Silk Fibroin: A New Approach in Drug Delivery System
Received: 03-Jul-2022 / Manuscript No. jabt-22-70532 / Editor assigned: 05-Jul-2022 / PreQC No. jabt-22-70532(PQ) / Reviewed: 19-Jul-2022 / QC No. jabt-22-70532 / Revised: 23-Jul-2022 / Manuscript No. jabt-22-70532(R) / Published Date: 30-Jul-2022 DOI: 10.4172/2155-9872.1000471
Abstract
Silk is definitely a natural biocompatible material with humans and has its role in medical treatments from prehistoric times. Silk fibroin, the fibrous structural-protein component in silk, has emerged as a accomplished treatment for these impaired processes by promoting functional tissue regeneration. Silk is a functional protein biomaterial obtained from a variety of insects like flies, silkworms, scorpions, spiders, and mites. Silk synthesized by silkworms is widely studied for its applications in tissue engineering and wound healing. The silk worm protein made up of two types of proteins which are fibroin and sericin. Silk fibroin has been known to stimulate wound healing by increasing cell proliferation and growth and migrating various types of cells which are involved in different stages of wound healing process. Impaired wound healing can cause local hypoxia or tissue necrosis and ultimately result in amputation or even death. Silk fibroin (SF) is a natural polymeric biomaterial that is broadly adopted for the preparation of drug delivery systems. It holds great potential due to its abundance, mechanical robustness, biocompatibility, and tunable degradation. Different forms of silk fibroin include nanoparticles,tissue scaffolds, wound dressings, and novel drug-delivery systems.
Keywords
Wound healing; Silk Fibroin; Nanoparticles; Polymers; Scaffolds; Sericin
Introduction
Silk fibroin (SF) has emerged as a active biomaterial that meets the above mentioned criteria for an ideal wound treatment. SF is a fibrous structural protein and is the component typically secluded for therapeutic uses. Fibroin and sericin are the two proteins found in silk from the Bombyx mori silkworm: Fibroin is produced by the silkworm’s posterior gland, while sericin is released by the silkworm’s middle and anterior glands Sericin creates a gum-like structure of glycoproteins surrounding the unprocessed SF and can exhibit immunogenicity; therefore, it is usually removed.
In order to separate these two elements, sericin is removed via a degumming method, and the remaining SF is regenerated via electro spinning, a fairly simple and economical process. SF can be useful to many wound treatment approaches, such as molecular scaffolds, dermatological applications, and novel drug delivery systems. This natural bioactive polymer is effective, readily available, and economical, making it an ideal candidate for wide usage. While SF has been studied as a regenerative therapeutic for a plethora of tissues, including bone, cornea, nerve, and cartilage [1].The cocoon silks spun by the domestic silkworm Bombyx mori have been used in textiles from thousands of years. Silk has long been an essential textile stock due to its unique feel, luster, dyeability, tensile strength and elasticity, good thermal stability, hygroscopic nature, and microbial resistance. For almost two decades, the regenerated liquid silk fibroin (LSF) solution has been widely applied to the research and development of various forms of biomaterials, such as drug/enzyme delivery systems [2, 3]. Because of its biocompatibility, slow disintegration, low immunogenicity, adaptability, and outstanding mechanical qualities, silk fibroin is increasingly being studied for biomedical applications[4-6]Silk is a natural protein polymer that has been recognized by the US Food and Drug Administration (FDA) for medical practice [4-6].
Physicochemical properties of Silk Fibroin
SF is made up of three components: a heavy chain, a light chain, and a glycoprotein, which are 350 kDa, 25 kDa, and 30 kDa, respectively [7]. The heavy chains consist of lipophilic domains, while the light chains are hydrophilic. These two secondary chain structures commonly found in SF: silk I and silk II [8]. Silk I consist of α helices, while silk II consists of β-sheets. In particular, the β-sheets form hydrogen bonds and, along with glycine and alanine bonds, lend the biomaterial its well-known mechanical strength. Genetic manipulation of SF can lead to tunable properties which influence how the biomaterial behaves [9]. These tunable properties include permeability, composition, and sequence [10]. Ultimately, the physicochemical properties of SF unite to create an ideal biomaterial for wound curing. High water retention keeps the wound hydrated, while antimicrobial properties stop infection. Enhanced cytocompatibility and efficient carbon dioxide and oxygen gas exchange allow cells to more efficiently multiply within the wound. Slight to no immunogenicity keeps inflammation low down, falling the risk of adverse reactions [11].
