Sickle Cell Trait, Malaria and Sensorineural Hearing Loss–A Case-Control Study from São Tomé and PrÃÂncipe
Received: 30-Oct-2016 / Accepted Date: 22-Nov-2016 / Published Date: 29-Nov-2016 DOI: 10.4172/2161-119X.1000278
Abstract
Background: Hearing loss is a problem with higher incidence in South Asia, Asia Pacific and sub-Saharan Africa. In these countries there is also associated history of anemia and malaria. Objective: This study aims to identify a putative role of Beta globin mutation - sickle cell trait and HL in São Tomé and Príncipe population. Methods: A retrospective case-control study of a convenience sample was collected during Otolaryngologist Humanitarian Missions in São Tomé and Príncipe. Control group includes individuals with normal hearing in both ears, and the case group has participants presenting bilateral or unilateral HL. It was evaluated the potential risk factors and sickle cell trait with HL, as well self-report of malaria infection, consanguinity, familial history of HL. The HbS gene point mutation (Glu6Val) was determined by PCR-RFLP. Results: Our results showed a statistical significance between HL - oral language and self-report of HL. Taken altogether, our data did not reveal association between sickle cell trait and HL. However, a statistical association between HL and self-report of malaria was found. Conclusion: No association between sickle cell trait and the high prevalence of HL was found. Self-report of Malaria was found as a risk factor for the development of HL in São Tomé and Príncipe population. The multifactorial profile of HL shall not exclude the relevance of other etiologic factors than Malaria to justify the high prevalence of HL in São Tomé and Príncipe and further investigation must be applied.
Keywords: Hearing loss; Sensorineural hearing loss; São tomé and príncipe; Sickle cell trait; Sickle cell disease; Malaria; Hemoglobinopathies
253657Introduction
More than 360 million people in the World have disabling hearing loss (HL). According to new World Health Organization (WHO) Global Estimates on prevalence of the HL [1,2], there is a higher incidence in South Asia, Asia Pacific and sub-Saharan Africa [1-3]. Sub-Saharan Africa holds 10% of the world’s population and two thirds of the world’s least developed nations.
More than 1.2 million of the children living in sub-Saharan Africa aged 5 to 14 years old have moderate to severe bilateral HL [3]. The consequences of hearing problems are well known. HL in children can result in developmental delays and lead to significant inability to engage in oral/aural communication.
Anemia has been proposed as an etiologic factor in sensorineural deafness for many years, but there is little supporting evidence [4]. In these African countries there is a high prevalence of anemia and this could be associated with infections, hemoglobinopathies or stunting [5].
Several gene mutations and polymorphisms in the human hosts confer survival advantage and have increased in frequency through natural selection over generations. These include hemoglobinopathies like Sickle Cell Disease (SCD), thalassemias and glucose-6-phosphate dehydrogenase (G6PD) deficiency. Sickle Cell Disease (SCD) refers to a group of symptomatic disorders associated with a specific mutation on the Beta globin (HBB) gene [6]. Sickle hemoglobin (HbS), a structural variant of normal adult hemoglobin, results in the substitution of glutamic acid with valine in the sixth position of β- chain of the hemoglobin (β6Glu-Val) [7]. HbS is the most common pathological hemoglobin variant worldwide [7].
Several studies have suggested that HbS has an effect of protection against Plasmodium falciparum, the etiologic agent of malaria [6], showing that heterozygous people carrying the sickle-cell trait (HbAS) are protected against severe malaria (prevalence of HbAS, in some populations, is above 90%) [6,8].
This mechanism act as natural selection and is co-responsible for the high prevalence of HbS in malaria endemic regions as a result of natural selection over generations [9].
The red cells of individuals with the mutant homozygous gene (HbSS) become sickle shaped in low oxygen tension pressure with reduced oxygen-carrying capacity. The basal turn of the cochlea is particularly sensitive to anoxia, due to the high oxygen consumption rate of the stria vascularis and poor capacity for anaerobic metabolism. In this cases, SNHL start with the loss on the higher frequencies, then the lower frequencies, resulting from the damage at the apical region (low frequencies) and finally loss of the function throughout the entire cochlea [8].
SNHL is one of the several complications of this disease and has been found to occur in 8% of SCD children in Nigeria, 12% in USA, 22% in Jamaica, 36.5% in Kenya and 60% in Ghana [3,8].
In some studies, HbAS in malaria exposure could be also a cause of SNHL [10].
