Research Article Open Access
Simple Analysis Method for Metallothionein-1, -2 and -3 in the Brain by One-Step Size-Exclusion Column HPLC On-Line Coupling with Inductively Coupled Plasma Mass Spectrometry
Satomi Kameo1,2*, Kunihiko Nakai3, Akira Naganuma4, Hiroshi Koyama2 and Hiroshi Satoh11Environmetal Health Sciences, Tohoku University Graduate School of Medicine, Japan
2Department of Public Health, Gunma University Graduate School of Medicine, Japan
3Development and Environmental Medicine, Tohoku University Graduate School of Medicine, Japan
4Laboratory of Molecular and Biochemical Toxicology, Graduate School of Pharmaceutical Sciences, Tohoku University, Japan
- *Corresponding Author:
- Satomi Kameo
Department of Public Health
Gunma University Graduate School of Medicine
3-39-22 Showa-machi, Maebashi, Gunma 371-8511, Japan
Tel: +81-(0)27-220-8011
Fax: +81-(0)27-220-8016
E-mail: skameo-t@gunma-u.ac.jp
Received date: October 17, 2014; Accepted date: November 14, 2014; Published date: November 18, 2014
Citation: Kameo S, Nakai K, Naganuma A, Koyama H, Satoh H (2014) Simple Analysis Method for Metallothionein-1, -2 and -3 in the Brain by One-Step Size-Exclusion Column HPLC On-Line Coupling with Inductively Coupled Plasma Mass Spectrometry. J Anal Bioanal Tech 5:224. doi: 10.4172/2155-9872.1000224
Copyright: © 2014 Kameo S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A simple analysis method of metallothionein (MT) isoforms MT-1, MT-2, and MT-3 was developed based on the use of one-step size-exclusion column (SEC) HPLC and on-line coupling with inductively coupled plasma mass spectrometry (ICP-MS). For the elucidation of the functions of MT isoforms in the brain, it is necessary to have a simple method to determine these isoforms. A SEC TSK gel G2000 SWXL PEEK (7.8 mm I.D. × 30 cm) system was used in this study. The HPLC system was connected with a quadrupole ICP-MS. All of the connections were made using PEEK tubing. To induce MTs, cadmium (Cd) chloride was administered to MT-1, 2 null and 129/Sv mice at a dose of 4 mg/kg body weight by i.p. injection. Mice were sacrificed 24 h after treatment under anesthesia. Brains and livers were collected, and all the samples were stored at −80°C until subsequent analyses. Soluble extracts of the livers and brains from 129/Sv mice either treated with cadmium or untreated were analyzed. MT-1, MT-2, and MT-3 were clearly separated by the one-step SEC HPLC-ICP-MS system (monitored at 65Cu, 66Zn, 113Cd and 55Mn for element) using an appropriate buffer (25 mM Tris-12.5 mM HCl containing 20 mM KCl) and an ultrafiltration membrane filter to eliminate high molecular weight proteins over 30,000 MW and Cu, Zn-SOD. Retention times (RTs) of peaks of each isoform were distinguishable; RTs of MT-1, MT-2, and rMT-3 were 8.6, 8.1, and 7.6 min, respectively. MT-1, MT-2, and MT-3 were separated clearly using this system. This system would be a powerful tool for the separation and metal-component analysis of MT isoforms to elucidate further the biological functions of MTs in the brain.
