Research Article Open Access
Silk Protein, Sericin as a Cognitive Enhancer in Alzheimer's Disease
Yellamma K*
Department of Zoology, Sri Venkateswara University, Tirupati, India
- Corresponding Author:
- Yellamma K
Department of Zoology
Sri Venkateswara University, Tirupati, India
Tel: 81-853-20-2260
E-mail: Yellamma55@gmail.com
Received date: March 14, 2014; Accepted date: October 01, 2014; Published date: October 06, 2014
Citation:Yellamma K (2014) Silk Protein, Sericin as a Cognitive Enhancer in Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 4:163.doi: 10.4172/2161-0460.1000163
Copyright: © 2014 Yellamma K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer’s disease (AD) a well-known neurodegenerative disorder characterized by formation of amyloid plaques and neurofibrillary tangles and by loss of neurons is the most common type of Dementia, also called as Senile Dementia of Alzheimer Type (SDAT) which frequently occurs in elder people. Globally it was noticed that every year 3, 60, 000 new cases were admitted with severe AD. AD was first described by a German Psychiatrist and Neuropathologist, Dr. Alois Alzheimer in 1906 and was named after him. According to Cholinergic hypothesis, AD is caused by reduced synthesis of the neurotransmitter ACh, where in the AChE levels were increased which causes damage to the cholinergic neurons finally leading to cognitive impairments. At present, AChE inhibitors, Anti- amyloid vaccine and Vitamin E are recommended to treat AD but long-term exposure to these drugs causes side effects. Since treatment of AD with single drug is not a realistic option due to it’s the complicated nature, combination therapy of AChE inhibitor along with Antioxidants is felt necessary to treat Alzheimer’s disease effectively. Therefore, it is worthwhile to identify new and selective AChE inhibitors with an Antioxidant nature from natural source. Hence, in the present investigation, the natural bioproduct of silk viz. the Silk Protein, Sericin, having several beneficial qualities viz., Anti-bacterial, Antioxidant, Wound healing, Anti-tumor activity etc. has been chosen to evaluate its potential as “Cognitive enhancer”, AD-induced rat model by conducting experiments on Morphometric changes along with Learning and Memory efficiency; on Cholinergic system, Antioxidant system and Histological aspects in selected brain regions of control and experimental rats. The results in the present study on the Cholinergic system revealed that while ACh content was significantly elevated, AChE level was inhibited in all selected brain regions of rats treated with As a corollary to these biochemical aspects, the Histological Studies in control and different groups of experimental rat brain demonstrated that Sericin could effectively reverse the AD-induced damage in neurons of CC and HC regions, the centres for most of the cognitive functions. From all these findings, it was finally concluded that, Sericin can be used as a potential cognitive enhancer in general and Alzheimer’s disease in particular.
Keywords
Alzheimer’s disease; Silk protein; Sericin; Morphometric and Behavioural aspects; Cholinergic and antioxidant systems; Histological aspects
Introduction
The most common human Neurodegenerative disease such as Huntington’s Disease, Parkinson’s Disease, Alzheimer’s Disease, Frontal temporal dementia, Creutzfeldt-Jakob disease, Spinocerebellar Ataxias, Epilepsy, Multiple Sclerosis and Neuroinfections etc., occur by progressive loss of a specific population of neurons in the brain, spinal cord or less frequently peripheral nerves or muscles, a genetic mutation in a specific neuronal protein that leads to a loss of function of that protein or slow accumulation of insoluble material in or around the cell. One constant factor in these Neurodegenerative diseases is that they are caused by aggregation of proteins, known as “Proteinopathies” and some protein residues can acquire toxic properties through a variety of ways, including irregular protein folding and degradation pathways, altered subcellular localization and abnormal interactions etc., while inherited Neurodegenerative diseases are caused by the expression of genes. Approximately 450 million people are suffering from them worldwide with a mortality rate of 14.7%. They are usually more common in older people aged above 60years. Alzheimer’s Disease (AD) is a neurodegenerative disorder characterized by formation of amyloid plaques and neurofibrillary tangles and by loss of neurons [1,2]. AD is the most common type of Dementia, also called as Senile Dementia of Alzheimer Type (SDAT) and frequently occurs in elder people. AD is a progressive and fatal brain disease. Globally it was noticed that every year 3,60,000 new cases were admitted with severe AD. The incidence of AD rises from 2.8% per every 1000 persons in the age group of 65- 69 to 56.1 % per every 1000 persons in people older than 90 years. AD was first described by a German Psychiatrist and Neuropathologist, Dr. Alois Alzheimer in 1906 and was named after him. The underlying neuronal histopathology consists of Neurofibrillary Tangles (NFT) comprised of hyperphosphorylated collections of the microtubuleassociated protein tau and Senile Plaques made up of aggregated Amyloid β-protein (Aβ) fibrils found in neocortex, hippocampus and in several subcortical areas, NFT density which correlates with disease duration and severity of dementia. The main causes of Alzheimer’s are include Age, Family history, Hereditary factors, Down’s syndrome, Whiplash or Head injuries, Aluminium, Poor education, Consumption of high fat and high calorific diet, Smoking, Cardiovascular disorders, Hyper cholesterolemia, Diabetes mellitus, Menopause and sedentary life style. The Biochemical basis of AD was explained by three different Hypotheses, they are: Cholinergic hypothesis, Amyloid hypothesis and Tau hypothesis. According to Cholinergic hypothesis, AD is caused by reduced synthesis of the neurotransmitter ACh, wherein the AChE levels were increased which causes damage to the cholinergic neurons finally leading to cognitive impairments. At present, AChE inhibitors, Anti-amyloid vaccine and Vitamin E are recommended to treat AD but long-term exposure to these drugs causes side effects. Since treatment of AD with single drug is not a realistic option due to it’s the complicated nature, combination therapy of AChE inhibitor along with Antioxidants is felt necessary to treat Alzheimer’s disease effectively.
The Silk cocoon produced from the silkworm, Bombyx morii consists of two major proteins, Fibroin and Sericin. Sericin contributes about 20-30% of total cocoon weight and remaining fibroin. There are 18 kinds of amino acids present in Sericin, among these 8 amino acids are essential for human, which plays a key role in different metabolic pathways. In Pharma industry, it has wide applications viz., Anti-bacterial, Antioxidant, Wound healing, Cell proliferation, Antitumor activity and UV protection. Sericin is a complete protein and the amino acids compositions are with appropriate proportions in line with FAO/WHO standards. A nutritional value of Sericin is 2 times higher than the pork and 3 times higher than the meat. Indian produce of ,600 tons of silk can be the source of about 250 to 300 tons of Sericin per year [3] which can be recovered and recycled to produce many value-added compounds. According to Cholinergic hypothesis, AD is caused by reduced synthesis of the neurotransmitter ACh, wherein the AChE levels were increased which causes damage to the cholinergic neurons finally leading to cognitive impairments. At present, AChE inhibitors, NMDA Receptor Antagonists, Memantine (Namenda), β-secretase (BACE) inhibitor, Anti-amyloid vaccine and Vitamin E are recommended to treat AD but long-term exposure to these drugs causes’ side effects. Hence, treatment of AD with single drug is probably not a realistic option due to the complicated nature of disease. Combination therapy of AChE inhibitor along with Antioxidants is felt necessary to treat Alzheimer’s disease effectively [4-6]. Therefore it is worthwhile to identify new and selective AChE inhibitors with an Antioxidant nature from natural source. Recent studies revealed that the Silk Protein, Sericin possesses the properties viz., Anti-bacterial, Antioxidant, Wound healing, Cell proliferation, Anti-tumor activity and UV protection [7]. Hence, the natural bioproduct from silk viz. the Silk Protein, Sericin has been thought of as a “Potential Neuroprotective Compound” against AD in a most accepted animal model, the Rat. Hence, in the present investigation, the natural by-product of silk viz. the Silk Protein, Sericin, having several beneficial qualities viz., Anti-bacterial, Antioxidant, Wound healing, Anti-tumor activity etc. has been chosen to evaluate its potential as “Cognitive enhancer”, AD-induced rat model by conducting experiments on Morphometric changes along with Learning and Memory efficiency; on Cholinergic system, Antioxidant system and Histological aspects in selected regions of control and experimental rat brain.
Materials and Methods
The present study was focused on the evaluation of the neuroprotective effect of Silk Protein, Sericin on Morphometricand Behavioural Aspects, Cholinergic system, Antioxidant system, Histological Aspects and Bioinformatics aspects.
Procurement and Maintenance of Experimental Animals
Healthy Wistar strain Albino rats, Rattus norvegicus of the same age group of 3 months, weighing 160 ± 20 grams, obtained from Sri Venkateswara enterprises, Bangalore were used as the experimental model in the present investigation. Prior to experimentation, the rats were acclimatized according to the instructions given by [8,9]. They were housed in polypropylene cages under the controlled conditions of 28 ± 2°C temperature with photoperiod of 12 hours light and 12 hours dark and 75% relative humidity maintained in the animal house of the Department Zoology, according to the ethical guidelines for animal protection and welfare bearing the Resolution No. 04/(i)/a/CPCSEA/ IAEC/ SVU/ KY- KPR / Dt. 28-03-2011. The rats were fed with standard pellet diet supplied by Sri Venkateswara Enterprises, Bangalore and water ad libitum throughout the period of experimentation.
Biological effect of D-Gal: Old Age can be induced by Intra peritoneal (IP) injection of D-Galactose, a reducing sugar, which reacts readily with the free amines of amino acids in proteins and peptides both in vivo and in vitro to form Advanced Glycation End-products (AGE) through non-enzymatic glycation [10,11]. The Advanced Glycation End-product activates its receptors, which are coupled to biochemical pathways that stimulate free radical production (Yan et al. [10]). D-Galactose is a physiological nutrient, but over supply of D-Galactose will result in abnormality of metabolism. The oxidative metabolism of D-Galactose produces Reactive Oxygen Species (ROS), which surpass the ability of the cells to eliminate them, consequently causing impairment of cellular membrane, structure and gene expression [12,13]. In addition, the non-enzymatic glycation is another pathway that can enhance oxidative lesions in ageing and age-associated disease such as Alzheimer’s disease [14]. Thus, long-term intraperitoneal injection of D-Galactose induces AD in normal rat [13,15].
Collection of silk cocoons and extraction of Sericin:
Extraction of Sericin
Raw Silk cocoons, purchased from local market of Chittoor were boiled in water for 1hour and the resulting solution was cooled and filtered. After repeating this process for 3 times, the filtrate was concentrated by using Hahnvapor Rotary Evaporator (HS-2005V). 95% ethanol was added to the extract to precipitate Sericin, collected by filtration, dried at 40oC, powdered and finally preserved in clean container for further use.8% SDS PAGE was done to confirm the presence of Sericin, based on the molecular weight.