São Tomé and Príncipe it’s an archipelago in western equatorial Africa, near Gabon, Equatorial Guinea, Camaroon and Nigeria, with Portuguese as official language [11]. São Tomé and Príncipe was discovered by Portuguese explorers in 1470. The resident population is about 187,000 inhabitants who have a low average age distribution (17-18 years) with low socioeconomic conditions and poor sanitary conditions, as well as a public health infection problem–malaria [12– 14].
Since 2011 an otolaryngologist group formed by 2 doctors, 2 nurses and 1 audiologist began humanitarian missions in São Tomé and Príncipe (Project “Health for all”–Instituto Marquês de Valle Flor (IMVF)). Apparently according their clinical registries, this was the first hearing evaluation performed in these islands. Upon the first mission, a high prevalence of SNHL in the population was identified and it was observed upon subsequent missions.
As far as we know this is the first study developed in São Tomé and Príncipe population to evaluate the causes inherent to the high incidence of HL in this population. Such way, this study proposes to answer the question: will it be possible to establish an association between the sickle cell trait (HbAS) and the high incidence of SNHL in São Tomé and Príncipe?
Materials and Methods
Subjects
We present a case-control study, with a convenience sample where the control group include individuals with normal hearing in both ears, and patients with unilateral or bilateral HL compose the case group. The project was submitted and approved by the Medical Ethics Committee of São Tomé and Príncipe and Ethics Research Committee NMS|FCM-UNL.
A total of 316 individuals (136 HL patients and 180 controls with bilateral normal hearing), ranging from 2 to 35 years old, agreed to participate in this study. The limitation to 35 years was chosen to avoid action of other risk factors of HL, like acoustic trauma, age and the effect of other diseases. We organized into two age groups based on WHO: 1) below 14 years old (2–14 years) as children group and 2) above 15 years old (15–35 years) as adults group. The patients were recruited during consultation provided by the humanitarian missions in São Tomé and Príncipe over a period from February 2012 to May 2014. The controls (normal hearing bilateral) were recruited at consultation at health services, primary schools from São Tomé and local hotel.
All patients signed an informed consent and answered a clinical questionnaire recording risk factors and clinical history. An otolaryngologist observed all. The risk factors included were family history of HL, consanguinity, self-report of malaria infection, pre-natal and perinatal history and history of infections.
All 316 individuals were evaluated regarding their hearing status with Pure Tone Audiogram (PTA) or Auditory Brainstem Response (ABR) depending on collaboration to participate on the audiometric exams.
Audiometric exams were carried out by an audiologist without an audiometric cabin, with earphones-TDH39, in a closed room, with a level of noise measured by iPhone using SchabelDoesIT GbR, Munich, Germany (version 1.0.0), considered acceptable (ANSI S3.1-1999) (R2013). The equipment used was the Madsen Midimate 622 and Vivosonic Integrity V500 audiometer (auditory brainstem response), calibrated according to calibration ISO389 1975/Oslo Recommendation. The IntegrityTM V500 system used to collect auditory brainstem is a modular equipment comprised by 4 main components: the computer, the VivoLink (SN: VL0026), the Amplitrode (SN: AJ0270) and the earphones. The earphones used were the ER-3A (ER-3A Left SN:63762 e ER-3A Right SN: 63763) and were calibrated according to ANSI S3.6-1996 and the stimulus used was the CLICK, calibrated in dB equivalent to the sound pressure level (dBpeSPL) according to the procedure IEC 60645-3 for the calibration of short duration stimulus.
Electrophysiological thresholds were translated into the audiometric thresholds for frequencies 2000 Hz and 4000 Hz. No correction factor was applied as is advocated in studies conducted by Jerger and Mauldin [15]; Gorga et al. [16]; van der Drift et al. [17]; Gorga et al. [18] in which establish a strong correlation between the electrophysiological thresholds with the "click" and the audiometric thresholds at 2000 and 4000 Hz.
The hearing loss of each patient was determined based on the better ear and following the WHO classification [19].
Patients with less than 2 years and more than 35 years of age, conductive hearing loss, syndromic hearing loss, obvious environmental causes such as meningitis, cerebral malaria, intrauterine or neonatal complications, ototoxicity, severe head trauma or developmental delay were excluded.
DNA extraction
Blood samples of all patients and controls were collected into Guthrie cards. Genomic DNA was obtained from each sample using a commercially available kit (QIAamp® DNA micro kit, Qiagen) according to the manufacturer’s instructions. All DNA samples were stored at –20°C.
