Research Article Open Access
Shanghai Cohort Study on Mild Cognitive Impairment: Study Desig n and Baseline Characteristics
Bin Zhou1*, Qianhua Zhao2, Shinsuke Kojima1, Ding Ding2, Yoji Nagai1, Qihao Guo3, Masanori Fukushima1 and Zhen Hong2,3
1Translational Research Informatics Center Foundation for Biomedical Research and Innovation, Kobe, Japan
2Institute of Neurology, Huashan Hospital, Fudan University, Shanghai, China
3Department of Neurology, Huashan Hospital, Fudan University, Shanghai, China
- Corresponding Author:
- Bin Zhou
Translational Research Informatics Center
Foundation for Biomedical Research and Innovation
1-5-4 Minatojimaminamimachi, Chuo-ku, Kobe, Japan 650-0047
Tel: +81-78-3039093
E-mail: zhoubin@tri-kobe.org
Received date: March 03, 2016; Accepted date: March 17, 2016; Published date: March 24, 2016
Citation: Zhou B, Zhao Q, Kojima S, Ding D, Nagai Y, et al. (2016) Shanghai Cohort Study on Mild Cognitive Impairment: Study Design and Baseline Characteristics. J Alzheimers Dis Parkinsonism 6:224. doi:10.4172/2161-0460.1000224
Copyright: © 2016 Zhou B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Objective: Establish cohort of patients with mild cognitive impairment (MCI) in order to identify the risk factors and validation of neuropsychological tests for early detection of Alzheimer disease.
Methods: Four hundred participants with MCI were enrolled in at Huashan Hospital in Shanghai, China. All patients will be followed once annually for 3years. The primary endpoint is the time of conversion from diagnosis of MCI to Alzheimer disease (AD).
Results: of the 400 subjects, 170 (42.5%) were male, and the average mean age and education was 68.7 (Standard Deviation, SD: 8.2, 45- 90 years old) and 12 years (SD: 3.1), respectively. At baseline age, education and being single influenced MMSE, ADAS-cog13 and most domains of cognition.
Conclusions: Baseline characteristics of the Shanghai MCI cohort study indicate the participants were younger, less educated and had a moderate gender ratio compared to other studies conducted so far and represent the target population. 3 years later when the cohort will be finished some risk factors Associated with the conversion will be validated and some novel factors will be identified.
Keywords
Alzheimer disease; Mild cognitive impairment; Cohort study; Design; Baseline characteristics
Introduction
Alzheimer’s disease (AD) is a progressive, neurodegenerative disease characterized by loss of memory and other cognitive function. Currently, there is no effective treatment. Although the cause of the disease is still unknown, basic research indicates that Beta amyloid accumulation is a crucial early player in disease pathogenesis, ultimately leading to the onset of dementia [1]. Amyloid precursor protein (APP) cleaving enzyme 1 (BACE1), the enzyme that initiates Aβ production by cleaving the extracellular domain of APP or antibody to clear beta amyloid, has been the target of drug development for decades. Unfortunately, the several inhibitors of BACE1 and immunotherapy used to lower cerebral Aβ concentrations and treat Alzheimer disease that have been tested in human clinical trials failed to show effectiveness, and many questions remain about the safety of these drugs [2]. 99% of Alzheimer drug trials in the past decade have failed [3], and there is an ‘urgent’ need to improve therapies.
Recent clinical research, histopathology and amyloid imaging results suggest that amyloid deposition might begin more than a decade before the appearance of cognitive deficits and diagnosis of Alzheimer disease [4,5]. Targeting the production of beta amyloid at the prodromal stage in order to prevent the onset of dementia may be an important strategy for conquering the disease. However, the MCI based on current diagnostic criteria showed MCI is heterogeneity. That MCI progresses to Alzheimer disease at a rate of roughly 10–15% per year depending on the clinical diagnosis of MCI [6]. It remains uncertain how to identify the right subjects in such a preventive approach. Individual risk estimation based on amyloid imaging (PiB) remains difficult because many normal control individuals have a brain amyloid increased the risk of cognition decline. Predictors with high sensitivity and specificity for the early detection of Alzheimer disease are always of interest for research. Zhou’s analysis using ADNI data indicated the combination of neuropsychological tests of clinical rating scale and ADAS-cog can predict the conversion rate of 92.7% (95%CI: 82.4-100.00), which is better than single cerebrospinal fluid Abeta42 or MRI findings [7].