Induction of Alzheimer’s Disease
D- Gal infection has been generally accepted to establish an aging model for brain aging or anti-aging pharmacological research [16,17]. In the present study Memory impairment was induced to rats by IP injection of D-Galactose (120mg/kg body weight) by dissolving in distilled water for one month [16,18-20].
Administration of tested substance: Silk Protein, Sericin (SP-P) extract (200 mg/kg body weight) was dissolved in distilled water and given to the rat. A gavage tube was used to deliver the substance by oral route, which is clinically expected route for administration. The volume of Sericin administered was kept at 1 ml to the animal.
Experimental Design:
• Animal model: Male Albino Rat
• Age: Three months old
• Weight of: 160 ± 20 grams
• Chemical agents used for
• Induction of AD in Rat: D-Galactose (D-Gal)
• Route of Administration: Intraperitoneal injection (IP)
• Test substance: Sericin
• Route of Administration: Oral
• Tissue selected: Brain
• Brain regions selected: Cerebral Cortex (CC) and Hippocampus (HC)
• Isolation of tissues for
• Biochemical estimation: 60th day and 90th day
Grouping of Animals: After the rats were acclimated to the laboratory conditions for 10 days before the experimentation, they were randomly divided into four groups. Each main group was again divided in to 2 sub-groups of six each and were housed in separate cages. These different groups of rats except control were treated with selected doses of Sericin and D-Gal as given below. Keeping in view the altered activity of rats during the nights compared to day time, all doses were given in (Table 1) once in the morning hours in between 8 A.M. to 9 A.M.
In the present study the experimental duration selected was 90 days. D-Gal was given for first 30 days period to observe AD symptoms with the assessment of cognitive skills in rats (AD group). Further AD induced rats were again treated with D-Gal as well as Sericin simultaneously. To observe the cognitive skills, the rats were subjected to behavioural studies on selected days (as per the experimental design) (Table 2).
Behavioural Aspects: Behavioural experiments were performed by using the water maze [23] which was originally designed to test the learning and memory ability in rodents. A great deal of knowledge has been obtained on the Neurochemical, Neuroanatomical and Neurophysiological basis for the behavior associated with this paradigm. The apparatus consisted of a circular tank, 100 cm in diameter and 50 cm in depth painted with non-toxic white paint. The tank was filled with water (21-26°C) up to a height of 30 cm and the transparent escape platform made of plexiglass measuring 10 cm in diameter and 29 cm in height was hidden 1.5 cm below the surface of water in a fixed location. Water was made opaque by mixing with powdered non-fat milk. The platform was not visible from just above the water level and transfer trials have indicated that escape on to the platform was not achieved by visual or other proximal cues [30]. The time spent by the animal to reach the hidden platform was called as the Escape Latency and used as the index of memory.
Isolation of tissues: For all biochemical estimations, the above mentioned four groups of rat were sacrificed on selected days i.e., on 60thdayand 90thdayby cervical dislocation. The brain was isolated immediately and placed on a chilled glass plate. Cerebral Cortex and Hippocampus were separated by following standard anatomical marks [31] and they were frozen in liquid nitrogen and stored at -80°C until further use. At the time of biochemical analysis, the tissues were thawed and used. The results obtained were analyzed statistically.
| Group-I | Control Rat |
|---|---|
| Group-II (SP-S) | Rat, orally administered with Sericin (200 mg/kg body weight) up to 60 days (31st day to 90th day) continuously once in a day. [21] |
| Group-III (AD) | Rat, Intraperitoneally (IP) administered with D-Gal (120 mg/kg body weight) up to end of the experiment (1st day to 90th day). [13,22] |
| Group-IV (AD+SP-S) | Rat, Intraperitoneally injected with D-Gal (120 mg/kg body weight) once daily for first 30 days. From 31stday onwards rats were administered with Sericin (orally; 200 mg/kg body weight) along with D-Gal (IP) up to 90th day. |
Table 1: Groupings of rats to view altered activity during nights.
| I | Behavioural Aspects | [23] |
|---|---|---|
| II | Â Cholinergic system Acetylcholine (ACh) Acetyl cholinesterase (AChE) | Metcalf method as given by [24,25] |
| III | Antioxidant system Superoxide dismutase (SOD) | Â [26] |
| Catalase (CAT) | [27] | |
| Glutathione Reductase(GR) | [28] | |
| Lipid peroxidation (MDA) | [29] | |
| IV | Histological Aspects | Light Microscopy |
Table 2: Standard methods employed for various parameters.
Biochemical Assays
Estimation of total proteins: The total protein content was estimated by the method of [32] 2% homogenates were prepared in 10 % TCA and centrifuged at 1000 xg for 15 minutes. The supernatant was discarded and the residue was dissolved in a known amount of 1N sodium hydroxide. From this, 0.2 ml was taken and 4 ml of alkaline copper reagent and 0.4 ml of folin phenol reagent (1:1 folin phenol and distilled water) was added. The contents were allowed to stand for 30 minutes at room temperature and the developed color was read at 600 nm in a spectrophotometer against a reagent blank. The amount of total proteins present in the sample was calculated by using bovine albumin standard and the values were expressed as mg/g wet weight of tissue.
Cholinergic system:
Acetylcholine (ACh)
Acetylcholine (ACh) content was estimated by the method of [33] as given by [24]. The rat brain regions such as Cerebral Cortex (CC) and Hippocampus (HC) were weighed accurately, transferred to test tubes and placed in a boiling water bath for 5 minutes to terminate the Acetyl cholinesterase enzyme activity and also to release the bound ACh. Then the tissues were homogenized in 1ml of distilled water. To the homogenate, 1 ml of alkaline hydroxylamine hydrochloride was added followed by 1 ml of 50% hydrochloric acid solution. The contents were mixed thoroughly and centrifuged. To the supernatant, 0.5 ml of 0.37 M ferric chloride solution was added and the brown colour developed was read at 540 nm against a reagent blank (1ml of alkaline hydroxylamine hydrochloride +1 ml of 50% hydrochloride + 1ml of distilled water + 0.5 ml of 0.37 M ferric chloride solution) in a spectrophotometer. The Acetylcholine content was expressed as μ moles of ACh/gm wet weight of tissue.
Acetyl cholinesterase (AChE) (E.C.: 3.1.1.7; Acetylcholine acetyl hydroxylase): Acetyl cholinesterase activity was estimated by the method of [25] 10% homogenates of different regions of rat brain were prepared in 0.25M ice cold sucrose solution. The reaction was started with the addition of 100 μ liters of homogenate to the reaction mixture containing 3.0ml of phosphate buffer (PH 8.0) + 20 μ moles of substrate (0.075M) + 100 μ moles of Dithiobis Trinitrobenzene (DTNB,0.01M). The contents were incubated at 37°C for 15 minutes. The developed colour was read at 412 nm in a spectrophotometer against a reagent blank containing 3.0ml of phosphate buffer (pH8.0) + 20 μ moles of substrate (0.075M) + 100 μ moles of Dithiobis Trinitrobenzene (DTNB,0.01M).The enzyme activity was expressed as μ moles of ACh hydrolyzed/mg protein/hour.
Antioxidant System
Superoxide dismutase : ( SOD EC: 1.15.1.6): Superoxide dismutase activity was determined according to the method of [26] at room temperature. Different areas of rat brain such as Cerebral Cortex (CC), and Hippocampus (HC) were homogenized in ice cold 50 mM phosphate buffer (pH 7.0) containing 0.1 mM EDTA to give 5% homogenate (w/v). The homogenates were centrifuged at 10,000 rpm for 10 mm at 4°C in ice cold centrifuge. The supernatant was separated and used for enzyme assay. 100 μl of tissue extract was added to 880 μl (0.05 M, PH 10.2, containing 0.1 mM EDTA) carbonate buffer and 20 μl of 30 mM epinephrine (in 0.05% acetic acid) was added to the mixture and measured the optical density values at 480 nm for 4 min in UV Spectrophotometer.SOD activity was expressed as the amount of enzyme that inhibits the oxidation of epinephrine by 50%, which is equal to 1 unit activity.
Catalase (CATEC 1.11.1.6): Catalase activity was measured by a slightly modified version of [27] at room temperature. Rat brain regions such as Cerebral Cortex (CC) and Hippocampus (HC)were homogenized in ice cold 50 mM phosphate buffer (pH 7.0) containing 0.1 mM EDTA to give 5% homogenate (w/v). The homogenates were centrifuged at 10,000 rpm for 10 min. at 4°C in ice cold centrifuge. The resulting supernatant was used as enzyme source. 10 μl of 100% ethyl alcohol was added to 100 μl of tissue extract and then placed in an ice bath for 30 min. After 30 minutes the tubes were kept at room temperature followed by the addition of 10 μl of Triton X-100 RS. In a cuvette containing 200 μl of phosphate buffer and 50 μl of tissue extract, 250 μl of 0.066 M H202 (in phosphate buffer) was added and optical density was measured at 240 nm for 60 s in a UV Spectrophotometer. The molar extinction coefficient of 43.6 M cm-1 was used to determine CAT activity. One unit activity is equal to the moles of H202 degraded / mg protein /min.
Glutathione Reductase (GR-EC: 1.6.4.2): Glutathione Reductase activity was determined by a slightly modified method of [28] at 37°C. Selected rat brain regions such as Cerebral Cortex (CC), and Hippocampus (HC)were homogenized (5%-w/v) in 50 mM phosphate buffer (pH 7.0) containing 0.1 mM EDTA. The homogenates were centrifuged at 10,000 rpm for at 4°C in ice cold centrifuge. The separated supernatant part was used as enzyme source. NADPH (50 μl, 2 mM) in 10 mM Tris buffer (pH 7.0) was added to the cuvette containing 50 μl of GSSG (20 mM) in phosphate buffer (0.5 M, pH 7.0 containing 0.1 mM EDTA) and 800 μl of phosphate buffer. The tissue extract (100 μl) was added to the NADPH-GSSG buffered solution and measured at 340 nm for 3 mm. The molar coefficient of 6.22 X 103 cm-1 was used to determine GR activity. One unit of activity is equal to the mM of NADPH oxidized I mg protein / mim. The enzyme activity was expressed in μ moles of NADPH oxidized / mg protein /min.
Lipid Peroxidation (LPO): The MDA levels were measured as described by [29]. Different areas of rat brain such as Cerebral Cortex (CC) and Hippocampus (HC) tissue were homogenized (5%- W/V) in 50 mM phosphate buffer (pH 7.0) containing 0.1 nM EDTA. The homogenates were centrifuged at 10,000 rpm for 10n min at 40C in cold centrifuge. The separated supernatant part was used for estimation. 200 μl of the tissue extract was added to 50 μl of 8.1% Sodium Dodecyle Sulphate (SDS), vortexed and incubated for 10 min at room temperature. 375 μl of twenty percent acetic acid (pH 3.5) and 375 μl of thiobarbituric acid (0.6%) were added and placed in a boiling water bath for 60 min. The samples were allowed cool at room temperature. A mixture of 1.25 ml of Butanol: Phyridine (15:1) was added vortexed and centrifuged at 1000 rpm for 5min. The colored layer (500 μl) was measured at 532 nm using ,,3,3-tetraethoxypropane as a standard. The values were expressed in μ moles of Malondialdehyde formed/gram wet weight of the tissue.