SNP screening
The HbS gene point mutation (Glu6Val) was determined by PCRRFLP. The primers and PCR conditions for the mutation site of this gene is shown in Table 1. For all of them the nucleotide mutated resulted in either gain or loss of restriction site, which therefore allowed the wild-type (HbAA) and variant alleles to be discriminated by RFLP after appropriate restriction enzyme digestion.
PCR was carried out with 50 ng of DNA in 50 ml reaction volume, containing 1.3x ImmoBuffer, 1.5 mM MgCl2, 0.6 mM dNTP, 1.0 mM of each primer, and 0.75 U of Immolase (Bioline). The amplification started with an initial denaturation step at 95°C for 7 min, cycling parameters were 35 cycles of 95°C for 30 s, specific annealing temperature (60°C) for 30 s, 72°C for 30 s and a final extension at 72°C for 10 min. After amplification, 10 ml of each PCR products were digested with appropriate restriction enzymes (DdeI) for 3 h at 37°C followed by an inactivation step at 65°C for 20 min and electrophoresed in 2% agarose gel with ethidium bromide (0.5 mg/ml) for visualization under ultraviolet light. The expected products for each genotype of the tested gene are shown in Table 1. All the genotype determinations were carried out twice in independent experiments and inconclusive samples were reanalyzed.
| Gene | Primers | PCR fragment | Patterns after restriction enzyme digestion |
|---|---|---|---|
| HbAS codon 6 (rs334) | Rv 5’- AGG GTG GGA AAA TAG ACC AA -3’ | 395 bp | HbAA: 202, 180, 13 bp |
| FW 5’- CGG CTG TCA TCA CTT AGA CCT -3’ | HbAS: 382, 202,180,13 bp | ||
| HbSS: 382, 13 bp |
Table 1: PCR-RFLP conditions for identification of HbAS (rs334) polymorphism.
Statistical Analysis
The Hardy-Weinberg equilibrium of HbS was assessed by Quisquare test, calculated by HW calculator™ - Michael H Court (2005-2008).
Description of the sample was made with descriptive statistics, considering frequency analysis, means and standard deviation (SD).
To study the association between HL and each of the following parameters as district origin, oral language, perception of HL, sex and history of malaria infection, have been used the Qui-squared test.
The association between HbS genotype and the degree of HL of each ear and was analysed with Qui-square test by Monte Carlo Simulation.
To identify risk factors of HL we adopted a Binary Logistic Regression, where HL is a response variable and independent variables were HbS, age groups and self-report of Malaria infection.
All analyses were performed using the IBM, SPSS 20 version.
Results
We evaluated 316 subjects (Table 2) of whom 146 (45.6%) were men and 172 (54.4%) were women, with an age mean of 17.4 ± 9.74 years and a median of 15 years.