Keywords
Metallothionein; MT-3; SOD; HPLC; ICP-MS; Brain
Introduction
Metallothioneins (MTs) are a class of ubiquitously occurring low molecular weight cysteine- and metal-rich proteins containing sulfurbased metal clusters. Mammalian MTs are comprised of four major isoforms designated MT-1 through MT-4. MT-1 and MT-2 are expressed in most tissues, including the brain, whereas MT-3 and MT-4 are expressed predominantly in the central nervous system and in keratinizing epithelia, respectively. MT-1 and MT-2 have been implicated in detoxification of heavy metal poisoning, disparate physiological functions such as zinc and copper metabolism, protection against reactive oxygen species, and adaptation to stress [1-3]. MT-3 was discovered unexpectedly while investigating the hypothesis that in the brains of patients with Alzheimer’s disease (AD) a loss of growth inhibitory factor (GIF) occurs, resulting in unbalanced total neurotrophic activity of the brain [4]. GIF was subsequently isolated from the normal human brain and was shown to belong to the MT family and designated MT-3 [5,6]. MT-3 is a non-inducible protein, primarily found in the brain, and seems to have important neurophysiological and neuromodulatory functions [1,7,8].
High-performance liquid chromatography (HPLC) and capillary electrophoresis (CE) are able to separate different MT isoforms; however, UV detection has relatively poor sensitivity and no metalspecific capabilities [9]. HPLC followed by metal-specific detection using atomic absorption spectrometry (AAS) or inductively coupled plasma atomic emission spectrometry (ICP-AES) was employed for the detection and determination of metal-binding proteins [10,11] as an early hyphenated technique. Recently, inductively coupled plasma mass spectrometry (ICP-MS) has been widely used as a detection tool with hyphenated techniques because of its high sensitivity and multi-element capabilities [9,12-16]. However, in previous studies, no simple system was developed for separating the MT isoforms (MT-1, MT-2, and MT- 3) of brain in one step. Typically, HPLC systems with the combination of two different types of chromatography, such as size-exclusion column chromatography (SEC) and ion-exchange chromatography, or tandem connection with SEC are used.
The applications of various HPLC separation techniques and the coupling to ICP-MS hyphenated techniques are attractive tools and valid alternatives to classical methods for the analysis of MTs. These new techniques have the potential to meet the key challenges in the characterization of MTs [17,18]. In the present study, we developed a simple separation system for analysis of MT-1, MT-2 and MT-3 based on the use of one-step SEC HPLC and on-line coupling with an ICP-MS system with using an appropriate buffer and filter.
Materials and Methods
Animals
MT-1, -2 null mice and 129/Sv mice (as wild type) were purchased from Jackson Laboratory, Bar Harbor, ME, USA. Mice were housed in plastic cages with free access to food and water. The light:dark cycle was 12:12 h and the room temperature was maintained at 21–23°C. The entire procedure was reviewed and approved by the Committees of Animal Experimentation of Tohoku University and Tohoku University School of Medicine.
Chemicals
Cadmium (Cd) chloride and standard solutions of Cd, zinc (Zn), copper (Cu) and manganese (Mn) were products of Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Ultrapure nitric acid was purchased from Kanto Chemicals Co., Inc. (Tokyo, Japan). Trizma base (T1503) and MT-1, -2 standards from rabbit liver (M7641 for metallothionen-1, -2 mixture, M5267 for MT-1, and M5392 for MT- 2) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were of commercially available analytical grade. Ultrapure water (resistance 18M-Ω/cm) from a Milli-Q Gradient (Millipore, Bedford, MA, USA) was used for all experiments.
Instrumentation: HPLC-ICP-MS
SEC with TSK gel G2000 SWXL PEEK (7.8 mm I.D. x 30 cm, MW range:5,000-150,000) and a TSK guard column SWXL PEEK guard column (Tosoh, Tokyo, Japan) for the HPLC system (Nanospace SI- 2, Shiseido, Tokyo, Japan) was used in this study. The HPLC system was connected to a quadrupole ICP-MS system (ELAN5000, Perkin- Elmer, Waltham, MA, USA). All of the connections were made using PEEK tubing to prevent metal-binding absorption to the equipment. Operating conditions for ICP-MS are described in Table 1.
| Condition | Value |
| RF power | 1300 W |
| Flow rate | |
| Plasma gas | 15 L m-1 |
| Auxiliary gas | 0.8 L m-1 |
| Nebulizer gas | 0.9 L m-1 |
| Sample uptake rate | 1 mL m-1 |
| Detection mode | Graphic mode |
| Isotope monitored | 65Cu, 66Zn, 113Cd, 55Mn |
Table 1: Operating conditions for ICP-MS.
