Signet-ring Adenocarcinoma of the Duodenum Spreading through Brunner's Gland Adenoma
Received: 05-Nov-2019 / Accepted Date: 04-Feb-2020 / Published Date: 12-Feb-2020 DOI: 10.4172/2161-069X.1000610
Abstract
We present a rare case of a 66-year-old male with evidence of a polypoid mass in the first section of the duodenum. He first received the diagnosis of Brunner’s gland adenoma with foci of signet-ring adenocarcinoma on
biopsy and after two months he underwent distal gastrectomy extended to the first section of duodenum. The resected specimen contained one polyp of 2.5 cm (maximum diameter). Morphological assessment and
immunohistochemistry were performed and the previous diagnosis of an infiltrating signet-ring cells adenocarcinoma arising from Brunner’s gland was confirmed. Brunner’s gland adenomas are rare tumors of the duodenum. These lesions have previously been described as benign. Some authors described epithelial dysplasia associated with surface erosions, suggesting a malignant potential. In the literature, just few cases of adenocarcinoma originating from Brunner ’ s gland adenoma are described. As far as we know, there have been no previous reports about pure signet-ring adenocarcinomas arising from Brunner’s glands polyp. First diagnosis on biopsy is difficult and could be even more insidious within surgical specimen. This is due to extensive erosions, massive loss of surface epithelium, inflammation, fibrosis and hyperplastic changes, especially when facing with an adenocarcinoma infiltrating in a single-cells manner. Based on our experience, for patient’s better surgical and oncologic management, we think it is important to look carefully at the polyp surface while at the same time considering all the potential pitfalls.
Keywords: Duodenum, Gastrectomy, Inflammation, Fibrosis
Introduction
Brunner’s gland adenoma is a very rare benign tumor. Some authors considered it as a hematoma, because the mass is not a true neoplasm, but rather a hyperplastic collection of mature glands with intervening bands of fibrous tissue and variable adipose and lymphoid tissue. Occasionally, they extensively involve the lamina propria and expand, resulting in a small sessile polyp. It mostly develops in the duodenal bulb, which mirrors the glands distribution [1]. The estimated incidence is 0.008% based on autopsy studies. They occur in middle age adults, with gastrointestinal hemorrhage, obstructive symptoms or as an incidental finding [1,2]. The glands are sub mucosal and are composed of neutral mucin-secreting cuboidal to columnar cells, which fluids are alkaline. Their role is to protect the duodenal epithelium from the acid chyme coming from the stomach. They are histologically indistinguishable from the glands located in the distal gastric mucosa and periampullary region [1]. The pathogenesis is unknown; it is probably due to an abnormal response to hyperchlorhydria, pancreatic insufficiency or H. pylori infection [1-3]. The bigger the polyp gets, the more it is exposed to surface damages. When injury happens, there could be a metaplastic change in the shallow intestinal-epithelium and gastric-foveolar metaplasia can occur [4,5]. Superficial erosive sites with metaplastic cells were associated with higher proliferative activity (8-9%) of the underneath sub mucosal glands. On the other hand, Brunner ’ s glands below regenerating intestinal epithelium showed a lower proliferative rate (0. 77%) [4].
Malignant transformation of Brunner ’ s gland adenoma is exceptionally rare. Duodenal adenocarcinoma is less common than in the stomach or large intestine and usually consists of an intestinal-type adenocarcinoma with tubular or villous architecture [5]. Less frequently, according to mucin phenotype, it consists of a gastric-type tumor (positive cells with MUC5AC antibody). In the literature, there are just few cases of gastric-type adenocarcinomas arising from Brunner ’ s gland adenoma [5-9]. These studies demonstrated that gastric-type tumors are divided into 2 subsets: foveolar gland-type and pyloric gland-type [6]. They have reported that the presence of epithelial tumors with gastric features is associated with generative cell zones and gastric metaplasia, formed in Brunner’s glands during the process of mucosal regeneration, with particular reference to foveolartype cells [7-12]. Signet-ring adenocarcinomas are rare tumors mostly find in the stomach, less than 30 cases of signet ring cell carcinoma of the duodenum have been reported so far [8]. Authors speculate they might derive from gastric-foveolar metaplasia or from duodenum goblet cells purchasing gastric-type mucins [8].