| Control-180 | Case-136 | Case Unil HL | Case Bil HL | p-Value | |
|---|---|---|---|---|---|
| Age range | 0.361 | ||||
| [2-14]: | 82 (45.6%) | 69 (50.7%) | 15 (34.1%) | 54 (58.7%) | |
| [15-35]: | 98 (54.4%) | 67 (49.3%) | 29 (65.9%) | 38 (41.3%) | |
| Mean Age SD | 17.8±9.77 | 16.9±9.73 | 20.1±9.29 | 15.4±9.61 | |
| Sex | 0.256 | ||||
| Male: | 87 (48.3%) | 57 (41.9%) | 17 (38.6%) | 40 (43.5%) | |
| Female: | 93 (51.7%) | 79 (58.1%) | 27 (61.4%) | 52 (56.5%) | |
| Oral Language | 0.0001 | ||||
| No: | 4 (2.2%) | 33 (24.3%) | 1 (2.3%) | 32 (34.8%) | |
| Yes: | 170 (94.5%) | 85 (62.5%) | 42 (95.4%) | 43 (46.7%) | |
| Indefined: | 6 (3.3%) | 18 (13.2%) | 1 (2.3%) | 17 (18.4%) | |
| Family History Hearing Loss | 0.278 | ||||
| No: | 140 (77.8%) | 115 (84.6%) | 40 (90.9%) | 75 (81.5%) | |
| Yes: | 34 (18.9%) | 20 (14.7%) | 4 (9.1%) | 16 (17.4%) | |
| Missing: | 6 (3.3%) | 1 (0.7%) | 1 (1.1%) | ||
| Consanguinity | 0.443 | ||||
| No: | 171 (95%) | 127 (93.4%) | 43(97.7%) | 84 (91.3%) | |
| Yes: | 3 (1,7%) | 4 (2.9%) | 1 (2.3%) | 3 (3.3%) | |
| Missing: | 6 (3.3%) | 5 (3.7%) | 5 (5.4%) | ||
| Malaria | 0.07 | ||||
| No: | 72 (40%) | 41 (30.1%) | 11 (25%) | 30 (32.6%) | |
| Yes: | 103 (57.2%) | 91 (66.9%) | 33 (75%) | 58 (63.1%) | |
| Missing | 5 (2.8%) | 4 (3%) | 4 (4.3%) | ||
| Hearing Loss | |||||
| Normal: | 180 (100%) | 44 (32.3%) | 44 (100%) | ||
| Mild: | 16 (11.8%) | 16 (17.4%) | |||
| Moderate: | 19 (14%) | 19 (20.7%) | |||
| Severe: | 17 (12.5%) | 17 (18.5%) | |||
| Profound: | 40 (29.4%) | 40 (43.5%) | |||
| Right Ear | |||||
| Normal: | 180 (100%) | 19 (14%) | 19 (43.2%) | ||
| Mild: | 26 (19.1%) | 13 (29.5%) | 13 (14.1%) | ||
| Moderate: | 17 (12.5%) | 3 (6.8%) | 14 (15.2%) | ||
| Severe: | 17 (12.5%) | 2 (4.5%) | 15 (16.3%) | ||
| Profound: | 57 (41.9%) | 7 (15.9%) | 50 (54.3%) | ||
| Left Ear | |||||
| Normal: | 180 (100%) | 25 (18.4%) | 25 (56.8%) | ||
| Mild: | 18 (13.2%) | 8 (18.2%) | 10 (10.9%) | ||
| Moderate: | 19 (14%) | 1 (2.3%) | 18 (19.6%) | ||
| Severe: | 16 (11.8%) | 2 (4.5%) | 14 (15.2%) | ||
| Profound: | 58 (42.6%) | 8 (18.2%) | 50 (54.3%) | ||
| Hb | 0.743 | ||||
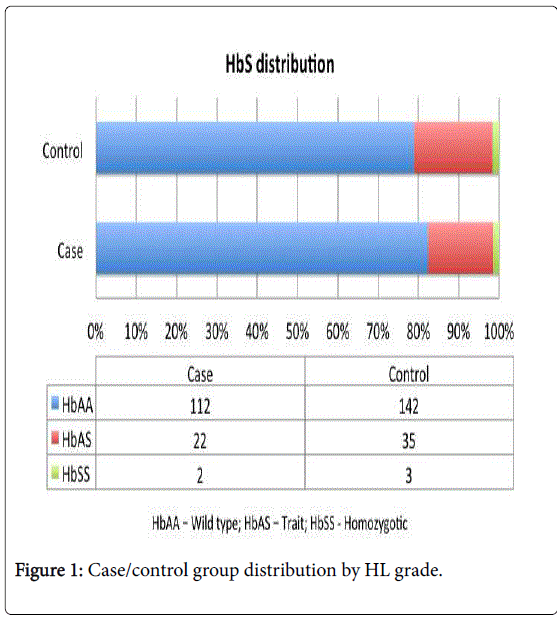
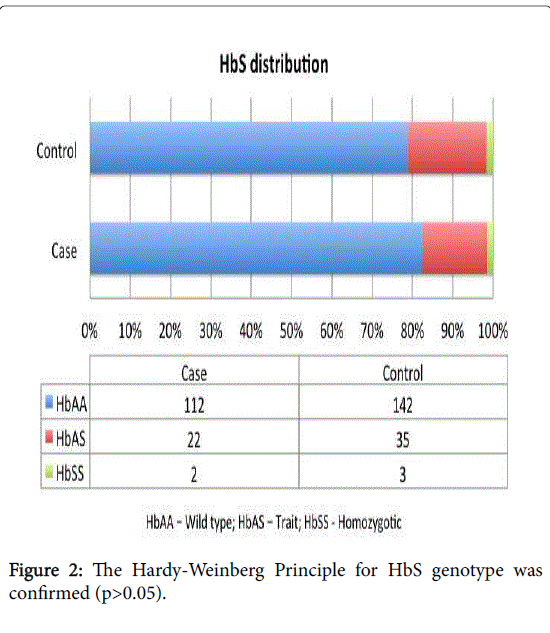
| HbAA: | 142 (78.9%) | 112 (82.3%) | 35 (79.5%) | 77 (83.7%) | |
| HbAS: | 35 (19.4%) | 22 (16.2%) | 9 (20.5%) | 13 (14.1%) | |
| HbSS: | 3 (1.7%) | 2 (1.5%) | 0 | 2 (2.2%) | |
| SD – Standard Deviation; HbAA - Wild Type; HbAS - Trait; HbSS - Homozygotic | |||||
Table 2: General characteristics for convenience sample of São Tomé and Príncipe population. Both groups (case-control) are homogeneous to gender (p=0.256) and age groups (p=0.361).
