Research Article Open Access
Short-Term Changes in Humus Fungal Biomass, Mesofauna and CO2 Efflux Following Liming in a Microcosm Experiment
| Rizvi SHH1*, Gauquelin T1, Gers C2 and Baldy V1 | |
| 1Mediterranean Institute of Biodiversity and Ecology Marine and Continental, UMR CNRS 7263, IRD 237, Aix Marseille University, Downtown St Charles, Case 4, Build Natural Sciences, 3 Place Victor Hugo, 13331 Marseille Cedex 3, France | |
| 2Laboratory of Functional Ecology, UMR 5245 CNRS INPT, University Paul Sabatier, Bldg IVR3 - 118 route de Narbonne, 31062 Toulouse Cedex 4, France | |
| Corresponding Author : | Rizvi SHH Institute of Mediterranean Biodiversity and Ecology marine and continental UMR CNRS 7263, IRD 237, Aix Marseille University Downtown St Charles, Case 4, Build Natural Sciences 3 Place Victor Hugo, 13331 Marseille Cedex 3, France Tel: +923009694855 E-mail: hussain_haider77@yahoo.com |
| Received June 06, 2014; Accepted July 10, 2014; Published July 20, 2014 | |
| Citation: Rizvi SHH, Gauquelin T, Gers C, Baldy V (2014) Short-Term Changes in Humus Fungal Biomass, Mesofauna and CO2 Efflux Following Liming in a Microcosm Experiment. J Earth Sci Clim Change 5:208. doi: 10.4172/2157-7617.1000208 | |
| Copyright: © 2014 Rizvi SHH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Earth Science & Climatic Change
Abstract
Our objective was to test how liming influence fungal biomass, abundance and diversity of organisms belonging to mesofauna and soil respiration associated to soil during a microcosms experiment. Soil was collected in the Vosges Mountains (North-eastern France) on acidified forested catchments lying on sandstone and granite of Vosges Mountain. After liming, we followed CO2 by using LiCor 6400, fungal biomass by quantifying soil ergosterol content during 8 weeks. At the end of the experiment, we extracted mesofauna by using Berlese method. Soil pH, rain, temperature and rate of liming were similar to natural conditions. Liming decreased the abundance of mesofauna including collembola on sansdstone substrate, while on granite, collembola abundance decreased but total mesofauna did not show any significant differences between acid control and limed samples. The ergosterol soil content showed immediate increasing tendencies on both geological substrates and followed by decreasing tendencies on both geological substrates. The CO2 efflux was significantly increased by liming on sandstone and granite substrates, indicating an increase of soil respiration in limed samples. However, this result is depending on the geological substrate.
| Keywords |
| Acidified forested catchments; Mesofauna; Ergosterol; Microcosms; Soil respiration; Liming |
| Introduction |
| The concentration of atmospheric CO2 is increasing continuously along with atmospheric temperature [1]. Increasing atmospheric temperature has strong relation with CO2 concentration, starting with photosynthesis respiration, ecosystem processes i.e. nitrogen mineralization and growth and development of living organisms [2,3]. Evidence provided by IPCC [4] demonstrated that if the average atmospheric concentration of CO2 gets doubled, the average temperature of the global surface will increase from 1.5°C to 4.5°C approximately. |
| Soils of earth sphere contain 28% of global carbon [5], and can be a sink of atmospheric carbon [6,7], playing a very important role in atmospheric carbon in context of climate change [8,9]. |
| Uptake of carbon in Europe’s terrestrial biosphere is higher in comparison with carbon stocks in terrestrial ecosystems [10]. Net carbon sink ranged from 135 to 205 teragrams per year in European terrestrial biosphere, corresponding to 7 to 12% of 1995 anthropogenic carbon emission. |
| CO2 efflux of terrestrial surface, with 50-75 Pg of C per year, is an important contributor of global carbon cycle [11]. The CO2 efflux is directly or indirectly affected by many factors, i.e. temperature, humidity and microbial activity in soil and also by the atmospheric CO2. CO2 efflux of soil surface is a result of byproducts of many reactions undergoing in soil which partially/fully dependent upon the availability and production of CO2 which is transported through the soil profiles [11]. |
| Two major types of soil respiration co-occur: autotrophic and heterotrophic respirations. Heterotrophic respiration, i.e. release of CO2 by the soil microorganisms during soil organic matter decomposition [12], is maximum in organic layers and decrease by depth [13,14]. It also strongly depends on soil temperature and moisture, soil nutrients, through the biological activity [15]. |