Histological Aspects
Histological examinations of the tissues Viz. Cerebral Cortex and Hippocampus were carried out according to [34].
Light Microscopy: On selected days of experimentation viz. 60th and 90th day, the rats were sacrificed by cervical dislocation and the selected brain regions such as CC and HC were isolated. Isolated tissue of control and experimental rats were gently rinsed with physiological saline (0.9% NaCl) to remove blood and debris adhering to the tissues. They were fixed in 5% formalin for 24 hrs. The fixative was removed by washing through running tap water overnight. After dehydrating through a graded series of alcohols, the tissues were cleared in methyl benzoate, embedded in paraffin wax. Sections were cut at 6μ thickness and stained with hematoxylin [35] and counter stained with eosin (dissolved in 95% alcohol). After dehydration and clearing, sections were mounted with DPX. The stained sections were observed under microscope and the histological changes were recorded with the help of a pathologist.
Statistical Analysis: Values of the measured in different parameters were expressed as Mean ± SEM. One way ANOVA was used to test the significance of difference among the four different groups with Dunnett’s post-hoc test for multiple comparisons using standard statistical software, SPSS (Version -16). The results were presented with the F-value and p-value. In all cases F-value was found to be significant with p-value less than 0.01. This indicates that the effects of factors are statistically significant.
Results and Discussion
Morphometric and Behavioural Aspects
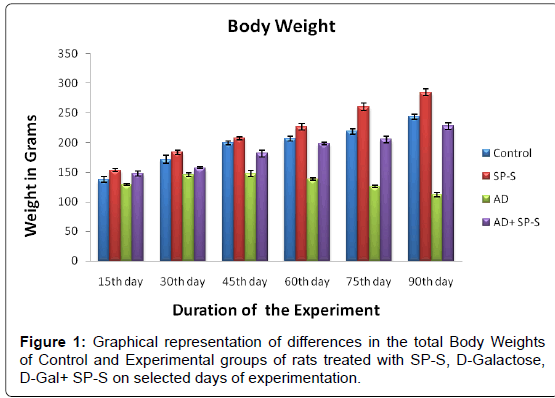
Morphometric Aspects: (Table 3 and Figure 1)
The total body weights of control and experimental rats were recorded at selected time intervals by using a digital balance. The control rats have registered a gradual gain in their body weights from 15th day to 90th day. However, in case of experimental rats treated with Sericin, the rats attained more body weights (16.67%) than the control. Contrary to this, AD-induced rats lost their body weights (54.00%) and become weak when compared to control while the AD-induced rats simultaneously treated with Sericin gained their body weights and reached to almost the control levels.
| Group | Days | |||||
|---|---|---|---|---|---|---|
| 15th day | 30th day | 45th day | 60th day | 75th day | 90th day | |
| Control | 138.00± 4.25 | 171.83± 6.74 | 193.83± 3.19 | 207.33± 4.40 | 219.33± 4.46 | 243.83 |
| SP-S | 153.50± 2.70 (-3.58) | 184.00± 3.43 (-7.08) | 207.83± 2.94 (-9.32) | 226.66± 5.21 (-10.86) |
260.33± 6.20 (-16.67) |
284.50 (-18.69) |
| AD | 129.50± 1.83 (6.15) | 145.83± 3.51 (15.13) | 148.16± 4.94 (25.85) | 138.83± 2.24 (33.03) | 125.83± 1.79 (42.62) | 112.16 (54.00) |
| AD+ SP-S | 148.16± 4.29 (-7.36) (-14.72)* |
157.50± 1.78 (8.33) (-8.00)* |
181.83± 5.51 (9.423) (-22.72)* | 199.16± 2.12 (3.94) (-44.31)* |
205.83± 5.48 (6.15) (-63.57)* |
228.00 (6.49) (-103.28)* |
Table 3: Differences in the total body weights (Grams) of Control and Experimental groups of rats treated with SP-S, D-Gal and D-Gal + SP-S on selected days ofexperimentation. Values are Mean ± SEM of six observations each from tissues pooled from 6 rats. Values in parentheses are percent changes from control (Except *). *Values in parentheses are percent changes from AD-induced rats. Values are significantly different from control at p < 0.01.
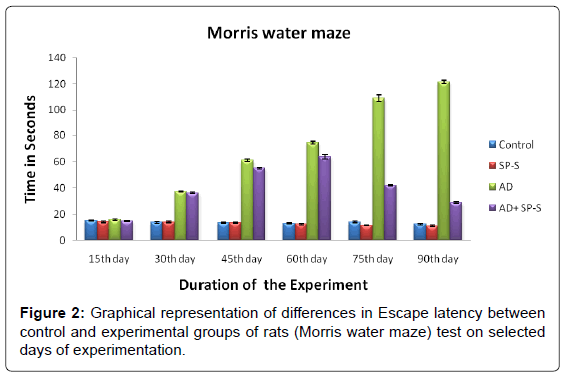
Behavioural Aspects: (Table 4 and Figure 2)
The control and experimental rats were subjected to Morris water maze task to measure cognitive skills viz., the spatial learning and memory ability. The results revealed that, Sericin treated rats have showed equal escape latency as that of the control one. In contrast to this, AD-induced rats showed a significant increase in the escape latency time from 15th day to 90th day with maximum latency on 90th day (872.00%). AD-induced rats treated with Sericin showed significant recovery tendency from 30th day (3.57%) to 90th day (76.13%) when compared to AD-induced rats thus demonstrating the positive effects of Sericin on total body weight and cognitive skills in AD induced rats.
| Group | Days | |||||
|---|---|---|---|---|---|---|
| 15th day | 30th day | 45th day | 60th day | 75th day | 90th day | |
| Control | 15.16 ± 0.31 | 13.66 ± 0.67 | 13.33 ± 0.33 | 13.00 ± 0.57 | 14.00 ± 0.57 | 12.50 |
| SP-S | 14.16 ± 0.60 (6.59) |
14.00 ± 0.58 (-2.48) |
13.50 ± 0.56 (-1.27) |
12.50 ± 0.67 (3.84) |
11.50 ± 0.22 (17.85) |
11.00 (12.00) |
| AD | 15.83 ± 0.60 (-4.41) |
37.50 ± 0.43 (-174.52) |
61.16 ± 0.87 (-358.81) |
74.66 ± 0.88 (-474.30) |
109.00 ± 2.28 (-678.57) |
121.50 (-872.00) |
| AD+ SP-S | 14.66 ± 0.33 (3.29) (7.39) * |
36.16 ± 0.65 (-164.71) (3.57)* |
55.16 ± 0.60 (-313.80) (9.81)* |
63.83 ± 1.53 (-391.00) (10.83)* |
42.00 ± 0.36 (-200.00) (61.46)* |
29.00 (-132.00) (76.13)* |
Table 4: Differences in the Escape latency (Seconds) between Control and Experimental groups of rats treated with SP-S, D-Gal and D-Gal + SP-S on selected days of experimentation. Values are Mean ± SEM of six observations each from tissues pooled from 6 rats. Values in parentheses are percent changes from control (Except *).*Values in parentheses are percent changes from ADinduced rats. Values are significantly different from control at p < 0.01.
The results of the present study clearly indicated that Sericin showed positive effects on body weight, cognitive skills, whereas AD-induced rats showed deficiency in learning and memory. Oral administration of Sericin significantly reversed the memory impairments in ADinduced rats, indicating that Sericin has the potential to enhance the cognitive skills. These observations derive strong support from many earlier similar reports by [36] who demonstrated that ethanol extract of Bacopa monniera has effectively reversed the D-Galactose induced learning and memory impairments. Similarly, [37] reported that alcoholic extract of Crocus sativus L reduced the learning impairments and memory deficit induced by D-Gal and NaNO2 in adult male mice. [38] who used the technique of prolonged systemic administration of Saffron extract (Crocins) to evaluate its prophylaxis and therapeutic effect on a model of chemically induced cognitive deficit, proved the saffron and its major constituents as cognitive enhancers on learning and memory consolidation processes [39]. Also have shown that aqueous Crocus sativus L. extract administration significantly inhibited learning and memory deficits in streptozotocin-induced dementia in passive avoidance test.
In rodents, spatial learning and memory are closely related to the function of the dorsal hippocampus [40] to which cholinergic neurotransmission contributes significantly [41]. Although especially prominent in AD, cholinergic deficits in the cortex and hippocampus occur during normal human ageing [42] and smaller numbers of neurons and atrophy of surviving cholinergic neurons in the basal forebrain were shown in aged animals with impaired learning and memory [43].
In support of the present study, [44] reported that the rats showed high levels of cognitive impairment when treated with D-Gal in passive avoidance and Morris water maze tests while silk peptides could increase the retention and retrieval of learned tasks in rats [44]. However, further investigations are needed to confirm Sericin as a cognitive enhancer. A recent study on forced swimming stress in diabetes-induced rat model has shown that consumption of silkworms and Silk Proteins improved lipid metabolism, thus demonstrating the protective effects of SP against tissue injury [45,46]. Especially, Silk Amino Acids (SAA) exerted neuroprotective effects on 6-hydroxydopamine-induced dopaminergic neurotoxicity and thereby improved movement functions of PDinduced animals [47].
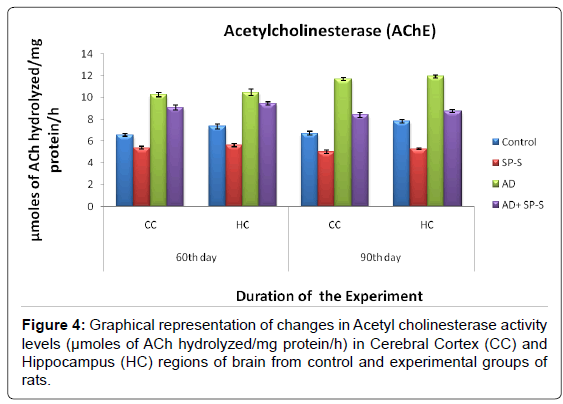
Acetylcholine Content (ACh) and Acetyl cholinesterase (AChE):
The results in the present study on the Cholinergic system revealed that ACh content was significantly elevated in both selected brain regions of rats treated with Sericin alone when compared with ADinduced and Control groups. Maximum increment (26.89%) in ACh content was found in HC region on 90th day. However, in case of ADinduced rats, simultaneously treated with Sericin, the ACh content almost reached the normal control levels. Contrary to Ach content, the AChE activity levels were significantly inhibited in CC and HC brain regions of Sericin alone treated rats. Whereas the AChE activity levels were increased significantly in selected AD-induced rat brain regions with a maximum elevation in CC region on 90th day (73.91%). ADinduced rats treated with Sericin showed significant inhibition in the AChE activity levels (maximum inhibition was in HC (11.89%) on 90th day) and reached to the control levels.