According to our results, HL is not associated with the district of origin (p=0.058) of each individual.
Both groups (case-control) are homogeneous to gender (p=0.256) and age groups (p=0.361).
According to our results, HL is not associated with the district of origin (p=0.058) of each individual.
Among the 316 subjects, we found 180 (57.0%) individuals with bilateral normal hearing, 44 (13.9%) unilateral HL (UHL) and 92 (29.1%) with bilateral HL (Figure 1). The individuals with UHL and bilateral HL were included in the case group.
The Hardy-Weinberg Principle for HbS genotype was confirmed based on the distribution of HbS genotype in control and case group (Figure 2).
When we calculate the media of the tone threshold, we found the left ear (40.02 dB) slight the same than the right (39.85 dB). The audiometric curve in the global sample has a horizontal shape, around mild HL.
The self-reported HL was confirmed by the audiometric evaluation, showing a positive association (p<0.0001) between both parameters. The tendency for self-reported HL was confirmed by the exams (ϕ=0.553; p<0.0001).
Considering oral language for communication, this was absent in 33 (24.3%) of the individuals from case group and in 4 (2.2%) of the control groups these showing a negative association (ϕ=-0.379; p<0.0001) between oral language and HL (p<0.0001). There was a tendency to have oral language in the control group.
A familial history of HL was found to be more prevalent in the control group than in the case group, respectively with 34 controls (18.9%) and 20 patients (14.7%). In the case group, 16 (80%) had bilateral HL and 4 (20%) unilateral HL.
From our sample 3 individuals (1.7%) in the control group and 4 patients (2.9%) in case group confirmed the existence of consanguinity in their families. Thus, no association between HL and consanguinity was performed.
In São Tomé and Principe we did not found any registry about history of malaria infection. At same time, we found that malaria infection is a public health problem and all individuals enrolled recognize malaria infection and can answer about their clinical history regarding this endemic infection. Self-report of malaria infection was more prevalent in the case group with 91 patients reporting a history of malaria (66.9%), being reported by 103 individuals (57.2%) of the control group.
The genotype distribution in our population was shown in Table 2. Concerning our results the HbAS showed no significant predisposition to HL (Table 3).
| Cases n (%) | Controls n (%) | P-value | Crude OR (95% CI) | P-value | Adjusted OR (95% CI) | |
|---|---|---|---|---|---|---|
| Age group | 0.362 | 0.07 | ||||
| [2-14]: | 69 (45.7%) | 82 (54.3%) | Reference | Reference | ||
| [15-35]: | 98 (59.4%) | 98 (59.4%) | 0.812 [0.520-1.269] | 0.633 [0.386-1.038] | ||
| Malaria infection | 0.071 | 0.021 | ||||
| No: | 41 (30.1%) | 72 (40%) | Reference | Reference | ||
| Yes: | 91 (66.9%) | 103 (57.2%) | 1.552 [0.964-2.497] | 1.840 [1.097-3.085] | ||
| HbS | 0.744 | 0.839 | ||||
| (HbAA) | 112 (82.3%) | 142 (78.9%) | Reference | Reference | ||
| (HbAS) | 22 (16.2%) | 35 (19.4%) | 0.449 | 0.797 [0.443-1.435] | 0.573 | 0.842 [0.463-1.530] |
| (HbSS) | 2 (1.5%) | 3 (1.7%) | 0.855 | 0.845 [0.139-5.145] | 0.832 | 0.821 [0.133-5.063] |
Table 3: Binary logistic regression between HbS and HL without and with risk factors (age group and history of malaria infection), HL: Normal hearing–0–Reference Category is a response variable; independent variables: HbS (0–HbAA wild type, 1- HbAS trait, 2–HbSS homozygotic), age groups (0–[2-14] years, 1–[15-35] years) and history of Malaria infection (0–no, 1–yes).