The objectives of the Shanghai cohort study on mild cognitive impairment for the early detection of Alzheimer disease were to explore the factors associated with the conversion from MCI to AD, to identify any special characteristics of the factors in Chinese people and to confirm the findings in ADNI for the global development of drug clinical trial. We conducted an MCI cohort at Huashan Hospital in Shanghai based on routine clinical practice. Here, we report the design and baseline characteristics of the subjects enrolled.
Material and Methods
Study design
The study is a prospective cohort study that included 400 subjects with MCI and a follow-up once annually for three years. The objectives are to identify individuals with MCI who convert to AD and to explore factors associated with the conversion. The observation time point is every 12 months and phone interview on 6th, 18th month. The primary endpoint was the time from diagnosis to the conversion from MCI to Probable AD Dementia. The secondary endpoints are the time to conversion from MCI to “Possible AD Dementia” or “Probable AD Dementia”, time to Conversion from MCI to “All-cause Dementia”, Overall survival, Changes in Neuropsychological examinations and Changes in MRI from baseline to the end of follow-up. The planned research duration was from Jan 2012 to Dec. 2016. The study is also registered in clinicatrial.gov as NCT01552265.
Subjects
Inclusion/exclusion criteria: MCI was diagnosed according to NIA criteria [8]. The inclusion criteria used are as follows: 1. Cognitive concern reflecting a change in cognition reported by the patient, informant or clinician; 2.Mini-Mental State Examination (MMSE) scores between 18-30 (inclusive); 3. Clinical Dementia Rating (CDR) = 0.5; Memory Box score of at least 0.5; 4. Essentially preserved the activities of daily living based on the Alzheimer’s Disease Cooperative Study - Activities of Daily Living (ADCS-ADL); 5. Does not meet criteria for dementia [9]; 6. Willing to and able to undergo all test procedures, including neuroimaging, and agreed to longitudinal follow up; 7. Written informed consent for participating in this study was obtained. Exclusion criteria: 1. Obvious vascular causes of cognitive decline; 2.Traumatic causes of cognitive decline; 3. Medical causes of cognitive decline (e.g. use of psychoactive medications, alcohol abuse and some types of disease including depression and epilepsy); 4. History of other diseases or unstable conditions which could lead to difficulty in complying with the protocol.
Enrollment: From February 2012 to June 2014, a total of 400 participants with mild cognitive impairment patients were enrolled in Huashan Hospital located at Fudan University in Shanghai, China. All participants underwent detailed informed consent procedures and provided written consent in accordance with the procedures approved by the institutional review boards on Jan. 12th, 2012 at Huashan Hospital and Dec. 9th, 2011 at Translational Research Informatics Center (TRI).
Study procedure
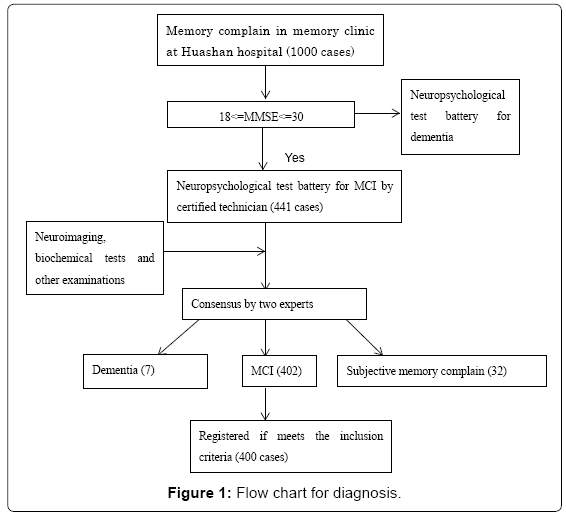
Clinical examination: The procedure of screening and diagnosis is presented in the follow-chart. At the memory clinic in Huashan Hospital, first the patients are interview by neurologist at memory clinic of extensive clinical examination and brief neuropsychological tests and then recommended to neuropsychological tests, blood laboratory and 3D MRI according to the results of first interview. Medical history, and family history. Information pertaining to the activities of daily living was gathered via clinical interviews of the patient and caregiver. Information of degree of education total education years, marital status, medical history (hypertension, diabetes, stroke, myocardial infarction, head injury, general anesthesia, depression), smoking and drinking status were collected. Type, frequency and period of physical activities was gathered via interview of the patient and caregivers. Neurological system and general physical examinations were performed although the data were not input in the database.