From the above findings, it was obvious that Sericin exerted Anti- Cholinesterase properties in AD-induced rats by elevating the levels of ACh and inhibiting the AChE activity in selected regions of brain. AChE is an important regulatory enzyme that controls the transmission of nerve impulses across cholinergic synapses by hydrolyzing the excitatory transmitter, ACh [48]. It was assumed that cholinergic deficit in AD was responsible for much of the short-term memory deficit [49] since Acetylcholine (ACh) is required for short-term memory. Markers for cholinergic neurons such as choline acetyltransferase and Acetyl cholinesterase, which are enzymes responsible for synthesis and degradation of ACh, respectively are decreased in the cortex and hippocampus, areas of the brain involved in cognition and memory [49]. The earliest loss of neurons in AD patients occurs in the nucleus basal is and the entorhinal cortex, where cholinergic neurons are preferentially affected [50] postulates that many of the cognitive, functional and behavioural symptoms experienced by patients with AD results from a deficiency in neurotransmitter, ACh.
Research findings on several animal models have demonstrated that loss of cholinergic function in these areas is associated with a decline in learning capacity and memory. The resultant decrease in ACh-dependent neurotransmission is thought to lead to the functional deficits of Alzheimer’s disease. Interest on the function of the basal forebrain cholinergic system greatly increased with the neuropathological demonstration that cholinergic markers in the hippocampus and cerebral cortex are changed in AD [5,52] and that these changes have been linked to its pathology and degree of cognitive impairment [51]. Therefore, much of research on cognitive decline has been focused on the cholinergic system [52].
Mouse aging models induced by Aluminium trichloride and D-Galactose have been widely used for studying mechanism of Alzheimer’s disease and for screening the drugs for many years [10,54-56]. Although aluminium trichloride and D-Galactose mouse models function their own pathways, they eventually cause similar pathological changes in mouse brains and lead to enhance amyloid-β peptide (Aβ) deposition [56,57]. Accumulation and deposition of Aβ (neuritic plaques) in the brain are the key pathological features of AD [58- 60]. Previous studies showed that D-Gal administration significantly accelerates inflammatory brain injury through activation and degeneration of astrocytes and causes cognitive deficits by activating AChE [16,56,61]. D-Gal administration (through IP and SC) has been gradually accepted to establish an aging model for brain aging or antiaging pharmacological research to induce AD [16,17]. Accordingly, in the present study, D-Gal was used as a model compound for facilitated AD animal model. AD therapy is largely based on compounds to increase ACh concentration, including AChE inhibition [62,63].
In the present study, D-Gal treated rats were used as AD model for experiment. Chronic IP injection of D-Gal to the rat induced a significant decrease of ACh content and increase of AChE activity in CC and HC regions of brain, indicating that there was oxidative damage in the brain. On the other hand treating with Sericin has significantly elevated the ACh levels and lowered the levels of AChE activity in AD-induced animals indicating the counteracting action of Sericin on cholinergic system. These observations demonstrated that Silk proteins (SP) and Silk Peptides exerted a moderate inhibitory activity on AChE levels with the elevation of ACh content in AD induced rats. In support of the present study, [44] reported that silk peptides (SP) acts as cognitive enhancers in aging rat model and also stated that the D-Gal (150mg/Kg) activated hippocampal astrocytes upto 1.7 folds (a marker of Brain injury and aging) and decreased AChE concentration upto 45-50%. Oral treatment with SP preparations showed recovery in the concentration levels of ACh. In the present study, sericin treatment might be evidence that the Silk peptide can improve damaged brain function, especially by suppressing the AChE activity levels and thus preventing the brain damage by inhibiting neuronal apoptosis.
| Name of the Group |
60th day | 90th day | ||
|---|---|---|---|---|
| CC | HC | CC | HC | |
| Control | 32.08 ±0.24 | 32.77 ±0.34 | 33.87 ±0.21 | 34.09 ±0.29 |
| SP-S Treated | 36.05 ±0.42 (-12.37) |
37.68 ±0.58 (-14.98) |
41.74 ±0.37 (-23.23) |
43.26 ±0.53 (-26.89) |
| AD-induced | 26.09 ±0.24 (18.67) |
24.56 ±0.68 (25.05) |
22.82 ±0.22 (32.62) |
20.43 ±0.41 (40.07) |
| AD+ SP-S Treated | 28.29 ±0.34 (11.81) (-8.43)* |
29.81 ±0.31 (9.03) (-10.68)* |
30.32 ±0.37 (10.48) (-32.86)* |
31.00 ±0.27 (9.06) (-51.73)* |
Table 5: Changes in ACh content of selected regions of Control and Experimental groups of rats treated with SP-S and D-Gal (AD-induced) separately and combined dose of D-Gal +SP-S on selected days of experimentation. Values are Mean ± SEM of six observations each from tissues pooled from 6 rats. Values in parentheses are percent changes from control (Except *). *Values in parentheses are percent changes from AD induced rats Values are significantly different from control at p < 0.01.
| Groups | 60th day | 90th day | ||
|---|---|---|---|---|
| CC | HC | CC | HC | |
| Control | 6.56 ± 0.14 | 7.35 ± 0.22 | 6.71 ± 0.16 | 7.82 ± 0.15 |
| SP-S | 5.39 ± 0.13 (17.83) |
5.61 ± 0.15 (23.67) |
5.01 ± 0.17 (25.33) |
5.29 ± 0.10 (32.35) |
| AD | 10.25 ± 0.19 (-56.25) |
10.46 ± 0.29 (-42.31) |
11.67 ± 0.16 (-73.91) |
11.91 ± 0.14 (-52.30) |
| AD+ SP-S | 9.07 ± 0.21 (-38.26) (11.51)* |
9.42 ± 0.15 (-28.16) (9.94)* |
8.40 ± 0.22 (-25.18) (28.02)* |
8.75 ± 0.13 (-11.89) (26.53)* |
Table 6: Changes in AChE activity of selected regions of Control and Experimental groups of rats treated with SP-S and D-Gal (AD induced) separately and combined dose of D-Gal + SP-S on selected days of experimentation. Values are Mean ± SEM of six observations each from tissues pooled from 6 rats. Values in parentheses are percent changes from control (Except *). *Values in parentheses are percent changes from AD induced rats. Values are significantly different from control at p < 0.01.
The results obtained in the present study were strongly supported by similar observations of [64] and [36] who have reported that the ethanol extract of Bacopa monniera (55-60% concentration of Bacosides) exerted anti-cholinesterase and anti-dementia properties in albino mice. Similar effects of standardized extract of Bacopa monniera were observed in scopolamine-induced dementia in mice which demonstrated a dose-dependent inhibitory effect on Acetylcholinesterase activity [64].
In the present study, in AD induced rats, D-Gal reduced the cholinergic neuronal activity in the hippocampus. It is evidenced by the depletion of cholinergic neurotransmitter (ACh) and decreased muscarinic cholinergic receptor binding in the frontal cortex and hippocampus in rats [66]. Alzheimer’s disease induced rats treated with Sericin showed significant increases the memory as well as reversal of AD-induced reductions of Acetylcholine in the frontal Cortex and Hippocampus regions. The activity of choline acetyltransferase and muscarinic receptor binding of Acetylcholine were also improved [44].
In general, the present investigation on cholinergic system in different regions of rat brain following the oral administration of sericin to AD-induced rats has shown the neuroprotective effect on cholinergic system by increasing the levels of ACh content and by inhibiting the AChE activity levels.
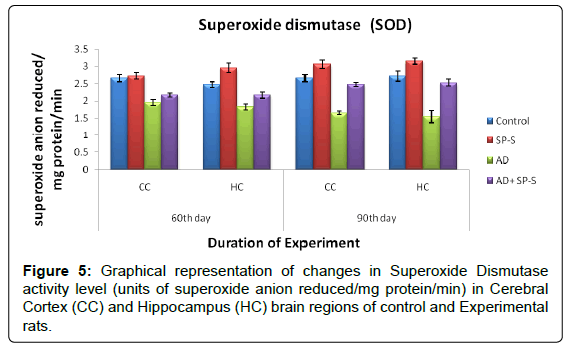
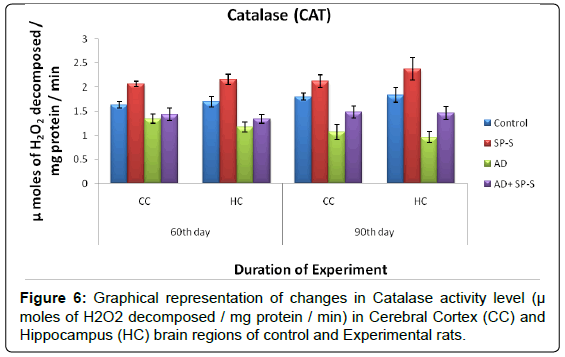
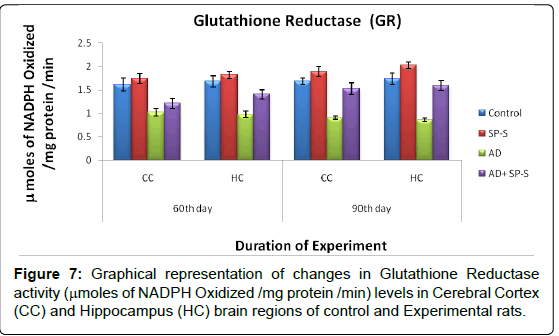
Antioxidant system: (Tables 7-11 and Figure- 5-8)
From the results presented in the table given below, it was observed that in rats treated with Sericin alone, all enzymes of the antioxidant system viz. Superoxide Dismutase, Catalase and Glutathione Reductase have registered maximum elevation in both selected brain regions viz., CC and HC on 90thday, on comparison to control. Conversely, AD induced rats brain regions showed significant reduction in all enzymes activity levels. However, progressive elevation of all antioxidant enzyme levels were observed in AD-induced rats treated with Sericin.
