Research Article Open Access
Simple and Fast Determination of Ammonia in Tobacco
Eugene Jansen*, Piet Beekhof, Johannes Cremers and Reinskje TalhoutCentre for Health Protection, National Institute for Public Health and the Environment, Netherlands
- *Corresponding Author:
- Eugene Jansen
Centre for Health Protection
National Institute for Public Health and the Environment
PO Box 1, 3720 BA Bilthoven, the Netherlands
Tel: +31-302742940
E-mail: eugene.jansen@rivm.nl
Received date: December 26, 2013; Accepted date: January 23, 2014; Published date: January 25, 2014
Citation: Jansen E, Beekhof P, Cremers J, Talhout R (2013) Simple and Fast Determination of Ammonia in Tobacco. J Anal Bioanal Tech 5: 178. doi: 10.4172/2155-9872.1000178
Copyright: © 2013 Jansen E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The presence of ammonia in tobacco is an important factor for the absorption of nicotine, and for product taste. The determination of ammonia in tobacco is usually performed by ion chromatography with conductivity detection devices.
Here a new method is presented to measure the concentration of ammonia in tobacco based on an automated enzymatic method. This method is easy to perform and can be used on routine clinical analyzers. The enzymatic ammonia determination showed an intra-assay and inter-assay variation of 4-7 and 5-8%, respectively as determined with 3 brands of cigarettes. A comparison with the established HPLC-IC method gave similar results with respect to both concentrations in cigarettes and the reproducibility of the method. In one working day 50-60 samples of cigarettes or tobacco can be processed and analyzed.
Keywords
Ammonia; Enzymatic assay; Tobacco
Introduction
Ammonia is an important component of tobacco, primarily, because it is believed to facilitate the absorption of nicotine [1-4]. In addition, ammonium compounds react with sugars during tobacco processing and smoking to form flavour components that ‘improve’ the taste of tobacco smoke [5]. For these reasons, ammonia has been selected as priority component for method development for the WHO Framework Convention on Tobacco Control [6]. The determination of ammonia in tobacco and tobacco products has been subject of several publications [7,8]. All these methods are rather complicated by the use of pre-purification techniques with subsequent detection by ion chromatography coupled with conductivity detection devices which are usually not available in a normal analytical laboratory.
The present report describes a new simple, specific and fast method for the quantitative determination of ammonia in tobacco by a specific enzymatic reaction, with glutamate dehydrogenase in which NADPH is oxidized to NADP+. This assay is used routinely on clinical auto analyzers.
Materials and Methods
Materials
Ammonia was measured on a clinical auto-analyzer (Synchron LX20) with a kit (AMM reagent # 439770) both supplied by Beckman- Coulter, Woerden, The Netherlands. The ammonia kit consists of α-ketoglutarate (3.23 mmol/L), adenosine diphosphate (ADP, 1.9 mmol/L), β-nicotinamide adenine dinucleotide phosphate (NADPH, 0.22 mmol/L) and glutamate dehydrogenase (GLDH) from beef liver (>10 U/L). The ammonia reagent is stable until the expiration date printed on the label when stored unopened at +2°C to +8°C (usually at least one year). Once opened, the reagent is stable for 30 days at +2°C to +8°C. The standard ammonium sulfate was dissolved in 0.01M sulfuric acid to a final concentration of 154 μmol/L. The extraction solution consisted of 0.025 mol/L sulfuric acid (pH 1.75).
The cigarettes used in this study were Chesterfield, Belinda and Gauloises, obtained from the local shops. The reference cigarette K3R4F and the Marlboro cigarettes were obtained from Kentucky Tobacco Research & Development Center, University of Kentucky, Lexington, USA. All cigarettes were conditioned for 48 h at 20°C and 60% relative humidity prior to the analyses.