Neuropsychological assessment: Participants underwent comprehensive cognitive examination during the baseline and followup visits. The neuropsychological assessments included the MMSE, Rey auditory-verbal learning test (RAVLT), logical memory (LM) subtest of the Wechsler memory scale (WMS), Stroop color-word conflict test (SCWT), Rey-Osterrieth complex figure test (CFT), verbal fluency test (VFT)], trail-making tests A and B (TMTA and TMTB), symbol digit modalities test (SDMT), clock drawing test (CDT), Boston naming test (BNT), clinical dementia rating (CDR) and ADAS-cog13. RAVLT included 5 sub-tests—RAVLT1, RAVLT2, RAVLT3, RAVLT4, and RAVLT5. The LM test included the assessment of immediate recall (LM I) and delayed recall (LM II) of a short story, for which the cut-offs were 7 and 5 for the diagnosis of dementia, respectively. The Stroop color-word conflict test included the 6 sub-tests of Atime, Acorrect, Btime, Bcorrect, Ctime and Ccorrect. The CFT had 2 components which are CFT-copy and CFT-delayed, respectively. VFT included the assessment of the correct number of animals (VFT1) and vegetables (VFT2) identified within 1 min. The results of TMTA and TMTB indicate the mean time taken to complete parts A and B. All neuropsychological tests have been validated for use and have a cut-off point for dementia according to the age group in the Chinese population [10]. Neuropsychiatric Inventory (NPI) and Center for epidemiological survey depression scale(CESD-C) were used to evaluated general psychiatric behavior and status of depression, respectively. The score of 13 items in ADAS-cog (ADAS-cog13) and sum of six boxes of CDR(CDR-sob) were used in analysis.
Diagnostic consensus conference: The primary endpoint is the time from diagnosis to the conversion of AD. The secondary endpoints are Time to Conversion from MCI to “Possible AD Dementia” or “Probable AD Dementia”
Time to Conversion from MCI to “All-cause Dementia”, overall survival, changes in Neuropsychological examinations and Changes in MRI. At baseline after all examinations, a consensus diagnosis and enrollment was made by two experts, then the subject was registered. During follow-up the data collected were reviewed and diagnosis was made by an international committee that includes 3 experts from China and Japan. Diagnostic consensus conferences were held at Huashan Hospital monthly to assess potential clinical core enrollees for cognitive diagnoses. These meetings were designed to ensure that enrollees meet the criteria for MCI diagnostic criteria and increase inter-rater reliability among clinicians, a neuropsychologist, behavioral neurologist, and study support personnel.
The Diagnosis of dementia and AD are according to DSM-IV (Diagnostic and Statistical Manual of Mental Disorders fourth edition) and McKhann’s criteria [9], respectively. If the test of delayed recall of the Wechsler Memory Scale and logical memory showed evidence of memory impairment, the subject was classified as amnestic MCI (aMCI). There are two sub-types of aMCI, single-domain (s-aMCI) and multiple-domain (m-aMCI), based on the number of domains impaired.
Laboratory measures/ imaging: Laboratory tests include chemical panel tests, complete blood count and Lipid Profile. MRI neuroimaging was collected via standard manualized procedures to ensure uniform collection. All patients had Siemens MRI scan to collect the Whole brain volume, hippocampus volume (R, L),temporal lobe volume (R, L), entorhinal cortex volume (R, L) are calculated. GE 1.5T• 14.0M4 TwinSpeed 8Ch,• 14.0M4 TwinSpeed 8Ch Phantom. GE 3.0T• 3T 14.0M4 TwinSpeed 8Ch• 3T 14.0M4 TwinSpeed 8Ch Phantom.
Data entry and data center: Cognitive and clinical data was reviewed by the psychometrist and study nurse and verified at the clinical consensus meeting. All data was input at Huashan Hospital via a web-based CRF with a data center at the Translational Research Informatics Center in Kobe, Japan. Source data verification (SDV) of the baseline data was conducted to ensure the research quality.
Analysis
The sample size is 400. The calculation was based on the conversion rate of about 12% per year based on prior data in the Huashan hospital (unpublished data). From the experience in the Huashan hospital, a total of 400 subjects may be enrolled during 2 years. If 400 subjects are enrolled for this study, a width of 95% confidence interval for the conversion rate is 9.8% (± 4.9%) at a maximum unless the conversion rate equals to 0 or 100%.)