| Name of the Antioxidant Enzyme | Sericin treated Rat | AD- Induced Rat | AD-Induced Rat treated with Sericin | |||
|---|---|---|---|---|---|---|
| Brain Regions | Brain Regions | Brain Regions | ||||
| CC | HC | CC | HC | CC | HC | |
| Superoxide Dismutase | 13.75% | 16.23% | 38.66% | 42.80% | 49.69% | 62.58% |
| Catalase | 17.22% | 29.50% | 40.55% | 47.54% | 38.31% | 52.08% |
| Glutathione Reductase | 11.90% | 16.76% | 46.42% | 50.28% | 68.88% | 84.88% |
Table 7: Changes in SOD activity in selected brain regions of Control and Experimental groups of rats treated with SP-S and D-Gal (AD induced) separately and combined dose of D-Gal + SP-S on selected days of experimentation.
| Name of  the Group | 60th day | 90th day | ||
|---|---|---|---|---|
| CC | HC | CC | HC | |
| Control | 2.66 ± 0.11 | 2.47 ± 0.09 | 2.66 ± 0.10 | 2.71 ± 0.14 |
| SP-S | 2.72 ± 0.09 (-2.25) |
2.95 ± 0.14 (-19.43) |
3.06 ± 0.12 (-15.03) |
3.15 ± 0.09 (-16.23) |
| AD | 1.95 ± 0.1 (26.69) |
1.82 ± 0.08 (26.31) |
1.65 ± 0.04 (37.96) |
1.55 ± 0.17 (42.80) |
| AD+ SP-S | 2.16 ± 0.06 (18.79) (-10.76)* |
2.19 ± 0.09 (12.55) (-15.87)* |
2.47 ± 0.06 (7.14) (-49.69)* |
2.52 ± 0.09 (7.01) (-62.58)* |
Table 8: Values are Mean ± SEM of six observations each from tissues pooled from 6 rats. Values in parentheses are percent changes from control (Except *). *Values in parentheses are percent changes from AD induced rats. Values are significantly different from control at p < 0.01.
| Name of  the Group |
60th day | 90th day | ||
|---|---|---|---|---|
| CC | HC | CC | HC | |
| Control | 1.63 ± 0.07 | 1.70 ± 0.11 | 1.80 ± 0.74 | 1.83 ± 0.14 |
| SP-S | 2.06 ± 0.55 (-26.38) |
2.15 ± 0.10 (-26.47) |
2.11 ± 0.13 (-17.22) |
2.37 ± 0.23 (-29.50) |
| AD | 1.34 ± 0.09 (17.79) |
1.17 ± 0.10 (31.17) |
1.07 ± 0.15 (40.55) |
0.96 ± 0.12 (47.54) |
| AD+ SP-S | 1.43 ± 0.13 (12.26) (-6.71)* |
1.34 ± 0.09 (21.17) (-14.52)* |
1.48 ± 0.12 (17.77) (-38.31)* |
1.46 ± 0.14 (20.21) (-52.08)* |
Table 9: Changes in CAT activity on selected brain regions of Control and Experimental groups of rats treated with SP-S and D-Gal (AD induced) separately and combined dose of D-Gal + SP-S on selected days of experimentation. Values are Mean ± SEM of six observations each from tissues pooled from 6 rats. Values in parentheses are percent changes from control (Except *). *Values in parenthese are percent changes from AD induced rats. Values are significantly different from control at p < 0.01.
| Name of  the Group |
60th day | 90th day | ||
|---|---|---|---|---|
| CC | HC | CC | HC | |
| Control | 1.62 ± 0.13 | 1.68 ± 0.11 | 1.68 ± 0.06 | 1.73 ± 0.11 |
| SP-S | 1.74 ± 0.10 (-7.40) |
1.81 ± 0.07 (-7.73) |
1.88 ± 0.09 (-11.90) |
2.02 ± 0.07 (-16.76) |
| AD | 1.02 ± 0.08 (37.03) |
0.97 ± 0.06 (42.26) |
0.90 ± 0.03 (46.42) |
0.86 ± 0.03 (50.28) |
| AD+ SP-S | 1.21 ± 0.10 (6.40) (-18.62)* |
1.41 ± 0.10 (16.07) (45.36)* |
1.52 ± 0.12 (9.52) (-68.88)* |
1.59 ± 0.10 (8.09) (-84.88)* |
Table 10: Changes in GR activity on selected brain regions of Control and Experimental groups of rats treated with SP-S and D-Gal (AD induced) separately and combined dose of D-Gal + SP-S on selected days of experimentation. Values are Mean ± SEM of six observations each from tissues pooled from 6 rats. Values in parentheses are percent changes from control (Except *). *Values in parentheses are percent changes from AD induced rats. Values are significantly different from control at p < 0.01.
| Name of the Groups |
60th day | 90th day | ||
|---|---|---|---|---|
| CC | HC | CC | HC | |
| Control | 4.45 ± 0.16 | 4.81 ± 0.14 | 5.35 ± 0.23 | 5.56 ± 0.12 |
| SP-S | 3.94 ± 0.10 (11.46) |
3.87 ± 0.09 (19.54) |
3.36 ± 0.17 (37.19) |
3.21 ± 0.06 (42.26) |
| AD | 6.63 ± 0.14 (-48.98) |
6.77 ± 0.20 (-40.74) |
7.17 ± 0.13 (-34.01) |
7.25 ± 0.19 (-30.39) |
| AD+ SP-S | 5.86 ± 0.10 (-31.68) (11.61)* |
5.78 ± 0.10 (-20.16) (14.62)* |
5.81 ± 0.15 (-8.59) (18.96)* |
5.68 ± 0.15 (-2.15) (21.65)* |
Table 11: Changes in LPO levels on selected brain regions of Control and vExperimental groups of rats treated with SP-S and D-Gal (AD induced) separately and combined dose of D-Gal + SP-S on selected days of experimentation. Values are Mean ± SEM of six observations each from tissues pooled from 6 rats.Values in parentheses are percent changes from control (Except *). *Values in parentheses are percent changes from AD induced rats. Values are significantly different from control at p < 0.010.
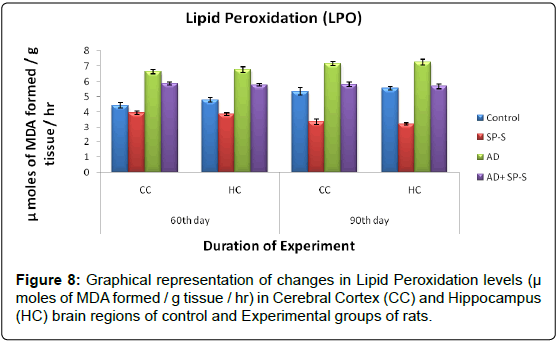
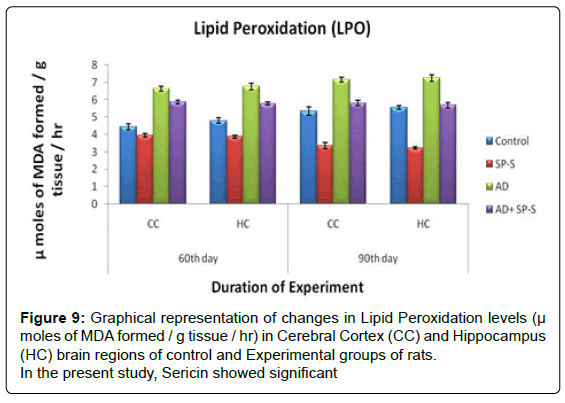
Lipid Peroxidation: (Table 12 and Figure 9)
In control rats, the LPO levels were relatively increased from CC (5.35 μ moles of MDA formed/g tissue/hr) to HC (5.56 μ moles of MDA formed/g tissue/hr) on 90th day of experimentation. When compared to control rats, LPO levels were significantly inhibited in CC and HC brain region of Sericin alone treated rats and maximum inhibition was noticed in HC region (42.26%) on 90th day.
In AD-induced rats, the increase in LPO levels was observed on 90th day in CC (34.01%) than in HC (30.39%) when compared to control. When the AD induced rats treated with sericin showed a significant inhibition in the LPO levels in HC (21.65%) than CC (18.96%) with compared to AD induced rats as follows.
| Name of the Group | 60th day | 90th day | ||
|---|---|---|---|---|
| CC | HC | CC | HC | |
| Control | 4.45 | 4.81 | 5.35 | 5.56 |
| Sericin treated Rat | Â 11.46 | Â 19.54 | Â 37.19 | Â 42.26 |
| AD- Induced Rat | Â -48.98 | -40.74 | Â -34.01 | -30.39 |
| AD-Induced Rat treated with Sericin | Â -31.68 11.61* |
 -20.16 14.62* |
 -8.59 18.96* |
 -2.15 21.65* |
Table 12: Table shows Percent changes of LPO levels. The values are percent changes from control (Except *). * The values are percent changes from AD induced rats.
In the present study, Sericin showed significant elevation in the activity levels of SOD, CAT, GR and with reduced LPO content in both CC and HC of rat brain. Silk cocoons have 2 major proteins, Sericin and fibroin [66] which is well known to have pharmacological activities including antioxidant effect [67-69]. Hydrolysis of silk proteins leads to different sizes of peptide, while enzymatic degradation results in specific sizes or compositions of silk peptides exerting diverse bioactivities including anti-diabetic, hypocholesterolemic and antioxidative actions [69-71]. Furthermore, Silk Amino Acids (SAA) have enhanced physical stamina by preventing tissues from oxidative injures [45,46]. Interestingly, it was reported that silk proteins inhibited Mono Amine Oxidase-B (MAO-B), a dopamine-degrading enzyme in Parkinson disease [72,73,47]. Especially, Brain Factor-7 (BF-7), a peptide obtained by enzymatic degradation of silk proteins, was found to increase cognitive function in both animal and human [74,75]. Fibroin, the water-insoluble protein has been recognized as a substrate for growth and adherence of cells in culture [76-81]. Sericin is the water-soluble component of silk and can be used as a biomaterial due to its antibacterial, antioxidant and UV resistant properties [82]. Sericin is also reported to suppress in vitro lipid peroxidation [83] and possesses antitumor properties [70] with no immunogenicity [13].
The above observations revealed that, the AD induced rats showed down regulation of SOD, CAT, GR activities with increased LPO levels. The AD-induced rats simultaneously treated with Sericin showed significant elevation in the activity levels of SOD, CAT, GR and with reduced LPO content in both CC and HC of rat brain. It might suggest that, Sericin has Antioxidant properties.