Methods
Determination of ammonia in whole tobacco: To approximate 100 mg tobacco, 10 mL of the extraction solution was added and shaken for 1 h. Then the extract solution was centrifuged for 10 min at 4000 rpm. The supernatant was diluted (1:3, v/v) with phosphate buffered saline (PBS, pH7.6). The pH must be between 7.2 and 7.6. Now the sample represents a concentration of 2.5 g tobacco/L and is ready to apply to the automatic measuring system (Synchron LX20), described above. The ammonia reagent, as described above, is used to measure ammonia by a timed endpoint method. In the assay reaction, glutamate dehydrogenase (GLDH) catalyzes the reaction of ammonia and α-ketoglutarate to glutamate with the concomitant oxidation of NADPH to NADP+. The amount of NADPH oxidized is directly proportional to the amount of ammonia in the sample. The Synchron LX20 automatically pipette the appropriate sample volume (40 μL) and reagent volumes (226 μL) into a cuvette. The system monitors the change in absorbance at 340 nm that is directly proportional to the concentration of ammonia in the sample and is used by the Synchron LX20 to calculate the ammonia concentration in the sample.
Results
Measuring principle
AMM reagent is used to measure ammonia by a timed endpoint method. In the assay reaction, glutamate dehydrogenase (GLDH) catalyzes the reaction of ammonia and α-ketoglutarate to glutamate with the concomitant oxidation of β-nicotinamide adenine dinucleotide phosphate (NADPH) to β-nicotinamide adenine dinucleotide phosphate (NADP+). The amount of NADPH oxidized is directly proportional to the amount of analyte in the sample. The chemical reaction scheme is shown below.
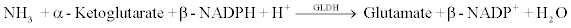
The Synchron LX-20 system automatically tranfers the appropriate sample and reagents into a cuvette. The ratio used is one part sample to 6 parts reagent (v/v). The system monitors the change in absorbance at 340 nm. This change in absorbance is directly proportional to the concentration of ammonia and its salts in the sample and is used by the Synchron LX-20 system to calculate and express the ammonia concentration.
Validation of the method
The extraction procedure has been adapted from a well-documented method [7]. Therefore recovery experiments related to the extraction procedure have not been performed in this study. Focus was put only on the detection method.
Samples can be measured in the analytical range from 160 μg ammonia/L (0.064 mg ammonia/g tobacco) to 17,000 μg ammonia/L (6.8 mg ammonia/g tobacco). Samples which exceeding the upper limit should be diluted with PBS buffer and reanalyzed.
Sensitivity for the ammonia determination is 160 μg ammonia/L (0.064 mg ammonia/g tobacco). Sensitivity is defined as the lowest measurable concentration that can be distinguished from zero with 95% confidence.
The reproducibility of the ammonia determination was very good with an intra-assay variation of 2.0% and an inter-assay variation of 3.0% as determined with plasma samples using the Beckman-Coulter system.
The reproducibility of the whole assay of ammonia in tobacco, including the weighing and extraction procedure was determined with three brands of cigarettes with 16 independent determinations/day during three days. The results have been depicted in Table 1.
| Brand | Average value (mg ammonia/g tobacco) |
Intra-assay CV (%) | Inter-assay CV (%) |
|---|---|---|---|
| Belinda | 0.83 | 5.97 | 7.38 |
| Chesterfield | 1.47 | 3.92 | 5.63 |
| Gauloises | 3.93 | 7.17 | 8.55 |
Table 1: Intra- and inter-assay coefficients of variation (%) as determined with 3 brands of cigarettes at 3 different days with 16 independent determinations.
The stability of the extracts prior to the analysis step was determined with three brands of cigarettes (N=16). The extracts were prepared and the ammonia concentrations were determined on the same day (day 1). Then the extracts have been stored at room temperature for another 2 days. At day 2 the average value was 101.8 % of the value measured at day 1. At day 3 this value was 101.1 %. So the extract can be prepared and kept at room temperature for at least several days before the final measurement.
In one working day, a well-trained technician can determine the ammonia concentration in 56 samples of tobacco from cigarettes. This includes weighing, extraction and measurement. The enzymatic measurement of ammonia with the auto-analyzer can be performed with a capacity of about 200 samples per hour.