By applying the model of binary logistic regression the self-report of malaria was identified as a risk factor for HL (Table 3). The history of malaria infection almost doubled the risk of HL (OR=1.840; CI 95% [1.097-3.085]). We also found that, even not statistically significant, the oldest age group presented a decreased risk of HL in 36.7%.
For sickle cell trait we did not verify a significant predisposition to develop HL (OR=0.842; CI95% [0.463-1.530]) as sickle cell disease (OR=0.821; CI95% [0.133-5.063]).
Discussion
The results of this study revealed a prevalence of bilateral SNHL in 92 (29.1%) individuals and unilateral SNHL in 44 (13.9%) of subjects from our convenience sample. The unilateral and bilateral SNHL, accounting for 43% of the total sample, were included in the case study group since the unilateral SNHL also contribute to worse language acquisition and decreased school performance [20].
The horizontal audiologic curve in tonal audiogram was not specific or characteristic to a specific risk factor, meaning that inumerous causes may be responsible for SNHL in São Tomé and Príncipe and not one in particular.
Analysing the risk factors, the history family of HL and consanguinity were excluded from the analysis because they have a small number of cases. They haven’t significant association in contrast to what we are expecting, since has been reported the relevance of these factors in HL [21, 22].
The individuals included in this study rarely reported to have consanguineous parents, even knowing that São Tomé and Príncipe are small islands, with high probability of consanguinity.
São Tomé and Príncipe is a population of Sub-Saharan Africa in which several haemoglobinopathies have been identified, including HbSS as the most prevalent [7,23]. It would be expected to obtain an increased prevalence of HbS homozygous (HbSS) and increased HbAS [4,8,10,24] in the São Tomé and Príncipe population. However in our results, HbSS genotype were found only in 16% and 18% for HbAS of the sample.
The study model adopted did not confirm this assumption that the HbAS can promote SNHL. In our study we found a significant association between SNHL and a self-report of malaria. Although the patient provides the clinical malaria episode information, we considered their report valid. We must also consider that in the São Tomé Príncipe population exists a strong public awareness of Malaria infection as public health problem and standard procedures and guidelines for infection evaluation are followed, including the collection of thick blood film Plasmodium sp. in the presence of fever and illness.
Our results are supported by others, suggesting also that mild clinical malaria may also be associated with SNHL [25,26].
The malaria infection may trigger the onset of SNHL by pathophysiological processes, thromboembolism and release of inflammatory mediators [26,27]. On the other hand, malaria treatment adopted may be ototoxic. Some studies report reversibility of ototoxicity of some antimalarial [28–32]. In the specific case of children it does not apply [33] especially when is not performed a drug weight adjustment, and children ingest high doses of antimalarial therapy which may induce irreversible ototoxicity [32,33]. Eventually the higher association of the youngest patients of the case group with HL could be supported by the report of children malaria infection in this country during last 14 years [34,35].
The epidemiological profile of Malaria in São Tomé and Príncipe reveals a significant decrease of malaria admissions and deaths over 2006-2007 [34]. Since then, the children were most affected, representing a high proportion relatively to all patients. In 2003 they started some measures to control the disease witch include indoor residual spraying (IRS). In 2004 initiated the intermittent preventive treatment (IPT) with and sulfadoxine and pyrimethamine and changed the antimalarial policy with association of artesunate (AS) and amodiaquine (AQ), as first line treatment, in 2005 implemented the use of insecticide-treated nets (ITN) [34].
Our results support the hypothesis that the sickle cell trait (HbAS) acts as a protective role against malaria and SNHL in São Tomé and Príncipe.
Moreover, although São Tomé and Príncipe region is also affected by other HBB mutations, their prevalence is not relevant [23], justifying no screening for them in our study. There are others haemoglobin diseases, like deficiency of glucose-6-phosphate dehydrogenase, which ototoxicity is well known when combined with primaquine antimalarial therapy [36,37].
Conclusion
No association between sickle cell trait (HbAS) and the high prevalence of HL was found. However, our study suggests that in this sample, HbAS is preventing HL because is protecting against malaria. Malaria was found as a risk factor for the development of HL in São Tomé and Príncipe population. The multifactorial profile of HL and the horizontal audiologic curve, highly suggests the relevance of other etiologic factors than Malaria to justify the high prevalence of HL in São Tomé and Príncipe and further investigation must be applied.