In the present study, the D-Gal was used to induce AD to experimental rat model is a nutrient derived from lactose which is hydrolyzed to monosaccharides viz., glucose and galactose. However, excessive D-Gal undergoes abnormal conversion to galactitol, causing osmotic stress and generation of ROS [84]. Though D-Gal is a normal substance in the body, at high levels, it can be turned into aldose and hydrogen peroxide under the catalysis of galactose oxidase, leading to the formation of a superoxide anion and Oxygen-derived Free Radicals (OFR) [85,86]. D-Gal, a reduced form of sugar, reacts with free amines of amino acids in proteins and peptides to form Advanced Glycation End Products (AGE) which in turn cause activation of their own receptors and facilitation of oxidative damage linked to the pathogenesis of degenerative diseases [10]. Especially, D-Gal makes cellular constituents vulnerable to oxidative reaction by decreasing various antioxidant components, enzymes, and reduces mitochondrial energy production [61]. The brain is susceptible to free-radical damage due to its comparatively high levels of oxygen metabolism and also relatively deficient in both free-radical scavenging enzymes and antioxidant molecules as compared with other organs. All these research reports strongly support the observations of the present study wherein the level of LPO was high in AD-induced rats brain regions such as CC and HC at all time periods due to the increased generation of ROS in the brain [44] also reported that D-Gal promotes the ROS generation in the brain which in turn causes LPO with decrement in the activity levels of enzymatic antioxidants like SOD, CAT and GR. In the present study the D-Gal-treated rats showed significant inhibition of SOD, CAT and GR which might be due to excess generation of ROS in the brain of AD-induced rats [87] reported that D-Gal induces cognitive impairments in rat with excessive formation of ROS. It might be due to the antioxidant property of sericin, which elucidates the action of free radical scavenging in the brain. My observations derive strong support from similar findings by [88] who also reported that Sericin enhanced the cell viability against H2O2-induced oxidative damage in feline skin fibroblasts. The protective effect of sericin may be due to its unique antioxidant potential. Consequently, exogenous antioxidants that scavenge ROS and restore normal redox state are supposed to be beneficial [89]. The mechanism of damage by hydrogen peroxide in fibroblast cultures involves ROS generation [90]. The level of ROS in cultures pre-incubated with Sericin was significantly decreased as indicated by cell viability tests. These studies proved that Sericin from mulberry and non-mulberry effectively suppressed H2O2-induced oxidative stress which reversed the activity levels of LPO and Catalase.
In general free radicals/ROS were nullified by enzymatic activity of antioxidants but the D-Gal suppressed the activity levels of antioxidants and causes the oxidation of Poly Unsaturated Fatty Acid (PUFA) in the cell membrane which leads to cell damage [91-93]. Malondialdehyde (MDA) alters the structure and function of the cellular membrane and blocks cellular metabolism leading to cytotoxicity [94].
SOD and CAT are the two critical antioxidant enzymes working in a co-operative way and are necessary for elimination of ROS, which in turn facilitates the survival of neurons. SOD dissimulates superoxide anion and generates H2O2 which can cleanup by the activity of CAT [95]. Catalase is involved in the decomposition of hydrogen peroxide to water and oxygen and is therefore important in protecting cells against oxidative stress [96].
Earlier in vitro studies on hydrogen peroxide-treated cells reported that the LPO levels were significantly inhibited with the treatment of Sericin through promotion of choline-uptake [62,97,98]. Furthermore, enhancers of ChAT mRNA expression recovered impaired learning and memory function [99-101].
From all the above findings, it was concluded that Sericin significantly reduced ROS generation which in turn increased the activity levels of enzymatic antioxidants such as SOD, CAT and GR thereby decreasing LPO. Thus, Sericin improves the cognitive functions and expresses the antioxidant properties. Further, it might be suggested that natural Silk Protein, Sericin may be a potential biological antioxidant and neuroprotective compound to scavenge free radicals as well as to improve the cognitive functions in Alzheimer’s Disease.
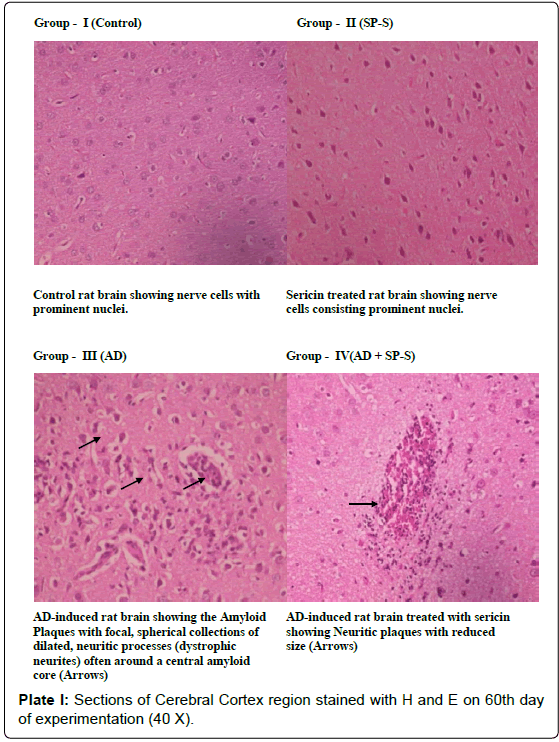
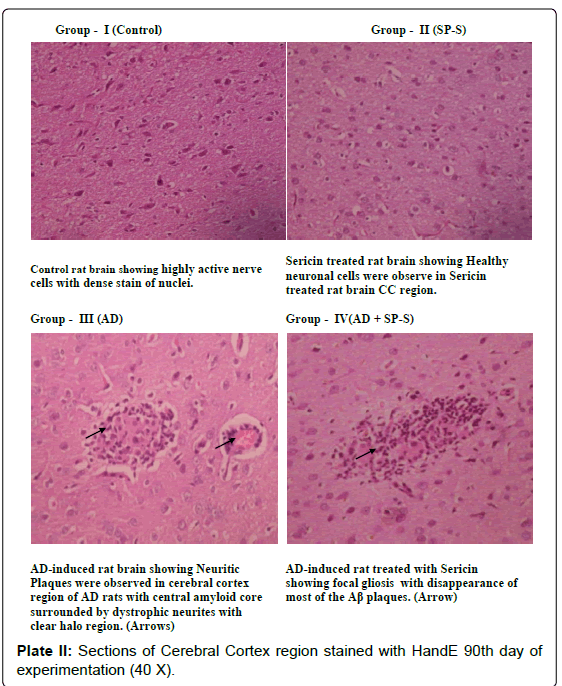
Histological Aspects: (Plate: 1- 4)
In the present study, histological examination of control as well as sericin treated rat brain region (CC) showed very prominent nerve cells in the cortex area with large nuclei. The surrounding support cells have small nuclei with densely stained, condensed chromatin. In contrast to this, the microscopic observation of the section of AD-induced rat showed dark neurons with corkscrew dendrites and besides this, many other neurons of the cortex area appeared in a degenerative form. On 90th day of experimentation, the Cerebral Cortex region showed Neuritic plaques with focal, spherical collections of dilated, tortuous, dystrophic neurites often around a central amyloid core, which may be surrounded by clear halo. Neuritic plaques ranged in size from 20 to 200 μm in diameter, Microglial cells and astrocytes were present at their periphery. In the histological sections of AD-induced rat brain region (CC), simultaneously treated with Sericin, the number and size of amyloid plaques was reduced with focal gliosis. With the increased period of Sericin treatment, the predominant neurodegenerative characteristics have gradually disappeared in AD-induced rats.
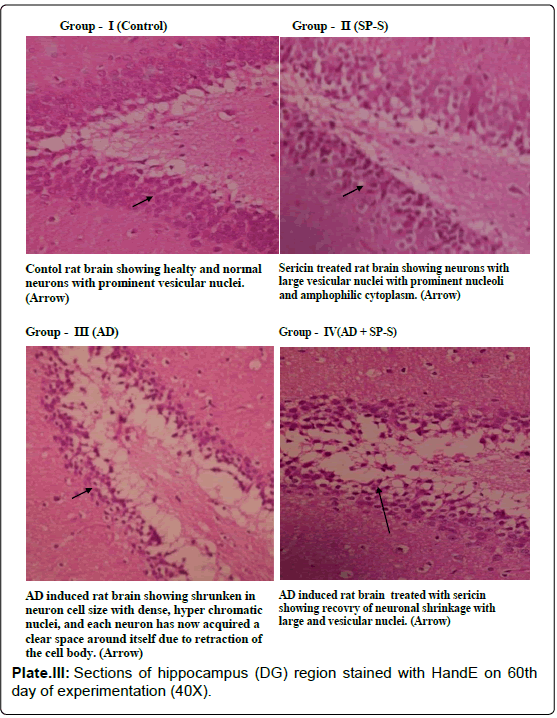
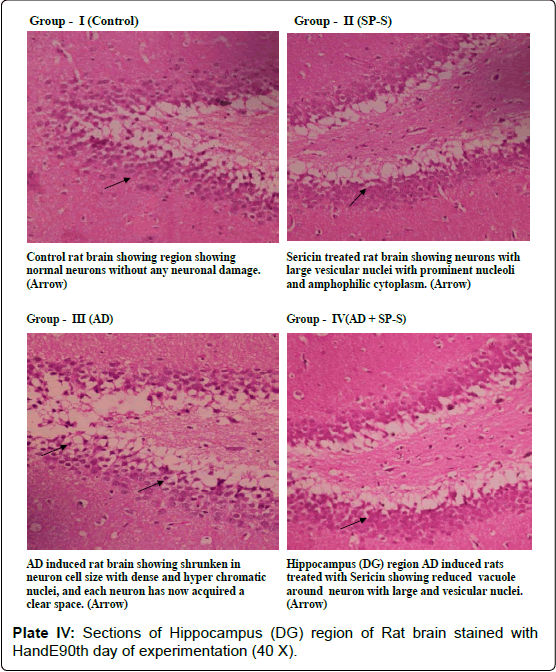
Microphotograph of control and Sericin treated rat hippocampus region showed the characteristic curvature of hippocampus. Cornu Ammonis layers (CA1 and CA3) were separated by compact glial cells. Between the nerve cells, a thick neuro fibrillary network (NFN) known a Neuro Pile (NP) is present. All the neurons have large round vesicular nuclei with prominent nucleoli and amphophilic cytoplasm. AD-induced Hippocampal region (DG) of rat brain showed neuronal shrinkage with dense and hyper chromatic nuclei, each neuron has acquired a clear space around itself due to retraction of the cell body. However, the hippocampal region of AD-induced rat treated with Sericin showed a reduction in the volume of the vacuole around the neuron. These AD-induced cytological changes in selected brain regions were reduced after prolonged treatment with Sericin.
Alzheimer’s disease (AD), a progressive neurodegenerative disorder that affects a large proportion of the elderly population, is characterised by the presence of many Amyloid Plaques in the hippocampus, amygdala and neocortex, although there is usually relative sparing of primary motor and sensory cortices (this also applies to Neurofibrillary Tangles). The amyloid core contained several abnormal proteins. The dominant component of the amyloid plaque core is Aβ, a peptide derived through specific processing events from a larger molecule, Amyloid Precursor Protein (APP). Two dominant species of Aβ, called Aβ40 and Aβ42, share an N-terminus and differ in length by two amino acids at the C-terminus. Other proteins in plaques were less abundance, including components of the complement cascade, pro-inflammatory cytokines, α1–antichymotrypsin, and apolipoprotein. In some cases, there was the deposition of Aβ peptides with staining characteristics of amyloid in the absence of the surrounding neuritic reaction.