Comparison with the HPLC-IC method
A comparison was made with the Health Canada methodology in which the ion chromatography with a suppressed ion conductivity detector was used. (Health Canada, 1999). This comparative study was performed in cooperation with Labstat International ULC. Kitchener. Ontario. Canada. The results with 2 brands of cigarettes have been summarized in Table 2.
| Brand | Labstat ULC | RIVM | |
|---|---|---|---|
| Marlboro Flip-Top Box | average value (µg/g) | 2007 | 2071 |
| standard deviation | 65 | 71 | |
| CV (%) | 3.2 | 3.5 | |
| # cigarettes | 7 | 9 | |
| KR 3R4F | average value (µg/g) | 1074 | 1074 |
| standard deviation | 15 | 32 | |
| CV (%) | 1.5 | 3.0 | |
| # cigarettes | 7 | 9 |
Table 2: The results with 2 brands of cigarettes.
For the Marlboro cigarettes RIVM measured an average value which is only 3% higher than Labstat ULC For the KR 3R4F cigarette both labs obtained exactly the same average value. The coefficients of variation are in the acceptable range (1.5-3.5%)
Discussion
With the present method, which uses an automated clinical chemical analyzer, the amount of total ammonia, including salts can be determined with great specificity, accuracy and reproducibility. In addition, the method is easy to perform and a large number of samples can be measured per day. The enzymatic method is specific for ammonia and can also be applied in a laboratory not equipped with an auto-analyzer by using the components of the automated assay. In our hands, however, the development of a suitable manual method for ammonia was not successful, using commercial kits and colorimetric detection devices, such as a spectrophotometer of a micro-titer plate reader. Most probably, the high accuracy of the auto-analyzer is a prerequisite to obtain the required assay performance.
Using two brands of cigarettes the ammonia content of the present method was compared with that obtained by the HPLC-IC method Based on this comparison, it was concluded that both the enzymatic and the HPLC-IC method gave similar results with high precision and reproducibility.
The present method to measure the concentration of ammonia in tobacco is a new and easy method which can be used on routineclinical analyzers. Almost all brands of these clinical analyzers have the possibility to measure ammonia with great accuracy, because of the use dedicated kits and calibration systems. Therefore this can be used in almost all research settings and tobacco control labs worldwide.
Declaration of Interest
The authors stated that there are no conflicts of interest regarding the publication of this article.
Acknowledgements
The authors are indebted to Dr. P. Joza from Labstat International ULC for the measurements of the HPLC-IC method.
This study was conducted with financial support of the Netherlands Foodand Consumer Product Safety Authority (NVWA) and the Tobacco Free Initiative of the World Health Organization (WHO HQ/TFI).
References
- Pankow JF, Mader BT, Isabelle LM, Luo W, Pavlick A, et al. (1997) Conversion of nicotine in tobacco smoke to its volatile and available free-base form through the action of gaseous ammonia. Environ Sci Technol 31: 2428-2433.
- Pankow JF (2001) A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem Res Toxicol 14: 1465-1481.
- Willems EW, Rambali B, Vleeming W, Opperhuizen A, Amsterdam JG van (2006) Significance of ammonium compounds on nicotine exposure to cigarette smokers. Food Chem Toxicol 44: 678-688.
- Amsterdam J van, Sleijffers A, Spiegel P van, Blom R, Witte M, et al. (2011) Effect of ammonia in cigarette tobacco on nicotine absorption in human smokers. Food Chem Toxicol 49: 3025-3030.
- Talhout R, Opperhuizen A, Amsterdam JGC van (2006) Sugars as tobacco ingredient: Effects on mainstream smoke composition. Food and Chemical Toxicology 44: 1789-1798.
- World Health Organization (2008) Conference of the Parties to the WHO Framework Convention on Tobacco Control, Durban, South Africa 2008.
- Health Canada (1999) Determination of ammonia in whole tobacco. Tobacco Control Programme. Health Canada Official method T-302.
- Tobacco Manufacturers Organization (2002) UK smoke constituent study. Annex A Part5 method: determination of ammonia yields in mainstream cigarette smoke using the Dionex DX-500 ion chromatograph, Report Nr GC15/M24/02.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16614
- [From(publication date):
February-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11939
- PDF downloads : 4675