| Liming to counteract soil acidification has numerous effects in soil including the pH [16,17], biological activity [18-20], which ultimately affect soil CO2 [15]. The CO2 can be elevated or decreased [21] in soil in situ by liming [22] through accumulative effect of moisture conditions, soil temperature and organic matter decomposition [23]. In the following years after liming the increase of CO2 proceeds downwards into OH horizon and into the mineral top soils [24]. |
| Moreover, it has been shown that the immediate change in soil pH value by liming [25] affects the prokaryotes communities which develop much faster than the fungal communities. These faster growing prokaryotes communities substitute the role of fungi as decomposers at higher level of pH values [25]. Zelles [26] and showed that the CO2 production increased under the elevating pH value of soil by liming. However, the adaptabilities of certain fungal species depend upon their ability to uptake nutrients, depending on the microclimate of soil after liming. However, there is still a controversy about the effect of liming on fungal biomass. Some studies reported significant effect of liming on fungal biomass [27], while others studies reported no significant effects of liming on fungal biomass [25]. Finally, Kjoller and Clemmensen [27] demonstrated that difference in fungal biomass by liming based on two major factors: fungal species and the geological nature of the substrate. |
| The aim of this study was to examine and compare the influence of liming on the soil fungal biomass, soil mesofauna and soil CO2 efflux during a microcosms experiment by using soils collected in acidified catchments of the Vosges Mountains (North-Eastern France), lying on two geological substrates i.e. sandstone and granite. To achieve that, half of the microcosms were limed while others were controlled for the both geological substrates. The layers of soil horizon, pH values and temperature were maintained in accordance with the natural conditions of the Vosges Mountains. The hypothesis is that liming increases the CO2 efflux through enhanced biological activity but this effect depends on geological substrate. |
| Materials and Methods |
| Experimental design |
| Thirty plastic containers were filled with the three organic horizons OL, OF and OH in two acidified catchments of the Vosges mountains: one lying on sandstone and one lying on granite. 12 containers (805 cm2) were used for sandstone, 12 for granite, and 6 containers were filled with sterilized soil (in order to evaluate the chemical reactions to liming) collected on both geological substrates (in same proportion in each container). These containers were perforated and then elevated to allow the drainage of extra water. For both catchments, we filled the containers firstly with a layer of sand in order to reduce the seepage of water, and then the layers of OL, OF and OH (or A) were laid down with thickness corresponding to what it has been observed on the field (sandstone 1.8 cm, 1.7 cm, 0.5 cm and granite 1 cm, 1.2 cm, 2.1 cm, respectively). For both substrates, six out of twelve containers (for nonsterilized soil) and three out of six (for sterilized soil) were limed with 2.5 t ha-1 of fine dolomite (CaMg[CO3]2), composed by 70%, 17%, 10% and 3% of CaCO3, MgCO3, CaSO4 and KCl, respectively. Remaining containers were controlled, e.g. without liming. The lime powder was dispersed homogenously by a simple powder-disperser. Artificial rain was elaborated based on the pH and chemical composition of the rain in Vosges forest (pH 5-5.5). All containers were watered at a rate of 1.4 l/week/container, according to the natural rainfall (100 mm per month) in Vosges forest [28] (Figure 1). The duration of the experiment was 8 weeks and soil samples were collected after 1, 14, 28, 32 and 34 days of liming. |
| Soil mesofauna |
| At the end of the experiment, content of all the 24 containers was placed on Berlese-Tullgren funnels in order to extract the soil mesofauna during 10 days and at ambient temperature (21 ± 2°C). After the extraction, soil samples were oven dried up to 24 hours at 80°C, in order to determine the % of humidity and to express the abundance in terms of numbers of organisms per g of soil dry mass. The arthropods were collected in small bottles half filled with ethanol 95%. Organisms were counted and identified to up to order level [29] and to species level for collembola [30]. |