Induction of metallothioneins
Cadmium chloride was administered to MT-1, -2 null and 129/Sv (wild type) mice at a dose of 4 mg/kg of body weight by i.p. injection to induce MTs for wild type mice. The mice were sacrificed 24 h after treatment under anesthesia. Untreated control mice also were sacrificed under anesthesia. The brains and livers were collected and all samples were immediately frozen using liquid nitrogen and stored at −80°C until subsequent analyses.
Sample preparation
The experimental protocol for sample preparation of mouse tissues and speciation of metal-binding proteins analyzed by SEC HPLC-ICPMS was as follows. The liver or brain tissue was homogenized in four volumes of 10 mM Tris(hydroxymehyl)aminomethane (Tris)-5 mM HCl using a glass-Teflon homogenizer on ice. The homogenate was centrifuged at 10,000 × g for 1 h at 4°C with a high speed refrigerated microcentrifuge (MX-150 with a TMP-11 rotor, Tomy Seiko Co. Ltd., Tokyo, Japan). The supernatant was divided into two portions. One portion was filtered with an ultrafiltration membrane filter (ultrafree- MC filter, Millipore) by centrifugation at 5,000 × g for 1 h at 4°C. The membrane filter which we used in this study was an ultrafiltration filter to cut the high molecular weight proteins. The cutoff point (Nominal Molecular Weight Limit, NMWL) which we used was 30,000 NMWL. The mechanism of the filter depends on the effects of size exclusion. The other portion was unfiltered. The ultrafiltration membrane-filtered samples or unfiltered samples were subsequently applied to the HPLC column as described below.
Analysis of metal-binding proteins and metallothioneins by SEC HPLC-ICP-MS
An aliquot of 30 μL of the supernatant from the liver or brain tissues of mice either treated with cadmium or untreated was injected into the SEC HPLC system (at a flow rate of 1 mL/min.). Eluates of the supernatants were analyzed by ICP-MS (SEC HPLC-ICP-MS system). As the elution buffer, 25 mM Tris-12.5 mM HCl containing 20 mM KCl was used. The metals in the eluates were continuously monitored at m/z 65(Cu), 66(Zn), 113(Cd) and 55(Mn) for element detection. The peaks of the Cd-complex or Zn, Cu-containing complex detected by HPLC-ICP-MS were confirmed with Sigma-Aldrich rabbit MTs as authentic samples for MT-1 and MT-2. As the MT-3 authentic sample, recombinant MT-3, which we previously produced, was used [19].