Different formulations of silk fibroin
SF Hydrogels
SF hydrogels are easy to make up. The pH of the SF solution is simply lowered with an acidic solution, and gelation is produced. As a drug carrier, this can be limiting as a lower pH may not be suitable for all drugs [12]. Additional methods, such as sonication, have been useful to the gelation of SF solutions [13]. These methods are more complex and less practical for the quick and widespread production of treatments; however, they do provide substitute to pH-restricted drug delivery. Hydrogels are three-dimensional polymeric networks with a high swelling ratio in an aqueous solution. Hydrogels are typically made from naturally occurring polymers, such as, and silk fibroin, hyaluronic acid, collagen, alginate, chitosan due to their relatively good biocompatibility [14-19]. Hydrogels have been extensively used in a variety of biomedical applications, including wound treatment.The valuable benefits of silk fibroin inducing cell attachment, development, migration, propagation, and creation of extracellular matrix and also improving the mechanical strength of hydrogels prepared from other natural polymers.
SF Scaffolds
Tissue scaffolds provide a three-dimensional structure to which cells can stick and subsequently repair wounds. The ideal tissue scaffold retains water, allows for enough gas exchange, and improves cell adhesion and motility. It also has the capability to deliver drugs or additional treatments, biodegrade, be non-immunogenic, and interact with the existing extracellular matrix (ECM). It is also important for these scaffolds to be simply prepared and economical, given the rising frequency of diseases leading to impaired wound treatment. SF tissue scaffolds can be united with other biomaterials, termed composite scaffolds, for a synergistic action on tissue repair. These biomaterials can include antimicrobial agents, therapeutics, growth factors and more. Composite scaffolds keep the strong mechanical properties of SF and give additional properties which help in wound curing. The dynamic capability of SF to be united with other biomaterials allows it to treat specific healing deficiencies within a wider range of ailing wounds. Instead of a particular treatment being applied to a wide range of wounds, extra targeted biomaterials can potentially facilitate specific wounds heal [20].
SF Nanoparticles
SF is presented as a nanoparticle for controlled delivery of bioactive therapeutics. The molecular and physical properties of SF permit for highly customizable particle size, ranging from about 10 nm to over 100 nm [21]. SF nanoparticles have also been prepared into different shapes as well as united with other biomaterials, depending on their intended applications [22]. It has been shown to capably deliver growth factors, proteins, and other novel therapeutics to wounds and other tissues. The use of SF nanoparticles used as a novel delivery system for therapeutics has grown in popularity due to the non-toxic different preparation methods to prepare the drug delivery systems; SF nanoparticles can be prepared without organic solvents or other cytotoxic chemicals through various processes including milling, electro spraying, freezing, and desolvation [23]. The nanoparticles can then be loaded with drugs and other therapeutics by the easy process of adsorption, which is enabled by SF’s porosity. Nanoparticles can be engineered for different release behaviors. Importantly, SF nanoparticles are small adequate to easily penetrate tissues, thus increasing the efficiency of drug uptake. After drug release, the high biocompatibility and low immunogenicity of SF nanoparticles leads to either passive clearance or natural degradation, without adverse effects [24].
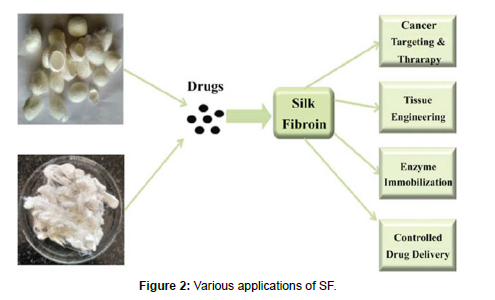
Applications of SF
• Silk fibroin nanoparticles in breast cancer [25]
• Silk fibroin for wound healing and Skin Regeneration [26]
• Silk fibroin nanoparticles for inflammatory bowel disease[27]
• Silk fibroin has also been used as a surgical suture material [28, 29] (Figure 1 and 2)
Conclusion
In the present review, we have summarized the physical and chemical properties, biocompatibility characters of SF and its applications in various drug delivery systems.
References
- Lehmann T, Vaughn AE, Seal S, Liechty KW, Zgheib C (2022) Silk Fibroin-Based Therapeutics for Impaired Wound Healing. Pharmaceutics 14: 651.
- Wang F, Zhang YQ (2015) Bioconjugation of Silk Fibroin Nanoparticles with Enzyme and Peptide and Their Characterization. Adv Protein Chem Struct Biol 98: 263-291.
- Vidya M, Rajagopal S (2021) Silk Fibroin: A Promising Tool for Wound Healing and Skin Regeneration. Int J Polym Sci 2021:1-10.
- Kundu B, Rajkhowa R, Kundu SC, Wang X (2013) Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev 65: 457-470.
- Zeplin PH, Maksimovikj NC, Jordan MC, Nickel J, Lang G, et al. (2014) Spider silk coatings as a bioshield to reduce periprosthetic fibrous capsule formation. Adv Funct Mater 24: 2658-2666.
- Rodriguez MJ, Brown J, Giordano J, Lin SJ, Omenetto FG, et al. (2017) Silk based bioinks for soft tissue reconstruction using 3-dimensional (3D) printing with in vitro and in vivo assessments. Biomaterials 117: 105-115,
- Dorishetty P, Dutta NK, Choudhury NR (2020) Silk fibroins in multiscale dimensions for diverse. RSC Adv 10: 33227-33247.