Acknowledgement
Authors would like to thank to: Democratic Republic of São Tomé and Príncipe, Instituto Marquês de Valle Flôr (IMVF), Instituto Camões, Nova University School – Faculdade de Ciências Médicas de Lisboa, Fundação Calouste Gulbenkian, Mota&Engil, José de Mello Saúde and Audiologists from Hospital CUF Infante Santo (Diogo Ribeiro, Tânia Martins and Vera Lourenço).
This project was part of a PhD for the first author, with a research Grant from Jose de Mello Saúde.
The authors warmly dedicate this study to the memory of their colleague and friend Prof. Jorge Gaspar (1963-2015) who so much contributed with his knowledge and vision to this study.
Funding
Jose de Mello Saúde PhD Grant - to data collection and analysis
Competing Interests
There are no conflicts of interest.
Ethics Approval and Consent to Participate
The project was submitted and approved by the Medical Ethics Committee of São Tomé and Príncipe and Ethics Research Committee NMS|FCM-UNL (nº02/2014/CEFCM). The Ethics Research Committee is aligned with the Declaration of Helsinki for the Protection of Human Subjects. A full consenting process was applied in respect of all participants. Consent to use the survey data was also obtained.
References
- WHO (2013) Media centre: Millions have hearing loss that can be improved or prevented. WHO Media Cent 1–2.
- Olusanya BO, Neumann KJ, Saunders JE (2014) The global burden of disabling hearing impairment: A call to action. Bull World Health Organ 92: 367-373.
- Tucci D, Merson MH, Wilson BS (2010) A summary of the literature on global hearing impairment: Current status and priorities for action. Otol Neurotol 31: 31-41.
- Al Okbi MH, Alkindi S, Al Abri RK, Mathew J, Nagwa AA, et al. (2011) Sensorineural hearing loss in sickle cell disease--a prospective study from Oman. Laryngoscope 121: 392-396.
- Tine RCK, Ndiaye M, Hansson HH, Ndour CT, Faye B, et al. (2012) The association between malaria parasitaemia, erythrocyte polymorphisms, malnutrition and anaemia in children less than 10 years in Senegal: A case control study. BMC Res Notes 5: 565.
- López C, Saravia C, Gomez A, Hoebeke J, Patarroyo MA (2010) Mechanisms of genetically-based resistance to malaria. Gene 467: 1-12.
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, et al. (2010) Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun 1: 104.
- Mgbor N, Emodi I (2004) Sensorineural hearing loss in Nigerian children with sickle cell disease. Int J Pediatr Otorhinolaryngol 68: 1413-1416.
- Driss A, Hibbert JM, Wilson NO, Iqbal SA, Adamkiewicz TV, et al. (2011) Genetic polymorphisms linked to susceptibility to malaria. Malar J 10: 271.
- GarcÃa Callejo FJ, Sebastián Gil E, Morant Ventura A, Marco Algarra J (2002) Presentation of 2 cases of sudden deafness in patients with sickle-cell anemia and trait. Acta Otorrinolaringol Esp 53: 371-376.
- Malheiro JB, Morais JS (2013) São Tomé e PrÃncipe - Património Arquitetónico, Caleidoscó.
- Instituto Nacional de EstatÃstica ST e P, Saúde M da, Macro I (2010) São Tomé e PrÃncipe Inquérito Demográfico e Sanitário, IDS STP 2008-2009.
- Instituto Nacional de EstatÃstica ST e P (2012) Seminário de divulgação dos dados. In: IV Recens. Geral da Popul. e habitação 2012 (RGPH-2012. Instituto Nacional de estatistica São Tomé e PrÃncipe 1-100.
- Jerger J, Mauldin L (1978) Prediction of sensorineural hearing level from the brain stem evoked response. Arch Otolaryngol 104: 456-461.
- Gorga MP, Worthington DW, Reiland JK, Beauchaine KA, Goldgar DE (1985) Some comparisons between auditory brain stem response thresholds, latencies, and the pure-tone audiogram. Ear Hear 6: 105-112.
- van der Drift JF, Brocaar MP, van Zanten GA (1987) The relation between the pure-tone audiogram and the click auditory brainstem response threshold in cochlear hearing loss. Audiology 26: 1-10.