Results
Metal-binding proteins and MTs induced by Cd in mouse liver
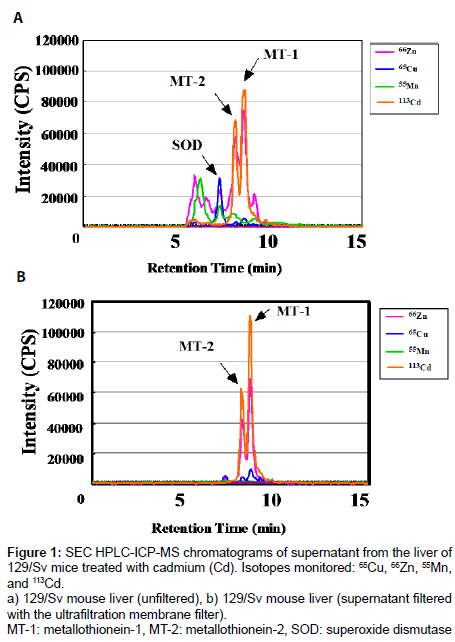
Figure 1 shows the SEC HPLC-ICP-MS elution profiles of the supernatant from the livers of the Cd-treated 129/Sv mouse. The chromatogram in Figure 1a shows the elution profile of the unfiltered supernatant, and the chromatogram in Figure 1b shows the elution profile of the ultrafiltration membrane filtered supernatant. A SEC gel with a low molecular weight range (5,000-150,000) was chosen to gain clear separation of MT isoforms. In this analysis system, 25 mM Tris- 12.5 mM HCl containing 20 mM KCl was used as the elution buffer. In HPLC-ICP-MS analyses, two major peaks of the Cd complex were detected. The peaks were confirmed using rabbit MTs as authentic samples for MT-1 and MT-2. Several high molecular weight proteins and Cu, Zn-superoxide dismutase (Cu, Zn-SOD; MW 32,000) also were eluted under these conditions (Figure 1a). To remove high molecular weight proteins and Cu, Zn-SOD from the supernatant, an ultrafiltration membrane filter was used before injection of the supernatant into the HPLC column. The resulting elution profile indicated clear separation of MT-1 and MT-2 (Figure 1b). We have also confirmed UV at 254 nm of the samples. Cd-metallothionein (Cd-MT) has a property of a specific absorption at 254 nm based on Cd-thiolate bond. Our results showed also absorptions at 254 nm of MT-1 and MT-2 peaks of SEC HPLC-ICP-MS in Figures 1a and 1b (data not shown). These results supported that the Cd-binding proteins in Figures 1a and 1b were Cd- MT which have many Cd-thiolate bonds.
Figure 1: SEC HPLC-ICP-MS chromatograms of supernatant from the liver of 129/Sv mice treated with cadmium (Cd). Isotopes monitored: 65Cu, 66Zn, 55Mn, and 113Cd.
a) 129/Sv mouse liver (unfiltered), b) 129/Sv mouse liver (supernatant filtered with the ultrafiltration membrane filter).
MT-1: metallothionein-1, MT-2: metallothionein-2, SOD: superoxide dismutase
SEC HPLC-ICP-MS chromatogram of mixture of MT-1, MT- 2, and recombinant MT-3 analyzed by HPLC-ICP-MS
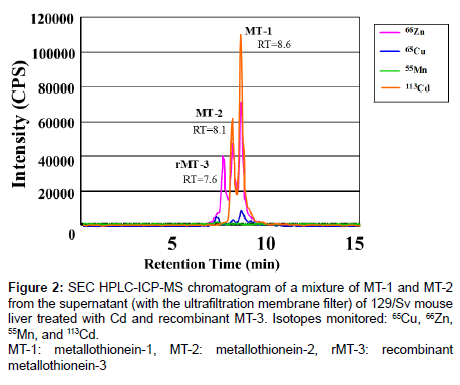
Figure 2 shows an SEC HPLC-ICP-MS chromatogram of a mixture of MT-1 and MT-2 from the ultrafiltration membrane filtered supernatant of the liver of the 129/Sv mouse treated with Cd and recombinant MT-3 (rMT-3). Retention times (RTs) of peaks of each isoform were distinguishable, and RTs of rMT-3, MT-2 and MT-1 were 7.6, 8.1, and 8.6 min, respectively. Thus, MT-1, MT-2, and MT-3 were clearly separated using this system.
Figure 2: SEC HPLC-ICP-MS chromatogram of a mixture of MT-1 and MT-2 from the supernatant (with the ultrafiltration membrane filter) of 129/Sv mouse liver treated with Cd and recombinant MT-3. Isotopes monitored: 65Cu, 66Zn, 55Mn, and 113Cd.