In the present study, rats treated with D-Gal showed significant neuroanatomical alterations which were always regarded as adverse and were more commonly observable signs of AD. The neuro histopathological observations and the literature cited above clearly illustrated the neurodegenerative potentiality of D-Gal. Histopathological changes in AD-induced rats brain regions were evidenced by the lesions, termed as diffuse plaques in superficial portions of cerebral cortex as well as in hippocampus. Appearance of diffuse plaques represented early stages of plaque development. It might be supported based on the studies of brain from individuals with trisomy 21. In this context, it may be recalled that in patients with trisomy 21 (Down syndrome), early onset of Alzheimer disease is common [16,6,102] reported that the transgenic mice presenting Amyloid Plaques (AP) are widely studied to improve our understanding of the pathophysiology of AD but also to investigate new therapeutics. In animal models of AD, i.e., β-amyloid protein-expressing transgenic and AF64A-injected mice, 25-30% decrease in brain ACh resulted in severe impairment of learning and memory function [103,97]. During aging, brain atrophy and malfunction of cholinergic nervous system occur, leading to cognitive deficit.
In the present study the senile plaques were observed in the AD induced rat brain but Neurofibrillary tangle formation was not observed. These findings are in consonance with the previous studies of [104] and [105] who demonstrated a significant correlation between the number of senile plaques and the degree of deficits in AD brains. In rodent models of AD, Neurofibrillary tangle formation was generally not observed in brain regions, it does; however possess similar neurotoxic and amyloidogenic activity as the complete Aβ peptide, thereby making it biologically relevant to the etiology of AD. In brain regions such as cerebral cortex and hippocampus, diffused plaques represented a major manifestation of the Alzheimer’s disease. From the histological investigation of the present study, it was obvious that the Sericintreated AD-induced rat brain regions showed significant reduction in the formation of β-Amyloid Plaques with decreased vacuole size around the neuron and deactivated astrocytes. These findings were in accordance with the earlier reports of [44,47].
Thus, the results of the present study clearly indicated that Sericin showed positive effects on body weight, cognitive skills such as memory and learning, whereas D-Gal caused learning and memory deficits in rats. Oral administration of Sericin significantly reversed the memory impairments in AD-induced rat, indicating neuroprotective properties of Sericin. Based on these findings supported by the previous evidences, Silk Protein and its derivatives such as Sericin and Fibroin and Silk peptides can be thought of as potential compounds for treatment of cognitive disorders such as Alzheimer’s disease, which of course need further investigations.
Acknowledgements
The author thanks the Head, Department of Zoology for providing the necessary Lab facilities to carry out the research work.
References
- Srinivas P (1999) Diagnosis and management of Alzheimer's disease--an update. Med J Malaysia 54: 541-549.
- Parihar MS, Hemnani T (2004) Alzheimer's disease pathogenesis and therapeutic interventions. J ClinNeurosci 11: 456-467.
- Gulrajani ML (2005) Sericin: A bio-molecule of value, Souveni 20th Congress of the international sericultural commission. Bangalore, India 15-18th Dec-2005. 21-29.
- Giacobini E (2000) Cholinesterase inhibitors stabilize Alzheimer disease. Neurochem Res 25: 1185-1190.
- Kem WR (2000) The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer's disease: studies with DMXBA (GTS-21). Behav Brain Res 113: 169-181.
- Korczyn AD (2000) Muscarinic M(1) agonists in the treatment of Alzheimer's disease. Expert OpinInvestig Drugs 9: 2259-2267.
- Teramoto H, Kameda T, Tamada Y (2008) Preparation of gel film from Bombyxmori silk sericin and its characterization as a wound dressing. BiosciBiotechnolBiochem 72: 3189-3196.
- Behringer MP (1973) Laboratory care of vertebrates, In: Techniques and materials in biology. McGraw Hill. Inc., New York. 171-174.
- Behringer MP (1973) Techniques and materials in biology. McGraw Hill, Inc. New York. 120-132.
- Song X, Bao M, Li D, Li YM (1999) Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev 108: 239-251.
- Bassi AM, Ledda S, Valentini S, De Pascale MC, Rossi S, et al. (2002) Damaging effects of advanced glycation end-products in the murine macrophage cell line J774A.1. ToxicolIn Vitro 16: 339-347.
- Wang Z (1999) Physiologic and biochemical changes of mimetic aging induced by D-Galactose in rats. Lab AnimSci Admin 16: 23-25.
- Zhang D, Liu G, Shi J, Zhang J (2006) Coeloglossumviride var. bracteatum extract attenuates D-galactose and NaNO2 induced memory impairment in mice. J Ethnopharmacol 104: 250-256.
- Baynes JW (2001) The role of AGEs in aging: causation or correlation. ExpGerontol 36: 1527-1537.
- Fang F, Liu G (2007) A novel cyclic squamosamide analogue compound FLZ improves memory impairment in artificial senescence mice induced by chronic injection of D-galactose and NaNO2. Basic ClinPharmacolToxicol 101: 447-454.
- Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J, Packer L and Liu J (2006) Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. Journal of Neuroscience Research. 83 : 1584��?1590.
- Ho SC, Liu JH, Wu RY (2003) Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 4: 15-18.
- Holden HM, Rayment I, Thoden JB (2003) Structure and function of enzymes of the Leloir pathway for galactose metabolism. J BiolChem 278: 43885-43888.
- Chen L, Jin T, Huang B, Chang X, Lei L, et al. (2006) Plasma metallothionein antibody and cadmium-induced renal dysfunction in an occupational population in China. ToxicolSci 91: 104-112.
- Chen CF, Lang SY, Zuo PP, Yang N, Wang XQ, et al. (2006) Effects of D-galactose on the expression of hippocampal peripheral-type benzodiazepine receptor and spatial memory performances in rats. Psychoneuroendocrinology 31: 805-811.
- Moon JH, Pyo KH, Jung BK, Chun HS, Chai JY, et al. (2011) Resistance to Toxoplasma gondii infection in mice treated with silk protein by enhanced immune responses. Korean J Parasitol 49: 303-308.
- Hua X, Lei M, Zhang Y, Ding J, Han Q, et al. (2007) Long-term D-galactose injection combined with ovariectomy serves as a new rodent model for Alzheimer's disease. Life Sci 80: 1897-1905.
- Morris R (1984) Developments of a water maze procedure for studying spatial learning in the rat. Neurosci Methods11: 47-64.
- Augustinsson KB (1957) In: Methods of Biochemical Analysis (ed. D. Click). Inter Science Publishers. New York. 5: 1.
- ELLMAN GL, COURTNEY KD, ANDRES V Jr, FEATHER-STONE RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. BiochemPharmacol 7: 88-95.
- Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J BiolChem 247: 3170-3175.
- Aebi H (1984) Catalase. In: Methods in enzymology. (L. Packer, Eds), Academic Press, Orlando. 105: 121 - 126.
- Carlberg I and Mannervik B (1985) Glutathione reduction. In: ��?Methods in Enzymology� ed., Colowick SP and Kaplan NO. Academic Press in Orlando. 113: 484-499.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Morris RGM (1981) Spatial location does not require the presence of local cues. Learn Motiv12: 239-260.
- Glowinski J, Iversen LL, Axelrod J (1966) Storage and synthesis of norepinephrine in the reserpine-treated rat brain. J PharmacolExpTher 151: 385-399.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J BiolChem 193: 265-275.
- Metcalf RL (1957) In: Methods of biochemical analysis, Vol: 5th Edition Click Interscience publishers Inc., New York.
- Humason GL (1972) Animal tissue techniques-3rd Ed., Co. San Fransisco: W.H. Freeman.
- Harris HF (1900) On the rapid conversion of haematoxylin into haemation in statining reaction. J. Appl. Microse. Lab. Met. 3 : 777.
- Yellamma Kuna and Kalyani Bai Kunte (2013) Neuroprotective effect of Bacopamonniera on memory deficits and ATPase system in Alzheimer��?s disease (AD) induced mice. Journal of Scientific and Innovative Research. 2 (4): 719-735
- Dashti MH, Zeinalib F, Anvaric M, Hosseinid SM (2012) Saffron (Crocus sativus L.) Extract prevents and improves D-galactose and NaNo2 induced memory impairment in mice. Excli. Journal.11: 328-337.
- Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, et al. (2007) Effects of the active constituents of Crocus sativus L., crocins on recognition and spatial rats' memory. Behav Brain Res 183: 141-146.
- Khalili M, Roghani M and Ekhlasi M (2009) The effect of aqueous Crocus sativus L. extract on intracerebroventricularstreptozotocin-induced cognitive deficits in rat: a behavioral analysis. Iran J Pharm Res. 8: 185-91.
- Moser E, Moser MB, Andersen P (1993) Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 13: 3916-3925.
- Bartus RT (2000) On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. ExpNeurol 163: 495-529.
- Colom LV (2006) Septal networks: relevance to theta rhythm, epilepsy and Alzheimer's disease. J Neurochem 96: 609-623.
- Muir JL (1997) Acetylcholine, aging, and Alzheimer's disease. PharmacolBiochemBehav 56: 687-696.
- Park Dongsun, Sun Hee Lee, Young Jin Choi, Dae Kwon Bae, Yun Hui Yang, Goeun Yang, Tae Kyun Kim, SunghoYeon, SeockYeon Hwang, Seong SooJoo and Yun Bae Kim (2011) Improving Effect of Silk Peptides on the Cognitive Function of Rats with Aging Brain Facilitated by D-Galactose.BiomolTher. 19(2): 224-230
- Shin S, Park D, Yeon S, Jeon JH, Kim TK, Joo SS, Lim WT, Lee JY and Kim YB (2009a) Stamina-enhancing effects of silk amino acid preparations in mice. Lab. Anim. Res. 25: 127-134.
- Shin S, Yeon S, Park D, Oh J, Kang H, et al. (2010) Silk amino acids improve physical stamina and male reproductive function of mice. Biol Pharm Bull 33: 273-278.
- Park D, Kim T K, Yeon S, Lee SH, Choi YJ, Bae DK, Yang YH, Yang G, Joo S S, Lim WT, Lee JY, Lee J, Jeong HS, Hwang SY and Kim YB (2010) Tyrosine-fortied silk amino acids improve physical function of Parkinson��?s disease rats. Food Sci. Biotechnol.20: 79-84.
- Milatovic D, Dettbarn WD (1996) Modification of acetylcholinesterase during adaptation to chronic, subacuteparaoxon application in rat. ToxicolApplPharmacol 136: 20-28.
- Francis PT, Palmer AM, Snape M, Wilcock GK (1999) The cholinergic hypothesis of Alzheimer's disease: a review of progress. J NeurolNeurosurg Psychiatry 66: 137-147.