- Gorga MP, Johnson TA, Kaminski JK, Beauchaine KL, Garner CA, Neely ST (2006) Using a combination of click- and toneburst-evoked auditory brainstem response measurements to estimate pure-tone thresholds. Ear Hear February 27: 60-74.
- WHO (2013) Prevention of blindness and deafness-Grades of hearing impairment. In: WHO.
- Lieu JE, Tye-Murray N, Fu Q (2012) Longitudinal study of children with unilateral hearing loss. Laryngoscope 122: 2088-2095.
- Driscoll C, Beswick R, Doherty E, D'Silva R, Cross A (2015) The validity of family history as a risk factor in pediatric hearing loss. Int J Pediatr Otorhinolaryngol 79: 654-659.
- Zakzouk S (2002) Consanguinity and hearing impairment in developing countries: A custom to be discouraged. J Laryngol Otol 116: 811-816.
- Williams TN, Weatherall DJ (2012) World distribution, population genetics and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med 2: a011692.
- Aderibigbe A, Ologe FE, Oyejola BA (2005) Hearing thresholds in sickle cell anemia patients: Emerging new trends? J Natl Med Assoc 97: 1135-1142.
- Zhao SZ, Mackenzie IJ (2011) Deafness: malaria as a forgotten cause. Ann Trop Paediatr 31: 1-10.
- Schmutzhard J, Kositz CH, Lackner P, Dietmann A, Fischer M, et al. (2010) Murine malaria is associated with significant hearing impairment. Malar J 9:159.
- Schmutzhard J, Kositz CH, Lackner P, Pritz C, Glueckert R, et al. (2011) Murine cerebral malaria: Histopathology and ICAM 1 immunohistochemistry of the inner ear. Trop Med Int Health 16: 914-922.
- Gürkov R, Eshetu T, Miranda IB, Berens-Riha N, Mamo Y, et al. (2008) Ototoxicity of artemether/lumefantrine in the treatment of falciparum malaria: a randomized trial. Malar J 7: 179.
- Carrara V, Phyo AP, Nwee P, Soe M, Htoo H, et al. (2008) Auditory assessment of patients with acute uncomplicated Plasmodium falciparum malaria treated with three-day mefloquine-artesunate on the north-western border of Thailand. Malar J 7:233.
- Hutagalung R, Htoo H, Nwee P, Arunkamomkiri J, Zwang J, et al. (2006) A case-control auditory evaluation of patients treated with artemether-lumefantrine. Am J Trop Med Hyg 74: 211-214.
- Roche RJ, Silamut K, Pukrittayakamee S, Looareesuwan S, Molunto P, et al. (1990) Quinine induces reversible high-tone hearing loss. Br J Clin Pharmacol 29: 780-782.
- Claessen FAP, Van Boxtel CJ, Perenboom RM, Tange RA, Wetsteijn JCFM, Kager PA (1998) Quinine pharmacokinetics: Ototoxic and cardiotoxic effects in healthy Caucasian subjects and in patients with falciparum malaria. Trop Med Int Heal 3:482–489.
- Freeland A, Jones J, Mohammed NK (2010) Sensorineural deafness in Tanzanian children--is ototoxicity a significant cause? A pilot study. Int J Pediatr Otorhinolaryngol 74:516-519.
- World Health Organization (2010) [Sao Tome and Prinicple]. Jeune Afr 36:79.
- Greenwood BM, Bojang K, Whitty CJ, Targett GA (2005) Malaria. Lancet 365: 1487-1498.
- Goo YK, Ji SY, Shin H I, Moon JH, Cho SH, et al. (2014) First evaluation of Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency in vivax malaria endemic regions in the Republic of Korea. PLoS One 9:1-6.
- Recht J, Ashley E, White N (2014) Safety of 8-aminoquinoline antimalarial medicines. World Health Organization, Switzerland.
Citation: Caroça C, de Lima JP, Campelo P, Carolino E, Caria H, et al. (2016) Sickle Cell Trait, Malaria and Sensorineural Hearing Loss–A Case-Control Study from São Tomé and Príncipe. Otolaryngol (Sunnyvale) 6:278 DOI: 10.4172/2161-119X.1000278
Copyright: © 2016 Caroça C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5603
- [From(publication date): 0-2016 - Jul 18, 2025]
- Breakdown by view type
- HTML page views: 4636
- PDF downloads: 967