Case Presentation
We report a rare case of a 66-year-old man with no history of diseases, presented with epigastric discomfort. Endoscopic examination of the upper tract of the digestive system showed a single, peduncolated, fleshy, pink-red polypoid mass of 2.5 cm with superficial erosions. Biopsy was recommended and performed. The first microscopic evaluation revealed intestinal epithelium surrounding Brunner’s glands hyperplasia, with foci of intramucosal signet-ring cells adenocarcinoma. The patient, after two months, underwent distal gastrectomy extended to the first section of duodenum, including the mass and some fat tissue including lymph nodes.
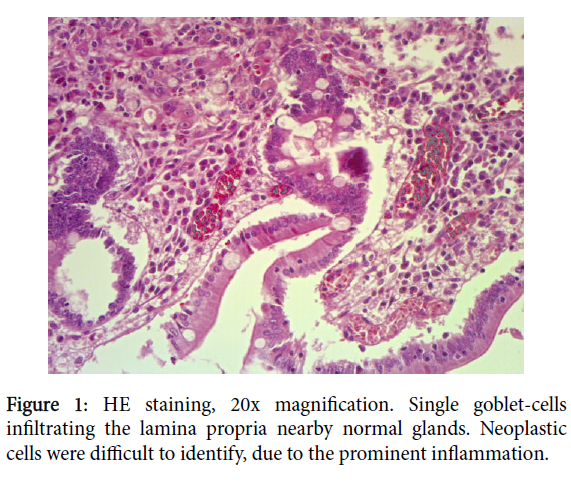
Grossly, the surgical specimen showed a polypoid mass of 2.5 cm located in the first section of duodenum, 1.5 cm far from distal surgical margins. There were found four lymph nodes. Histological examination identified a Brunner’s gland adenomatous proliferation, with densely packet mucinous gland, with no signs of dysplasia. On surface, extensive erosions didn’t allow to scan properly the whole intestinal mucosa, which was partly lost. Between atypical intestinal epithelium, including early goblet-cells dysplasia and gastric metaplasia, foci of adenocarcinoma were identified. This neoplastic proliferation was characterized by a pure signet-ring adenocarcinoma, with central globoid cytoplasmic droplet of mucin with eccentrically placed, scalloped, nucleus (Figure 1).
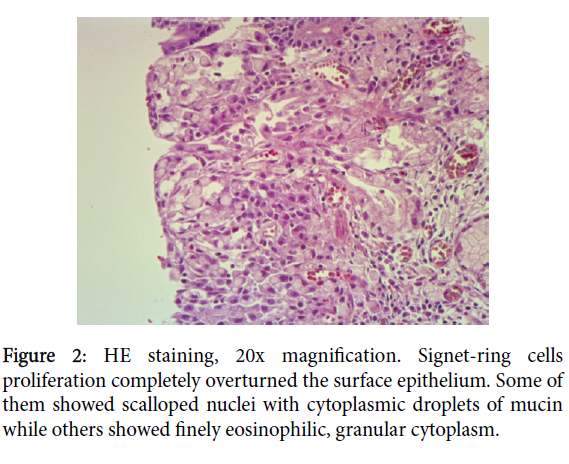
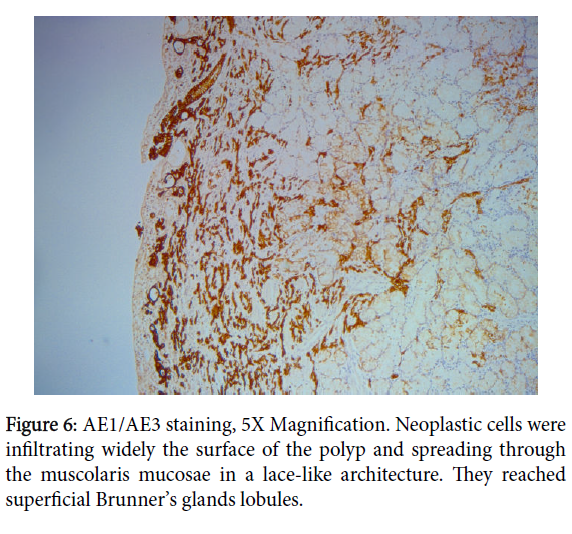
Neoplastic cells were infiltrating the lamina propria and focally the muscolaris mucosae, extending to superficial Brunner’s gland with a lace-like architecture. This was interpreted as micro invasion (Figure 2).