MT-1: metallothionein-1, MT-2: metallothionein-2, rMT-3: recombinant metallothionein-3
Separation of MT-1, MT-2, and MT-3 in the 129/Sv mouse brain (non-Cd treated) analyzed by HPLC-ICP-MS
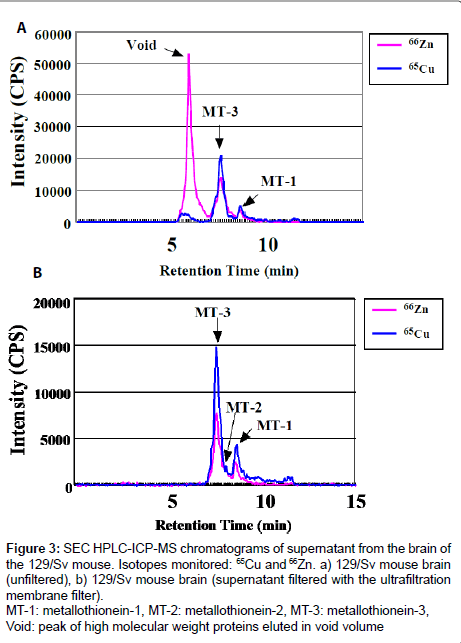
Figure 3 shows the elution profile of the supernatant from the brain of the 129/Sv mouse (Figure 3a: unfiltered) and elution profile of the ultrafiltration membrane filtered supernatant from the brain of the same mouse (Figure 3b: with ultrafiltration membrane filter) analyzed by SEC HPLC-ICP-MS. Peaks of high molecular weight and low molecular weight Zn, Cu-binding proteins were observed in the HPLC-ICP-MS chromatogram from the brain supernatant of the 129/ Sv mouse (Figure 3a). The second peak of Zn, Cu-containing proteins eluted at RT=7.6 contained MT-3 and also might contain Cu, Zn- SOD. Using the ultrafiltration membrane filter, high molecular weight metalbinding proteins (MW over 30,000) and especially Cu, Zn-SOD (MW 32,000) were removed from the supernatant in Figure 3b. Thus, the problem of Cu, Zn-SOD and MT-3 overlapping has been solved by using the ultrafiltration membrane filter before applying HPLC-ICPMS. The Zn, Cu-containing proteins were confirmed with authentic samples for MT-1, MT-2 and MT-3. The first peak of Zn, Cu-containing proteins eluted at RT=7.6 in Figure 3b was MT-3. The second peak of the Zn, Cu-containing proteins eluted at RT=8.1, which was a small peak, appeared to be MT-2. The third peak of the Zn, Cu-containing proteins eluted at RT=8.6 was MT-1.
Figure 3: SEC HPLC-ICP-MS chromatograms of supernatant from the brain of the 129/Sv mouse. Isotopes monitored: 65Cu and 66Zn. a) 129/Sv mouse brain (unfiltered), b) 129/Sv mouse brain (supernatant filtered with the ultrafiltration membrane filter).
MT-1: metallothionein-1, MT-2: metallothionein-2, MT-3: metallothionein-3, Void: peak of high molecular weight proteins eluted in void volume
Elution profile of MT-3 in the MT-1, 2 null mouse brain analyzed by HPLC-ICP-MS
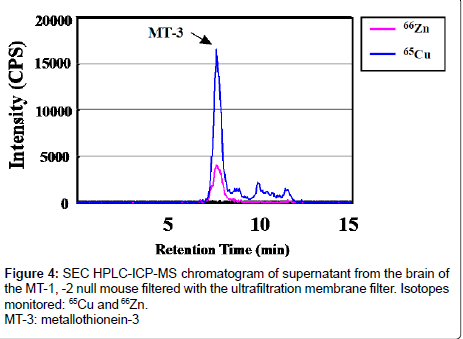
Figure 4 shows a chromatogram of the elution profile of the supernatant from the MT-1, -2 null mouse brain after the ultrafiltration membrane filtration analyzed by SEC HPLC-ICP-MS. Because this chromatogram was from the brain supernatant of MT-1, -2 null mice, peaks of MT-1 and MT-2 containing Cu and Zn disappeared. An MT-3 peak containing Cu and Zn was clearly observed in the HPLC-ICPMS chromatogram obtained using 25 mM Tris-12.5 mM HCl buffer containing 20 mM KCl using the ultrafiltration membrane filter. These results revealed that the Cu component of MT-3 compared with Zn in the MT-1, -2 null mouse was larger than that of wild type (129/Sv) mouse (Figure 3b).