| Carbon dioxide efflux |
| The CO2 efflux was measured in a series of seven readings by using a LI-COR Inc.(Lincoln, NE, USA), on the controlled, the limed containers, and also on the containers filled with sterilized soil to evaluate the part of CO2 efflux due to chemical reaction of liming on soil. A portable chamber (specialized for measuring soil CO2 efflux) was connected to LI-COR. This chamber was placed on pre-installed soil collars of the same dimension than the chamber (inside diameter 10 cm and of 6 cm height) during 2-3 min (one cycle of measurements). Each soil sample was measured 3-times and an average reading was taken. Measurement of CO2 efflux was based on linear increase (r²>0.95) of CO2 concentration in chamber over time. Flux tends to increase as the chamber CO2 concentration decrease. The LI-COR system is designed to draw down chamber CO2 concentration prior to measurement and to log data while CO2 concentration raises trough ambient concentrations. Artificial raining was done after every two days in order to keep the soil samples moist, and the CO2 efflux measurement was done approximately one day before the watering in order to have the constant conditions. |
| Soil fungal biomass |
| For determination of soil fungal biomass, a set of three soil samples were analyzed from each of the 24 containers. Soil samples were taken 5 times from OL, OF and OH, and then mixed and homogenized before sieved to 4 mm in order to integrate spatial heterogeneity. Fungal biomass associated with soil was estimated using ergosterol concentration. Ergosterol is a fungal indicator which offers an efficient measure of living fungal biomass [31]. Analyses were performed with 150 mg of lyophilized soil. Ergosterol was extracted from soil by 30 min refluxing in alcoholic base and purified by solid-phase extraction. Final purification and quantification of ergosterol was achieved by high-performance liquid chromatography (HPLC) on a HP series 1050 chromatograph. The system was run with HPLC-grade methanol at a flow rate of 1.5 ml min-1. Ergosterol eluted after 9 min and was detected at 282 nm; peak identity was checked on the basis of retention times of commercial ergosterol purchased from Flukas (98% purity). |
| Statistical analyses |
| Two-way analysis of variance (ANOVA) combined with Tukey test were used to study treatment (limed or acid control) and geological substrates (sandstone or granite bedrock) effects on the different soil parameters. If any interaction occurred between the two studied factors, one-way ANOVA was performed for each geological substrate to study liming effect. Test assumptions were verified before analysis. In some cases, normality and homocedasticity were not met and then we performed Mann-Whitney tests. All statistical analyses were conducted with the software Minitab and significant level was considered to be 95%. |
| Results |
| Soil mesofauna |
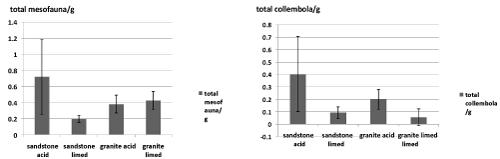
| The total abundance of mesofauna is significantly (one-way ANOVA, p<0.05) lower after liming on sandstone substrate, while on granite there is no significant (one-way ANOVA, p>0.05) change found by liming. Concerning collembola, we observe the same decrease after liming on sandstone and no change on granite (one-way ANOVA, p>0.05) (Figure 2). |
| Soil fungal biomass |
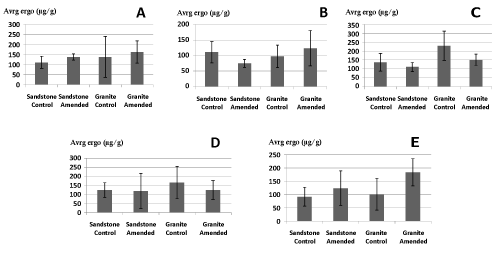
| Soil ergosterol content was higher in granite compared to sandstone (one-way ANOVA, p<0.05). Effect of liming depends on the sampling date and the geological substrate. 28 days after liming, there is a tendency to decrease on granite (one-way ANOVA, p=0.05) and no effect on sandstone. 34 days after liming, liming increased soil ergosterol content on granite (one-way ANOVA, p<0.05) but no significant effect of liming on sandstone is found (Figure 3, Table 1). |
| CO2 Efflux |
| The average CO2 efflux is significantly higher (Mann-Whitney test, p<0.05) in the limed microcosms for both geological substrates, i.e. sandstone and granite (Figure 4). For sterilized samples where measures begin only seven days after liming, we observe no significant difference between limed and non-limed containers (Figure 4). The main tendency is, both for granite, sandstone and sterilized containers, a high decrease of CO2 efflux in function of time. Differences between limed and not limed samples, very high in the beginning of the experiment, disappear after twenty days. |