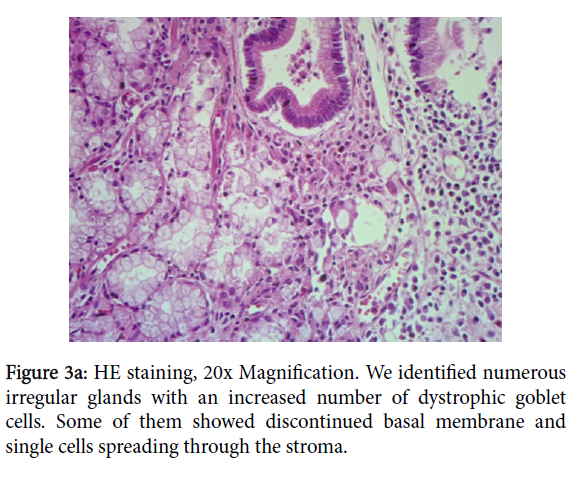
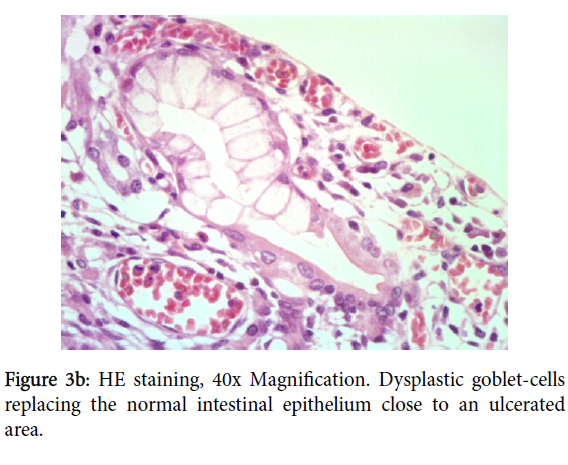
No necrosis neither hemorrhages was found. Nearby the adenocarcinoma, atypical cells with pleomorphic nuclei, macronucleoli, eosinophilic cytoplasm, have been readily identified. They were arranged in irregular glands (Figure 3a), some of them with an increased number of dystrophic goblet cells, replacing upwards the gland (Figure 3b). This could reflect either mild epithelium dysplasia or reactive and regenerative changes due to the continuous mechanical stress and inflammation.
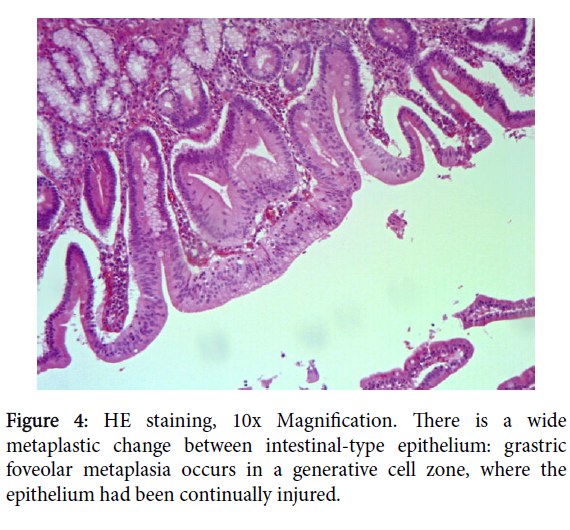
On the epithelium above the cancer, we found foci of gastricfoveolar metaplasia in close relation with densely packed Brunner’s glands extending to the mucosa, those latter showed no signs of dysplasia (Figure 4).