- Koh LD, Cheng Y, Teng CP, Khin YW, Loh XJ, et al. (2015) Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci 46: 86-110.
- Zielinska SG, Sionkowska A (2021) How to improve physico-chemical properties of silk fibroin materials for biomedical applications?-Blending and cross-linking of silk fibroin-A review. Materials 14:1510.
- Lehmann T, Vaughn AE, Seal S, Liechty KW, Zgheib C (2022) Silk Fibroin-Based Therapeutics for Impaired Wound Healing Tanner. Pharmaceutics 14: 651.
- Farokhi M, Mottaghitalab F, Fatahi Y, Khademhosseini A, Kaplan DL (2018) Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol 36: 907-922.
- Mottaghitalab F, Farokhi M, Shokrgozar MA, Atyabi F, Hosseinkhani H (2015) Silk fibroin nanoparticle as a novel drug delivery system. J Control Release 206: 161-176.
- Hu X, Lu Q, Sun L, Cebe P, Wang X, et al. (2010) Biomaterials from Ultrasonication-Induced Silk Fibroin−Hyaluronic Acid Hydrogels. Biomacromolecules 11: 3178-3188.
- Ju HW, Lee OJ, Moon BM, Sheikh FA, Lee JM, et al. (2014) Silk fibroin based hydrogel for regeneration of burn induced wounds. J Tissue Eng Regen Med 11: 203-210.
- Lee JM, Sultan MT, Kim SH, Kumar V, Yeon YK, et al. (2017) Artificial auricular cartilage using silk fibroin and polyvinyl alcohol hydrogel. Int J Mol Sci 18: 1707.
- Burdick JA, Prestwich GD (2011) Hyaluronic acid hydrogels for biomedical applications. Adv Mater 23: 41-56.
- Niiyama H, Kuroyanagi Y (2014) Development of novel wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor and vitamin C derivative. J Artif Organs 17: 81-87.
- Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, et al. (2001) Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog 17: 945-950.
- Murakami K, Aoki H, Nakamura S, Nakamura SI, Takikawa M, et al. (2010) Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials vol. 31: 83-90.
- Lehmann T, Vaughn AE, Seal S, Liechty KW, Zgheib C (2022) Silk Fibroin-Based Therapeutics for Impaired Wound Healing. Pharmaceutics 14: 651.
- Mathur AB, Gupta V (2010) Silk fibroin-derived nanoparticles for biomedical applications. Nanomedicine 5:807-820.
- Zhang YQ, Wang YJ, Wang HY, Zhu L, Zhou ZZ (2011) Highly efficient processing of silk fibroin nanoparticle-l-asparaginase bioconjugates and their characterization as a drug delivery system. Soft Matter 7: 9728-9736.
- Zhang W, Chen L, Chen J, Wang L, Gui X, et al. (2017) Silk fibroin biomaterial shows safe and effective wound healing in animal models and a randomized controlled clinical trial. Adv Healthc Mater 6: 700121.
- Mottaghitalab F, Farokhi M, Shokrgozar MA, Atyabi F, Hosseinkhani H (2015) Silk fibroin nanoparticle as A Novel Drug Delivery System. J Control Release 206: 161-176.
- Moin A, Wani SUD, Osmani RA, Lila ASA, Khafagy E, et al. (2021) Formulation, characterization, and cellular toxicity assessment of tamoxifen-loaded silk fibroin nanoparticles in breast cancer, Drug Deliv 28: 1626-1636
- Cestari M, Caldas BS, Fonseca DP, Balbinot RB, Bidóia DL, et al. (2022) Silk fibroin nanofibers containing chondroitin sulfate and silver sulfadiazine for wound healing treatment. J Drug Deliv Sci Technol 70: 103221.
- Echave PD, Malagón AJR, Tijeras JAM, García LH, Vezza T, et al. (2021) Silk fibroin nanoparticles enhance quercetin immunomodulatory properties in DSS-induced mouse colitis. Int J Pharm 606: 120935.
- Koh LD, Cheng Y, Teng CP, Khin YW, Loh XJ, et al. (2015) Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci 46: 86-110.
- Wani SUD, Veerabhadrappa GH (2018) Silk Fibroin Based Drug Delivery Applications: Promises and Challenges. Curr Drug Targets 19: 1177-1190.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Rupvate SR, Adavadkar PR, Ukhade SS, Patil LP, Pachorkar SS, et al. (2022) Silk Fibroin: A New Approach in Drug Delivery System. J Anal Bioanal Tech 10: 471. DOI: 10.4172/2155-9872.1000471
Copyright: © 2022 Rupvate SR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2303
- [From(publication date): 0-2022 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1967
- PDF downloads: 336