| Discussion |
| From the beginning up to the end of experiment the soil ergosterol content showed fluctuating behavior on both substrates (sandstone and granite). Even if ergosterol contents depend to some extent on the physiological state of fungi, this membrane sterol is widely accepted as an indicator of total fungal biomass [31,32]. Thus, these fluctuations of soil ergosterol content by the reaction of liming could be the result of microclimate influence in form of soil pH [33]. Another factor which could explain these dynamics is mesofauna population, as at high mesofauna population densities, the microbial biomass could decrease [34,35]. According to our results, the decreased abundance of mesofauna in limed samples pointing out towards a fact of microbial activity domination, on both substrates (sandstone and granite). Different fungal species have different reactions to liming [36]. They also demonstrated that out of 150 fungal species, some found to be increased while other found to be decreased by liming which narrates the possible fluctuation of ergosterol’s behavior on different substrates. Shah et al. [18] narrated that liming increase bacterial biomass for short-period while fungal biomass remained unaffected but according to Zelles [25] results, liming significantly decreases the fungal biomass while increasing tendencies on bacterial biomass. As liming can affect fungal species richness [37], the decreased tendency of fungal biomass is due to increase in pH value [25] by liming, because some fungal species are not used by this increased pH, as different fungal species have different traits [25]. Increased pH could also increase activity of many prokaryotes which suppress the activity of fungi. |
| Decrease abundance of mesofauna, including collembola, on sandstone and granite substrate can be the result of cumulative effect of different factors after liming. Firstly, mesofauna are less efficient to adapt to the pH as liming negatively affected mesofauna abundance on both substrates. Secondly, as we described above, the microbial community could have changed with pH, and this change can in turn affect the mesofauna abundance [38,39]. As Rusek [40] pointed out, collembolans are dependant on microorganisms and the enhanced microbial activity in limed samples could be explained by significant decreased mesofauna abundance [25]. |
| Concerning CO2 efflux, the high increase observed from the beginning of the experiment in limed containers as well as the steady decline of this CO2 efflux in function of time strongly suggests than CO2 efflux is closely linked to the degradation of lime (CaCO3) by used artificial acid rain [41]. Thus, CO2 efflux linked to respiration and then to change in microbial activity or collembolan density seems difficult to demonstrate because this is masked by release of CO2 by lime. |
| Conclusion |
| Concerning fungal biomass and mesofauna, this experiment show that liming can affect very quickly these communities, liming appearing then like a perturbation. The effects are quite different according to the substrate and we find here the same difference between the two substrates than in situ. Concerning CO2 efflux, the experiment finally does not allow to study soil respiration because of the release of CO2 by lime degradation. It is however very interesting to see than the lime can be very quickly degraded by acid rain. This result can be really helpful for the choosing of the date of liming in situ. |
| Acknowledgements |
| This work was supported by the French ANR program (ANR Biodiversité, RECOVER-Project) and Higher Education Commission, Pakistan (HEC). |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
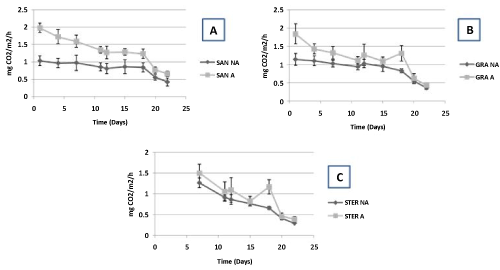 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Atmosphere
- Atmospheric Chemistry
- Atmospheric inversions
- Biosphere
- Chemical Oceanography
- Climate Modeling
- Crystallography
- Disaster Science
- Earth Science
- Ecology
- Environmental Degradation
- Gemology
- Geochemistry
- Geochronology
- Geomicrobiology
- Geomorphology
- Geosciences
- Geostatistics
- Glaciology
- Microplastic Pollution
- Mineralogy
- Soil Erosion and Land Degradation
Recommended Journals
Article Tools
Article Usage
- Total views: 14087
- [From(publication date):
September-2014 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 9479
- PDF downloads : 4608