Distribution of the test scores were assessed by the mean and standard deviation (SD) for the sample as a whole.
A composite Z score of the five cognitive domains was calculated to evaluate the factors associated with cognition. The five domains were memory (RAVLT5, RAVLT1-4, LMI &II, CFT- delayed recall), spatial processing (CFT-copy and CDT), language (AFT animal, BNT), attention (SDMT, TMT-A), and executive function (SCWT-C-time, SCWT-C-right, TMT-B).
Z score = (sample value-sample mean)/standard deviation. The z scores of the tests in each domain mentioned above were averaged to obtain a z score for all domains. T-test exact tests were used to determine the differences in demographic factors and measure each neuropsychological domain and global cognition. The higher score indicated good performance in AVLT, CFT-copy and delayed, CDT, AFT, SDMT and BNT; bad in TMT and SCWT. All analyses were conducted using SAS (9.0 SAS Corp.)
T-tests or ANOVA were used to assess the relationship between baseline demographic factors associated with cognition. Multiple linear regression models fit with the independent effects on each domain, and the three comprehensive tests MMSE, CDR-sob and ADAS-cog13. Factors which showed significant by univariate analysis were determined into multiple stage. Analyses were conducted in SAS version 9.3 (SAS Institute Inc, Cary, North Carolina).
Results
441 patients in the memory clinic in Huashan Hospital in Shanghai were screened, and 400 diagnosed with MCI were enrolled from February 2012 to June 2014. Figure 1 shows the flow chart of screening and diagnosis.
Cohort demographics and distribution of neuropsychological features
Tables 1-3 provide the demographic and neuropsychological features of the total cohort, respectively. Of the 400 subjects, 170 (42.5%) were male, and the average mean age was 68.7 (SD 8.2, ranged from 45 to 90 years old). The average education level was 12 years (SD: 3.1), and 5.3%, 27.8%, 32% and 365, respectively, graduated from elementary school, junior high school, senior high school and university or higher. The top three medical history diseases were hypertension (41.8%), diabetes (13.5%) and general anesthesia (10.5%). Average education was 12 years (SD: 3.1). Almost 80% never smoked or drank alcohol.
| Characteristics | N. (%) or mean (SD) |
|---|---|
| Female | 230 (57.5) |
| Age (years) | 68.7 (8.2) |
| Education | 12.0 (3.1) |
| Elementary school | 17 (4.3) |
| Junior high school | 110 (27.6) |
| Senior high school | 127 (31.9) |
| University and above | 144 (36.2) |
| Hypertension | 167 (41.8) |
| Diabetes | 54 (13.5) |
| Stroke | 14 (3.5) |
| Myocardial infarction | 2 (0.5) |
| Head injury | 15 (3.8) |
| General anesthesia | 42 (10.5) |
| Depression | 7 (1.8) |
| BMI | 22.9 (3.1) |
| Family history of AD | 56 (14.0) |
| Smoking status | |
| Never | 320 (80.0) |
| Former | 54 (13.5) |
| Current | 26 (6.5) |
| Drinking status | |
| Never | 318 (79.5) |
| Former | 39 (9.8) |
| Current | 43 (10.8) |
| Marital status | |
| single | 43 (10.7) |
| married | 357 (89.3) |
| Live alone | 21 (5.3) |
| ApoE4 | |
| 0 | 268 (68.2) |
| 1 | 99 (25.2) |
| 2 | 26 (6.6) |
Table 1: Baseline characteristics.