Discussion
In this study, we developed a simple separation system for the analysis of MT-1, MT-2 and MT-3 in the brain based on the use of one-step SEC HPLC and on-line coupling with an ICP-MS system. Conventional methods such as metal-saturation assays [20,21], immunochemical methods [22] (radioimmunoassay or ELISAs), and differential pulse polarography have been used by biochemists for the analysis of MTs. However, these techniques suffer from a lack of selectivity to separate different MT isoforms, and are unable to provide information on metal composition. Recently, HPLC followed by metalspecific detection on-line coupling with ICP-MS has been widely used for the detection and determination of metal-binding proteins and MTs in mammalian tissues. Analytical methods of MTs with these hyphenated techniques have been thoroughly reviewed by Prange et al., Ferrarello et al., and Lobinski et al. [9,12,16]. However, in previous studies, no simple system was developed for separating and detecting MT isoforms (MT-1, MT-2, and MT-3) of the brain. HPLC systems with the combination of two different types of chromatography such as SEC and ion-exchange chromatography, or tandem connection with SEC, have often been used [23]. In many studies, it was difficult to separate all of the MT isoforms in the brain completely by one-step chromatography. Peaks of MT-3 and a mixture MT-1 and MT-2 were observed; however, HPLC peaks of MT-1 and MT-2 were eluted with similar RTs and overlapped [13,15,24]. The peaks of MT-3 and Cu, Zn- SOD also might overlap.
SEC (GFC) HPLC is one of the orthodox methods for the separation of metallothioneins that many metallothionein researchers used in the previous studies. We would like to follow the previous research results by using SEC (GFC) HPLC method and to add some modifications to the SEC (GFC) HPLC-ICP-MS method. Therefore, in this study we have tried to develop a simple method by using one-step of SEC HPLC without using anion exchange chromatography after SEC HPLC or reverse-phase HPLC. Certainly reverse-phase HPLC has a benefit to clear separation of many of compounds. However, reverse-phase HPLC needs to use organic solvents like acetonitrile or methanol as mobile phase. If an organic solvent injected into plasma of ICP-MS, due to high carbon content in organic solvents lead to deposition of carbon on the sampling cone. Eventually it leads a reduction in sensitivity, if the ICP-MS instrument is without any incineration system of organic solvents. We would like to develop a simple system with standard ICPMS instrument.
In the system we developed, SEC gel, TSK gel G2000 SWXL PEEK, a low molecular weight range (5,000-150,000) was chosen to gain clear separation of MT isoforms. TSK gel G2000 SWXL PEEK does not only involve a size exclusion mechanism, but also may have an ion-exchange mechanism. It was reported that multi-mode gel filtration columns not only have size exclusion but also ion-exchange and reversed phase properties depending on the elution conditions [25]. The TSK gel G2000 SWXL PEEK used in this study behaved like a multi-mode gel filtration column. We have used the buffer ”25 mM Tris-12.5 mM HCl buffer containing 20 mM KCl” in this study. In our previous studies, HPLCICP- MS results with the buffer 25 mM Tris-12.5 mM HCl without KCl showed non-clear separation of MT-1 and MT-2. After addition of 20 mM KCl to the buffer, we have achieved the clear separation of MT-1 and MT-2. We have considered that the function may due to not only size exclusion mechanism, but also come from the multi-mode gel filtration feature like ion-exchange effects. Table 2 shows advantages and disadvantages of using an ultrafiltration membrane filter before HPLC-ICP-MS analysis of brain MT isoforms. With an ultrafiltration membrane filter, there is an advantages to the removal of high molecular weight proteins and Cu, Zn-SOD.