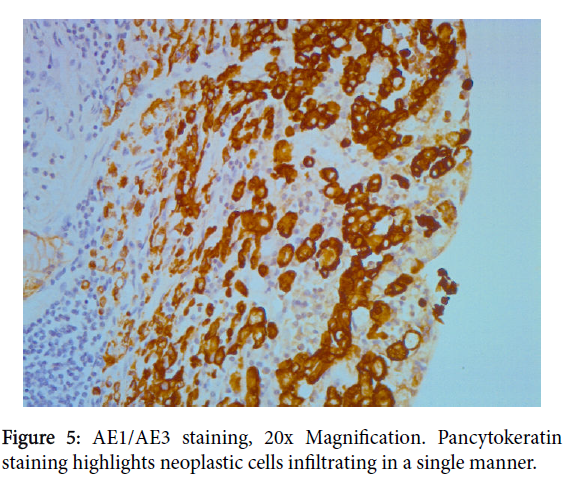
Immunoistochemical analyses demonstrated diffuse and strong cytoplasmic positivity for cytokeratin pool antibody (AE1/AE3) and EMA (Epithelium Membrane Antigen) in the epithelial cells and highlighted every single neoplastic cell (Figures 5 and 6). Anti-CD68 was also performed to differentiate signet-ring cells from histiocytes.
We made the diagnosis of multiple foci of micro-invasive adenocarcinoma of the duodenum, signet-ring type, risen on Brunner’s glands adenoma, pT1 staging according to WHO. Four detected lymph nodes exhibited just reactive follicular hyperplasia. Surgical margins were free from neoplasia. The patient ’ s postoperatory course was very good and he is still free from disease after 3 years following the surgery.
Discussion
Duodenal tumors, both malignant and benign, are quite rare. Brunner’s glands hamartomas/adenomas account for up to 1% of small bowel tumors with an incidence of 0.008% on autopsies studies, with tendency to be predominant in the fifth or sixth decade of life [2,10,11].
The majorities of cases are asymptomatic or present with nonspecific, vague symptoms such as abdominal discomfort and nausea, some of them develop gastrointestinal bleeding and obstructive symptoms. Usually it is an incidental finding during EGDS. The long-term outcome is favorable [11].
Duodenal adenocarcinomas are rare neoplasms and usually consists of an intestinal-type adenocarcinoma with tubular or villous architecture; on the other hand, few of them present an identical phenotype to gastric adenocarcinomas [6]. Very rarely, they arise from Brunner’s gland, gastric metaplasia or heterotopic gastric mucosa [5]. In the literature, there have been reported few studies discussing the possible origin of these adenocarcinomas; they examined the mucingenes expression in Brunner’s glands, in intestinal-type glands and in gastric-type glands [4-8].
By immunoistochemical studies, it is demonstrated that adenocarcinomas, showing intestinal-type cells, can be stained, at least focally, with MUC-6 antibody, as a normal Brunner’s gland does. Furthermore, some adenocarcinomas arising from Brunner’s glands showed a strong expression of MUC-5AC, which is typically found in gastric foveolar-type cells [5,12].
Kushima et al. described the histogenic pathway of gastric metaplasia in the duodenum: they demonstrated the close relationship with regenerating Brunner’s glands, following duodenal ulcer. They found MUC-5AC positivity in the mucin-producing cells in the regenerative zone, which were definitely gastric foveolar-type cells [12]. These results suggested that a consistent superficial damage on a polypoid mass could induce gastric metaplasia of the duodenum.
Crompton et al. [13], already then, tried to clarify the main pathways of epithelial differentiation in the intestine, considering Paneth, mucous, endocrine and columnar cells the indigenous lineages. They proposed another native crucial cell lineage with its definite stem cell, the “Ulcer-Associated Cell Lineage” (UACL) that commonly refers to the so called “pseudo pyloric metaplasia”. Because it is only induced by chronic inflammatory and ulcerative conditions, it would appear to be a primary defense reaction. The new forming glands, at the end, seem to develop the same proliferative organization as gastric gland tubules: the proliferative compartment is situated near the top of the duct with a bi-directional stream of the cells [12].
At early stage of the development, they seems to originate as Brunner’s primordial gland does, i.e. little buds in the lamina propria containing crypt stem cells with an upwards migration.
In our case, we had a polyp consisting of Brunner ’ s glands proliferation. We found little foci of gastric foveolar-type glands that replaced the villous surface epithelium above the infiltrating adenocarcinoma. Some glands may had more numerous dystrophic goblet cells. Signet-ring adenocarcinoma is extremely uncommon in duodenum. It mostly occurs in the stomach [8]. The relationship between gastric metaplasia, Brunner ’ s gland hyperplasia with dystrophic goblet cells and malignant transformation into a signet-ring adenocarcinoma is still unproven. In the literature, there have been described just few cases of these coexisting findings [8].