| Item | N | Mean (SD) | Median |
| MMSE | 400 | 26.5 (1.9) | 30 |
| CDR-sob | 400 | 1.6 (1.1) | 1.5 |
| Logical memory I-C* | 399 | 4.9 (2.8)* | 5.0 |
| Logical memory II-C* | 399 | 3.4 (2.7)* | 3.0 |
| AVLT-long-term delayed recall* | 400 | 2.0 (2.0) | 2.0 |
| AVLT-recognition* | 400 | 17.5 (3.7)* | 18.0 |
| AVLT-Total N1~ N5* | 400 | 15.8 (6.8) | 15.0 |
| CESD-C | 400 | 8.8 (10.9) | 5.0 |
| ADAS-cog13 | 400 | 20.1 (6.3) | 68.0 |
| Category fluency test-C : animals | 400 | 13.8 (3.8) | 14.0 |
| Category fluency test-C : cities | 400 | 12.1 (3.9) | 12.0 |
| Boston naming test | 400 | 20.1 (4.7) | 21.0 |
| Clock Drawing Test* | 400 | 21.2 (5.3) | 22.0 |
| Trail Making Test -A (Sec) | 398 | 87.3 (42.0) | 76.0 |
| Trail Making Test- B (Sec) | 394 | 234.7 (101.9) | 222.5 |
| Symbol Digit Modalities Test | 398 | 27.8 (10.8) | 28.0 |
| Rey-Osterrieth CFT-C - Copy | 400 | 32.3 (4.5) | 33.5 |
| Rey-Osterrieth CFT-C -Delayed Recall | 398 | 10.0 (6.8) | 9.0 |
| Stroop-C color-word (Sec) | 393 | 97.9 (33.6) | 92.0 |
| Stroop-C color-word (number) | 399 | 40.0 (9.6) | 43.0 |
| NPI | 224 | 5.1 (7.9) | 1.0 |
| NPI of caregiver distress | 224 | 1.2 (2.8) | 0.0 |
| ADCS-ADL: Total Score | 400 | 67.6 (5.9) | 68.0 |
| Brain Venticle Volume (mm3) | 262 | 37234.56 | 32736.45 |
| Left-Hippocampus (mm3) | 262 | 3392.21 | 3389.3 |
| Right-Hippocampus (mm3) | 262 | 3624.89 | 3651.5 |
| lh_entorhinal_volume (mm3) | 262 | 1508.76 | 1529 |
| rh_entorhinal_volume (mm3) | 262 | 1422.78 | 1383.5 |
| Left-Temporal Lobe (mm3) | 262 | 45656.30 | 45930.5 |
| Right-Temporal Lobe (mm3) | 262 | 46217.69 | 46069.5 |
| Whole Brain Volume (mm3) | 262 | 1027436.14 | 1022962 |
* modified in the Shanghai cohort.
Table 2: Distribution of neuropsychological tests and brain volume at baseline.
| Age group N (%) | ||||
|---|---|---|---|---|
| Education | ≤65 | 65-74 | ≥75 | Total |
| Elementary school | 12 (3.0) | 4 (1.0) | 1 (0.3) | 17 (4.3) |
| Junior high school | 56 (14.1) | 39 (9.8) | 15 (3.8) | 110 (27.6) |
| Senior high school | 32 (8.0) | 71 (17.8) | 24 (6.0) | 127 (31.9) |
| University | 16 (4.0) | 73 (18.3) | 55 (13.8) | 144 (36.2) |
| Total | 116 (29.2) | 187 (47.0) | 95 (23.9) | 398 (100.0) |
Table 3: Distribution of age and education.
The average scores of MMSE, CDR-sob and ADAS-cog were 26.5 (SD: 1.9), 1.6 (SD: 1.1) and 20.1 (SD: 6.3), respectively.
The distribution of age crossed with education in the whole cohort of 400 cases divided into the three age groups of ≤ 65, 65~74 and ≥ 74 years old was 116 (29.2%), 187 (47.0%) and 23.9%, respectively. Subjects with senior high school or higher education numbered 127 (31.9%) and 144 (36.2%). In the subjects educated above university level, 40% of those were aged over 75 years greatly exceeded by more than 11% those aged less than 65 years.
The association of demographic factors and medical history with cognition
Language was influenced factors of sex, education and living alone; memory by age and living alone; visuospatial by education; attention by sex and education; executive function by age and sex. No one factor involved in the study, such as sex, age and education, was associated with CDR-sob. MMSE was influenced by education and living alone more than 5 years; ADAS-cog 13 by age and marital status (Table 4).