| Unfiltered | with the ultrafiltration membrane filter | |
| advantages | Able to see distribution of all soluble fracion metal-biinding proteins | To remove high molecular weight proteins and Cu, Zn-SOD Benefit of a gentle preparation instead of precipitation by heating |
| disadvantages | Risk of overlap of MT-3 and Cu, Zn-SOD | Not able to see distribution of all soluble fracion metal-biinding proteins |
Table 2: Advantages and disadvantages of using the ultrafiltration membrane filter for HPLC-ICP-MS.
In this study, we had a clear separation of MT-isoforms in the brain using an appropriate buffer (25 mM Tris-12.5 mM HCl containing 20 mM KCl) for the TSK gel G2000 SWXL PEEK column using an ultrafiltration membrane filter. High molecular weight metal-binding proteins and Cu, Zn-SOD were removed from the supernatant using the ultrafiltration membrane filter before injection into the HPLC column. Furthermore, an ultrafiltration membrane filter has the benefit of a gentle preparation instead of precipitation using heating given that heat treatment might alter the MT metal composition. Commercially available columns and equipment are sufficient for analyzing MT isoforms in this system.
It is well known that MT-3 is the dominant isoform of MTs in the brain and we found that MT-1 also was one of the main isoforms of MTs. Although MT-2 accounts for only a small part of the MT isoforms in the brain physiologically, it is detectable using this system. The functional differences of MT-1 and MT-2 have not been well elucidated in the brain, and MT-3 is believed to have a very important role in the central nervous system. The study of MT isoforms with metal component information has great significance. Thus, this analysis system would be a powerful tool for the separation and metal-component analysis of MTs and further research on the biological functions of MT isoforms, especially MT-3 in the brain.
Conclusions
In this study, we developed a simple separation system for analysis of MT-1, MT-2 and MT-3 based on the use of one-step SEC HPLC and on-line coupling with an ICP-MS system. The metal components of the metal-binding proteins eluted by HPLC were continuously detected by ICP-MS. MT-1, MT-2, and MT-3 were clearly separated by this system using an appropriate buffer and membrane filter. This system would be a powerful tool for the separation and metal-component analysis of MTs and further research on the biological functions of MT isoforms in the brain.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The study was carried out in accordance with the Guide of Animal Experimentation, Tohoku University and the Tohoku University School of Medicine. The authors thank Ms. Chieko Satoh and Ms. Sato Yashima for their excellent technical assistance.
References
- Vasák M (2005) Advances in metallothionein structure and functions. J Trace Elem Med Biol 19: 13-17.
- Meloni G, Zovo K, Kazantseva J, Palumaa P, Vasák M (2006) Organization and assembly of metal-thiolate clusters in epithelium-specific metallothionein-4. J Biol Chem 281: 14588-14595.
- Vasák M, Hasler DW (2000) Metallothioneins: new functional and structural insights. Curr Opin Chem Biol 4: 177-183.
- Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M (1991) The growth inhibitory factor that is deficient in the Alzheimer's disease brain is a 68 amino acid metallothionein-like protein. Neuron 7: 337-347.
- Palmiter RD, Findley SD, Whitmore TE, Durnam DM (1992) MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A 89: 6333-6337.
- Klaassen CD (1999) Metallothionein IV. Birkhauser Verlag, Basel.
- Aschner M, Cherian MG, Klaassen CD, Palmiter RD, Erickson JC, et al. (1997) Metallothioneins in brain--the role in physiology and pathology. Toxicol Appl Pharmacol 142: 229-242.
- Hidalgo J, Aschner M, Zatta P, Vasák M (2001) Roles of the metallothionein family of proteins in the central nervous system. Brain Res Bull 55: 133-145.