In the present case, we presumed the precursor lesion took place either in the Brunner’s glands surface epithelium, from the so called “globlet-cells dysplasia” or from metaplastic gastric epithelium.
Conclusion
We report a rare case of primary signet-ring adenocarcinoma of the duodenum arising on Brunner ’ s gland adenoma. Three other components were identified: Brunner’s glands hyperplasia, foveolar metaplastic epithelium and regenerating intestinal epithelium with various degree of atypia, including early goblet cells dysplasia. So there is condition of glands with mixed gastric and intestinal phonotypes.
The damage suffered by the overlying intestinal cells, according to the literature, in our case might had an important role in the pathogenesis of a signet-ring adenocarcinoma, exhibiting the same histotype as the one seen more typically in the stomach.
This article is written without any conflict of interests.
References
- Yantiss RK, Atonioli DA (2009) (2nd edn.) Surgical pathology of the GI tract, liver, biliary tract and pancreas. Elsevier.
- Marinacci L (2017) Brunner Gland Adenoma. Mayo Clinic Proceedings 92: 1737-1738.
- Rocco A, Borriello P, Compare D, Colibus PD, Picaet L (2006) Large Brunner's gland adenoma: case report and literature review. World J Gastroenterol 12: 1966-1968.
- Akaki M, Taniguchi S, Hatakeyama K, Kushima R, Kataoka H (2014) Duodenal mucosal damage is associated with proliferative activity of Brunner’s gland hamartoma: a case report. BMC Gastroenterol 14: 14.
- Kushima R, Stolte M, Vieth KDM, Okabe H, Hattori T (2002) Gastric-type adenocarcinoma of the duodenal second portion histogenetically associated with hyperplasia and gastric-foveolar metaplasia of Brunner’s glands. Virchows Arch 440: 655-659.
- Mitsuishi T, Hamatani S, Hirooka S, Fukasawa N, Aizawa D, et al. (2017) Clinicopathological characteristics of duodenal epithelial neoplasms: Focus on tumors with a gastric mucin phenotype (pyloric gland-type tumors). PLoS One 12: e0174985.
- Khor TS, Brown I, Kattampallil J, Yusoff I, Kumarasinghe MP (2010) Duodenal adenocarcinoma arising from a pyloric gland adenoma with a brief review of the literature. BMJ Case Rep pii: bcr1020103385.
- Mochizuki K, Kondo T, Tahara I, Inoue T, Kasai K, et al. (2015) Signet ring cell carcinoma of the non-ampullary duodenum: A case report. Pathol Res Pract 211: 801-804.
- Iwamuro M, Kobayashi S, Ohara N, Kawano S, Kawahara Y, et al. (2017) Adenocarcinoma in situ Arising from Brunner’s Gland Treated by Endoscopic Mucosal Resection. Case Rep Gastrointest Med 17: 7916976.
- Brookes M, Manjunatha S, Allen C, Cox M (2003) Malignant potential in a Brunner's gland hamartoma. Postgrad Med J 79: 416–417.
- Levine JA, Burgart LJ, Batts KP, Wang KK (1995) Brunner's gland hamartomas: clinical presentation and pathological features of 27 cases. Am J Gastroenterol 90: 290-294.
- Kushima R, Manabe R, Hattori T, Borchard F (1999) Histogenesis of gastric foveolar metaplasia following duodenal ulcer: a definite reparative lineage of Brunner's gland. Histopathology 35: 38-43.
- Crompton MJ, Dexter TM, Wright NA (1998) Aspects of the biology of regeneration and repair in the human gastrointestinal tract. Phil Trans p: 353.
Citation: Masu L, Pinamonti M, Coverlizza S (2020) Signet-ring Adenocarcinoma of the Duodenum Spreading through Brunner ’ s Gland Adenoma. J Gastrointest Dig Syst 10: 610. DOI: 10.4172/2161-069X.1000610
Copyright: © 2020 Masu L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2887
- [From(publication date): 0-2020 - Nov 10, 2025]
- Breakdown by view type
- HTML page views: 2006
- PDF downloads: 881