact that there is a strong relationship between breast cancer and meningioma development as some authors [19] found out that female patients with meningioma present nearly 10-fold higher odds of breast cancer when compared with the general female population. Although there is difficult to differentiate between these two tumor types on imaging [9], a correct diagnosis can be obtained by the pathologist in all cases due to their pathological and immunohistochemical features, which differ considerably both among themselves, but also with other brain metastases [20-22]. Regarding the spatial relationship between the two surgically excised tumors. In the literature there are mentioned three mechanisms for the development of a cerebral carcinoma metastasis associated with a intracranial meningioma : (1) A carcinoma metastasis can occur within a meningioma - the so-called „collision tumor”. It occurs by metastatic embolization into the rich vasculature of the meningioma. This is an accidental discovery [9, 23, 24]. Symptoms appear when a previously known meningioma grows rapidly. The diagnosis imaging is very difficult because a possible hypodense area identified into the meningioma can lead to the diagnosis of a metastasis, even though it is only necrosis due to the high grade of meningioma. 2). Metastasis from a carcinoma can develop in the brain parenchyma in the immediate vicinity of a meningioma and this is an accidental event. Metastasis occurs by seeding the nervous tissue with metastatic emboli coming by way of the large dural vessels. 3). The simultaneous association of a meningioma with a metastatic tumor located at a distance from the meningioma [7]. Brain metastasis is often symptomatic because it progresses more rapidly and causes perilesional edema, but the meningioma is incidentally identified, or, when it is large enough, may contribute to symptoms of intracranial hypertension [25]. In the case of our case presentation, meningioma was located at some distance from the metastasis and we can take into consideration the third situation, i.e. meningioma was incidentally identified in a known oncological patient having a suspicion of brain metastasis.
Conclusion
The simultaneous occurrence of an intracranial meningioma and a single metastasis in the same patient is an unusual event. Our case demonstrated that when multiple intracranial tumors are identified in a patient with a known extracranial cancer, they should not always be regarded as metastases. The present case confirms the scant data in the literature that there is a predominant association between meningioma and cancers of the reproductive system, especially breast cancer.
Acknowledgment
None
Conflict of Interest
None
References
- Dario A, Marra A, Cerati M (1995) Intracranial meningioma and astrocytoma in the same patient. Case report and review of the literature. J Neurosurg Sci 39:27-35.
- Furtado SV, Venkatesh PK, Ghosal N, Hegde AS (2010) Coexisting intracranial tumors with pituitary adenomas. Genetic association or coincidence? J Cancer Res Ther 6: 221-223.
- Naydenov E, Marinov M, Nachev S (2012) Two different primary brain tumors, glioblastoma multiforme and pituitary adenoma, in association with colorectal carcinoma: an unusual case of nonfamilial Turcot's syndrome? J Neurol Surg A Cent Eur Neurosurg 73: 410-412.
- Honegger J, Buchfelder M, Schrell U (1989) The coexistence of pituitary adenomas and meningiomas: three case reports and a review of the literature. Br J Neurosurg 3:59-69.
- Roman A, Najera E, Spiriev T (2017) Concomitant Occurrence of Vestibular Schwannoma and Epidermoid Cyst in Neurofibromatosis: Case description and Review of the Literature. Braz Neurosurg 36 230-233.
- Campos Paiva AL, Araujo JLV, Ricieri Ferraz V, Esteves Veiga JC (2017) Simultaneous meningioma and brain metastases from renal cell carcinoma - a rare presentation. Case report. Sao Paulo Med J 135:296–301.
- Maiuri F, Cappabianca P, Iaconetta G, D'Acunzi G (2002) Meningiomas Associated with Brain Metastases. Cent Eur Neurosurg 63: 111-115.
- Kuksis M, Gao Y, Tran W (2021) The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro-Oncol 23:894-904.
- Ren Y, Cheng Y, Fan J (2022) A meningioma and breast carcinoma metastasis collision tumor. Br J Neurosurg 36: 272-273.
- Weil RJ, Palmieri DC, Bronder JL (2005) Breast Cancer Metastasis to the Central Nervous System. Am J Pathol 167: 913-920.
- Walsh KM. Epidemiology of meningiomas. In: Michael W. McDermott (Edited by) Handbook of Clinical Neurology. Volume 169, Elsevier, Amsterdam, 2020, pages 3-15.
- Bhala S, Stewart DR, Kennerley V (2021) Incidence of Benign Meningiomas in the United States: Current and Future Trends. JNCI Cancer Spectr 5: 30-35.
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours [Internet] In WHO Classification of Tumours Series, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6, Available online.
- Petrovanu C, Coman AE, Murariu GC (2008) Petrovanu R. Metabolic syndrome and breast cancer risk in post-menopausal women. Rev Med Chir Soc Med Nat Iasi 112:630-634.
- Coman EA, Petrovanu R (2009) Sindromul metabolic in practica de ambulator, PIM, Iași.
- Lin J-W, Su F-W, Wang H-C (2009) Breast carcinoma metastasis to intracranial meningioma, J Clin Neurosci 16 :1636-1639.
- Kubo M, Fukutomi T, Akashi-Tanaka S (2001) Association of Breast Cancer with Meningioma: Report of a Case and Review of the Literature, Japanese Journal of Clinical Oncology 31:510-513.
- C Bianchi, A Vassallo, L Di Bonito, Giammusso (1980) Association breast cancer-meningioma. Report of two cases. Sem Hop 56:488-489.
- Degeneffe A, De Maertelaer V, De Witte O, Lanfranc F (2023) The Association Between Meningioma and Breast Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open.
- Costea CF, Anghel K, Dimitriu G (2014) Anatomoclinical aspects of conjunctival malignant metastatic melanoma. Rom J Morphol Embryol 55:933-937.
- Buzdugă CM, Costea CF, Cărăuleanu A (2023) Protean cytological, histological and immunohistochemical appearances of medullary thyroid carcinoma: Current updates. Rom J Morphol Embryol 60: 369-381.
- Cosman M, Pantiru IM, Serban R (2019) Correlations between histological subtypes and neurocognitive assessment of language area tumors. our 43 case series and review of the literature. Rom J Morphol Embryol 60: 1143-1151.
- Sættem M, Sundstrøm T, Sæle AKM, Mahesparan R (2024) Review of metastasis to meningiomas with case examples. Brain Spine 4:102862.
- Mogere E, Mutebi M, Njau A (2024) A rare case of breast carcinoma metastasis into a meningioma in a 64-year-old female patient. Radiol Case Rep 19:1519-1523.
- Seckin H, Yigitkanli K, Ilhan O (2006) Breast carcinoma metastasis and meningioma. A case report. Surg Neurol 66:324-327.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Dumitrescu AM, Dima-Cozma LC, Gavrilescu CM, Barbu RM, Șorodoc V, et al. (2025) Simultaneous Atypical Meningioma and Cerebral Metastasis from Breast Carcinoma a Case Report. J Health Care Prev, 8: 304.
Copyright: © 2025 Dumitrescu AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 255
- [From(publication date): 0-0 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 146
- PDF downloads: 109