- Perry G, Kawai M, Tabaton M, Onorato M, Mulvihill P, et al. (1991) Neuropil threads of Alzheimer's disease show a marked alteration of the normal cytoskeleton. J Neurosci 11: 1748-1755.
- Greig N, Utsuki T, Ingram D, Wang Y, Pepeu G and Scali C (2005) Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer �?-amyloid peptide in rodent. Proceeding of the National Academy of Sciences.102: 17213-17218.
- Niewiadomska G, Baksalerska-Pazera M, Riedel G (2009) Thesepto-hippocampal system, learning and recovery of function. ProgNeuropsychopharmacolBiol Psychiatry 33: 791-805.
- Bacciottini L, Passani MB, Mannaioni PF, Blandina P (2001) Interactions between histaminergic and cholinergic systems in learning and memory. Behav Brain Res 124: 183-194.
- Shen YX, Xu SY, Wei W, Sun XX, Yang J, et al. (2002) Melatonin reduces memory changes and neural oxidative damage in mice treated with D-galactose. J Pineal Res 32: 173-178.
- Wei H, Li L, Song Q, Ai H, Chu J, et al. (2005) Behavioural study of the D-galactose induced aging model in C57BL/6J mice. Behav Brain Res 157: 245-251.
- Lei M, Hua X, Xiao M, Ding J, Han Q, et al. (2008) Impairments of astrocytes are involved in the d-galactose-induced brain aging. BiochemBiophys Res Commun 369: 1082-1087.
- Banks W, Niehoff ML, Drago D, Zatta P (2006) Aluminum complexing enhances amyloid beta protein penetration of blood-brain barrier. Brain Res 1116: 215-221.
- Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81: 741-766.
- Mattson MP (2004) Pathways towards and away from Alzheimer's disease. Nature 430: 631-639.
- Goedert M, Spillantini MG (2006) A century of Alzheimer's disease. Science 314: 777-781.
- Kumar A, Prakash A, Dogra S (2010) Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food ChemToxicol 48: 626-632.
- Terry AV Jr, Buccafusco JJ (2003) The cholinergic hypothesis of age and Alzheimer's disease-related cognitive deficits: recent challenges and their implications for novel drug development. J PharmacolExpTher 306: 821-827.
- Musia�?��? A, Bajda M, Malawska B (2007) Recent developments in cholinesterases inhibitors for Alzheimer's disease treatment. Curr Med Chem 14: 2654-2679.
- Das A, Shankar G, Nath C, Pal R, Singh S and Singh H (2002) A comparative study in rodents of standardized extracts of Bacopamonniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacology, Biochemistry and Behaviour. 73: 893-900.
- Enz A, Amstutz R, Boddeke H, Gmelin G, Malanowski J (1993) Brain selective inhibition of acetylcholinesterase: a novel approach to therapy for Alzheimer's disease. Prog Brain Res 98: 431-438.
- Lee K, Yeo JH, Lee YW, Kweon HY, Woo SO, Han SM and Kim JH (2003) Studies on industrial utilization of silk protein. Korean J. Food Ind. 36: 25-37
- Park JD, Cherrington NJ, Klaassen CD (2002) Intestinal absorption of cadmium is associated with divalent metal transporter 1 in rats. ToxicolSci 68: 288-294.
- Park KJ, Hong SE, Do MS and Hyun CK (2002) Stimulation of insulin secretion by silk fibroin hydrolysate in streptozotocininduced diabetic rat and db/db mice. Korean J. Pharmacogn. 33: 21-28
- Lee Y, Park M, Choi J, Kim J, Nam M and Jeong Y (2007b) Effects of silk protein hydrolysates on blood glucose level, serum insulin and leptin secretion in OLEFT rats. J. Korean Soc. Food Sci. Nutr.36: 703-707
- Zhaorigetu S, Yanaka N, Sasaki M, Watanabe H, Kato N (2003) Silk protein, sericin, suppresses DMBA-TPA-induced mouse skin tumorigenesis by reducing oxidative stress, inflammatory responses and endogenous tumor promoter TNF-alpha. Oncol Rep 10: 537-543.
- Kim YT, Yi YJ, Kim MY, Bu Y, Jin ZH, et al. (2008) Neuroprotection and enhancement of spatial memory by herbal mixture HT008-1 in rat global brain ischemia model. Am J Chin Med 36: 287-299.
- Kang YK, Nam SH, Sohn HO and Lee DW (2006) Inhibitory effect of silkworm-extract (SE) on monoamine oxidase activity in vitro and in vivo. Entomol. Res. 35: 189-193.
- Kang YK, Oh HS, Cho YH, Kim YJ, Han YG and Nam SH (2010) Effects of a silkworm extract on dopamine and monoamine oxidase-B activity in an MPTP-induced Parkinson��?s disease model. Lab. Anim. Res.26: 287-292.
- Lee JY, Lee SH, Sung JJ, Kim ET, Cho HJ, Kim KH, Kang YK, Kim SS, Kwon O S and Lee WB (2005) The effect of BF-7 on the ischemia-induced learning and memory deficits. Korean J. Anat. 38: 181-188
- Kim JK, Choi SJ, Cho HY, Hwang HJ, Kim YJ, Lim ST, Kim CJ, Kim HK, Peterson S and Shin DH (2010) Protective effects of kaempferol (3,4',5,7-tetrahydroxyflavone) against amyloid beta peptide (Abeta)-induced neurotoxicity in ICR mice. BiosciBiotechnolBiochem. 74(2): 397-401
- Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, et al. (2003) Silk-based biomaterials. Biomaterials 24: 401-416.
- Kim SG, Dai W, Xu Z, Li G (2011) Effects of montmorillonite on alleviating dietary Cd-induced oxidative damage in carp (Carassiusauratus). Biol Trace Elem Res 141: 200-206.
- Jin HJ, Park J, Valluzzi R, Cebe P, Kaplan DL (2004) Biomaterial films of Bombyxmori silk fibroin with poly(ethylene oxide). Biomacromolecules 5: 711-717.
- Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, et al. (2005) The inflammatory responses to silk films in vitro and in vivo. Biomaterials 26: 147-155.
- Vepari C, Kaplan DL (2007) Silk as a Biomaterial. ProgPolymSci 32: 991-1007.
- Wang X, Baughman KW, Basso DM, Strittmatter SM (2006) Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol 60: 540-549.
- Zhang YQ (2002) Applications of natural silk protein sericin in biomaterials. BiotechnolAdv 20: 91-100.
- Kato N, Sato S, Yamanaka A, Yamada H, Fuwa N, et al. (1998) Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. BiosciBiotechnolBiochem 62: 145-147.
- Hsieh CY, Miaw CL, Hsieh CC, Tseng HC, Yang YH, et al. (2009) Effects of chronic 4-n-nonylphenol treatment on aortic vasoconstriction and vasorelaxation in rats. Arch Toxicol 83: 941-946.
- Xu XH, Zhao TQ (2002) Effects of puerarin on D-galactose-induced memory deficits in mice. ActaPharmacol Sin 23: 587-590.
- Zhang C, Wang SZ, Zuo PP, Cui X, Cai J (2004) Protective effect of tetramethylpyrazine on learning and memory function in D-galactose-lesioned mice. Chin Med Sci J 19: 180-184.
- Wang W, Li S, Dong HP, Lv S, Tang YY (2009) Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci 85: 127-135.
- Dash R, Acharya C, Bindu PC, Kundu SC (2008) Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Rep 41: 236-241.
- Katiyar SK, Afaq F, Perez A, Mukhtar H (2001) Green tea polyphenol (-)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 22: 287-294.
- Zuliani T, Denis V, Noblesse E, Schnebert S, Andre P, et al. (2005) Hydrogen peroxide-induced cell death in normal human keratinocytes is differentiation dependent. Free RadicBiol Med 38: 307-316.
- Serrano F, Klann E (2004) Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev 3: 431-443.
- Lu J, Zheng YL, Luo L, Wu DM, Sun DX, et al. (2006) Quercetin reverses D-galactose induced neurotoxicity in mouse brain. Behav Brain Res 171: 251-260.
- Sun J, Li LS, Liu MQ, Wang MJ, Ding MQ, Deng SR, Lu CF, Zhou XY, Shen X, Zheng XJ and Chen SL (2010)Hydrogen peroxide and nitric oxide mediate K+ /Na+ homeostasis and antioxidant defense in NaCl-stressed callus cells of two contrasting poplars.Plant Cell, Tissue and Organ Culture. 103: 205��?215
- Ennamany R, Marzetto S, Saboureau D, Creppy EE (1995) Lipid peroxidation induced by bolesatine, a toxin of Boletus satanas: implication in m5dC variation in Vero cells related to inhibition of cell growth. Cell BiolToxicol 11: 347-354.
- Serrano F, Klann E (2004) Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev 3: 431-443.
- Ahmad S, Pardini RS (1990) Mechanisms for regulating oxygen toxicity in phytophagous insects. Free RadicBiol Med 8: 401-413.
- Bessho T, Takashina K, Eguchi J, Komatsu T, Saito K (2008) MKC-23, a choline-uptake enhancer: (1) long-lasting cognitive improvement after repeated administration in AF64A-treated rats. J Neural Transm 115: 1019-1025.
- Takashina K, Bessho T, Mori R, Eguchi J, Saito K (2008) MKC-23, a choline uptake enhancer: (2) Effect on synthesis and release of acetylcholine in AF64A-treated rats. J Neural Transm 115: 1027-1035.
- Egashira N, Yuzurihara M, Hattori N, Sakakibara I, Ishige A (2003) Ninjin-yoei-to (Ren-Shen-Yang-Rong-Tang) and Polygalae radix improves scopolamine-induced impairment of passive avoidance response in mice. Phytomedicine 10: 467-473.
- Karakida F, Ikeya Y, Tsunakawa M, Yamaguchi T, Ikarashi Y, et al. (2007) Cerebral protective and cognition-improving effects of sinapic acid in rodents. Biol Pharm Bull 30: 514-519.
- Wang Z (2012) Physiologic and biochemical changes of mimetic aging induced by D-Galactose in rats. Lab AnimSci Admin16: 23-25
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK (2008) The DISC locus in psychiatric illness. Mol Psychiatry 13: 36-64.
- Tsai J, Grutzendler J, Duff K, Gan WB (2004) Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci 7: 1181-1183.
- Winkler J, Connor DJ, Frautschy SA, Behl C, Waite JJ, et al. (1994) Lack of long-term effects after beta-amyloid protein injections in rat brain. Neurobiol Aging 15: 601-607.
- Shen ZX (2004) Brain cholinesterases: II. The molecular and cellular basis of Alzheimer's disease. Med Hypotheses 63: 308-321.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 25715
- [From(publication date):
November-2014 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 20650
- PDF downloads : 5065