- Prange A, Schaumlöffel D (2002) Hyphenated techniques for the characterization and quantification of metallothionein isoforms. Anal Bioanal Chem 373: 441-453.
- Klaassen CD, Lehman-McKeeman LD (1991) Separation and quantification of isometallothioneins by high-performance liquid chromatography-atomic absorption spectrometry. Methods Enzymol 205: 190-198.
- Suzuki KT (1991) Detection of metallothioneins by high-performance liquid chromatography-inductively coupled plasma emission spectrometry. Methods Enzymol 205: 198-205.
- Ferrarello CN, Fernández de la Campa MR, Sanz-Medel A (2002) Multielement trace-element speciation in metal-biomolecules by chromatography coupled with ICP-MS. Anal Bioanal Chem 373: 412-421.
- Polec K, Perez-Calvo M, Garcia-Arribas O, Szpunar J, Ribas-Ozonas B, et al. (2002) Investigation of metal complexes with metallothionein in rat tissues by hyphenated techniques. J Inorg Biochem 88: 197-206.
- Ogra Y, Suzuki KT (1999) Biological significance of non-acetylated metallothionein. J Chromatogr B Biomed Sci Appl 735: 17-24.
- Nischwitz V, Michalke B, Kettrup A (2003) Identification and quantification of metallothionein isoforms and superoxide dismutase in spiked liver extracts using HPLC-ESI-MS offline coupling and HPLC-ICP-MS online coupling. Anal Bioanal Chem 375: 145-156.
- Lobinski R, Chassaigne H, Szpunar J (1998) Analysis for metallothioneins using coupled techniques. Talanta 46: 271-289.
- Infante HG, Van Campenhout K, Blust R, Adams FC (2006) Anion-exchange high performance liquid chromatography hyphenated to inductively coupled plasma-isotope dilution-time-of-flight mass spectrometry for speciation analysis of metal complexes with metallothionein isoforms in gibel carp (Carassius auratus gibelio) exposed to environmental metal pollution. J Chromatogr A 1121: 184-190.
- Michalke B (2005) Capillary electrophoresis-inductively coupled plasma-mass spectrometry: a report on technical principles and problem solutions, potential, and limitations of this technology as well as on examples of application. Electrophoresis 26: 1584-1594.
- Kameo S, Nakai K, Kurokawa N, Kanehisa T, Naganuma A, et al. (2005) Metal components analysis of metallothionein-III in the brain sections of metallothionein-I and metallothionein-II null mice exposed to mercury vapor with HPLC/ICP-MS. Anal Bioanal Chem 381: 1514-1519.
- Onosaka S, Cherian MG (1981) The induced synthesis of metallothionein in various tissues of rat in response to metals. I. Effect of repeated injection of cadmium salts. Toxicology 22: 91-101.
- Klein D, Sato S, Summer KH (1994) Quantification of oxidized metallothionein in biological material by a Cd saturation method. Anal Biochem 221: 405-409.
- Mizzen CA, Cartel NJ, Yu WH, Fraser PE, McLachlan DR (1996) Sensitive detection of metallothioneins-1, -2 and -3 in tissue homogenates by immunoblotting: a method for enhanced membrane transfer and retention. J Biochem Biophys Methods 32: 77-83.
- Yasutake A, Nagano M, Hirayama K (2003) Alterations of metallothionein isomers in Hg(0)-exposed rat brain. Arch Toxicol 77: 12-16.
- Richarz AN, Brätter P (2002) Speciation analysis of trace elements in the brains of individuals with Alzheimer's disease with special emphasis on metallothioneins. Anal Bioanal Chem 372: 412-417.
- Ogra Y, Suzuki KT (2005) Speciation of selenocompounds by capillary HPLC coupled with ICP-MS using multi-mode gel filtration columns. J. Anal At Spectrom 20: 35-39.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16299
- [From(publication date):
December-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11636
- PDF downloads : 4663