| Factors | Cognition Mean (SD) | ||||||||
| N | MMSE | CDR-sob | ADAS-cog | Memory | Spatial | Language | Attention | Executive | |
| Age | |||||||||
| <65 | 116 | 26.65 (1.76) | 1.53 (1.00) | 18.36 (5.92)* | 0.16 (0.81)* | 0.05 (0.74) | -0.04 (0.79) | -0.04 (0.45) | -0.15 (0.52)* |
| 65-75 | 188 | 26.52 (1.87) | 1.55 (1.02) | 20.56 (6.33) | -0.01 (0.78) | 0.05 (0.78) | 0.03 (0.77) | -0.00 (0.45) | 0.06 (0.53) |
| ≥ 75 | 96 | 26.45 (1.98) | 1.81 (1.18) | 21.41 (6.25) | -0.17 (0.70) | -0.17 (0.91) | -0.01 (0.83) | 0.05 (0.48) | 0.04 (0.53) |
| Sex | |||||||||
| Male | 170 | 26.55 (1.9) | 1.62 (1.10) | 20.55 (5.78) | -0.02 (0.74) | 0.05 (0.87) | 0.19 (0.81)* | -0.06 (0.41)* | 0.08 (0.62)* |
| Female | 230 | 26.53 (1.84) | 1.60 (1.03) | 19.81 (6.63) | 0.01 (0.81) | -0.03 (0.76) | -0.14 (0.75) | 0.05 (0.49) | -0.07 (0.46) |
| Education | |||||||||
| <Elementary | 17 | 25.88 (1.69)* | 1.59 (0.91) | 21.35 (9.31) | 0.09 (0.79) | -0.53 (0.83)* | -0.72 (0.76)* | -0.12 (0.63)* | 0.09 (0.41) |
| Junior | 111 | 26.47 (1.84) | 1.64 (1.14) | 19.86 (5.37) | -0.07 (0.80) | -0.16 (0.81) | -0.25 (0.70) | -0.13 (0.42) | -0.11 (0.53) |
| Senior | 128 | 26.16 (1.78) | 1.63 (0.99) | 19.58 (6.03) | -0.03 (0.78) | 0.07 (0.59) | 0.03 (0.74) | 0.01 (0.45) | 0.04 (0.48) |
| University | 144 | 27.01 (1.88) | 1.56 (1.08) | 20.68 (6.74) | 0.07 (0.75) | 0.12 (0.92) | 0.25 (0.80) | 0.10 (0.45) | 0.02 (0.59) |
| Live alone | |||||||||
| No | 377 | 26.53 (1.87) | 1.61 (1.05) | 20.10 (6.18) | -0.01 (0.77) | 0.00 (0.80) | -0.02 (0.79)* | -0.01 (0.45) | -0.01 (0.54) |
| yes | 21 | 26.81 (1.63) | 1.36 (1.11) | 20.90 (8.34) | 0.13 (0.81) | 0.01 (0.90) | 0.35 (0.65) | 0.11 (0.54) | 0.02 (0.51) |
| Years living alone | |||||||||
| <5 | 10 | 27.7 (0.95)* | 1.05 (0.76) | 18.40 (7.26) | 0.59 (0.58)* | 0.17 (0.80) | 0.38 (0.86) | 0.16 (0.59) | -0.11 (0.49) |
| ≥ 5 | 11 | 26.0 (1.73) | 1.64 (1.32) | 23.18 (8.93) | -0.29(0.79) | -0.15 (1.00) | 0.33 (0.43) | 0.07 (0.52) | 0.15 (0.53) |
| Marital status | |||||||||
| Single | 43 | 26.28 (1.62) | 1.62 (1.15) | 22.09 (8.18)* | 0.06 (0.85) | -0.21 (0.96) | -0.12 (0.84) | 0.07 (0.55) | 0.14 (0.61) |
| Married | 357 | 26.57 (1.89) | 1.61 (1.05) | 19.89 (5.99) | -0.01 (0.77) | 0.03 (0.79) | 0.02 (0.78) | -0.01 (0.45) | -0.02 (0.53) |
* p < 0.05
Table 4: Demographic factors associated with cognition by univariate analysis.
Multivariable models
Based on the results of the univariate analysis, five factors were included in the multiple linear regression models. In Table 5 the results show that age and education influenced MMSE and most of the domains of cognition. In addition, being single was associated with poor performance in ADAS-cog13 and executive function and as well as poor attention and executive function in males; CDR-sob was not influenced by any of the factors included in the study used in multiple models.
| Factors | Covariate | Coefficient | SE | P |
|---|---|---|---|---|
| MMSE | Age | -0.025 | 0.012 | 0.045 |
| Gender | 0.107 | 0.192 | 0.578 | |
| Education (year) | 0.107 | 0.032 | 0.001 | |
| Live alone | 0.813 | 0.536 | 0.130 | |
| marriage | 0.579 | 0.389 | 0.138 | |
| CDR-sob | Age | 0.011 | 0.007 | 0.093 |
| Gender | 0.009 | 0.110 | 0.937 | |
| Education (year) | -0.008 | 0.019 | 0.686 | |
| Live alone | -0.476 | 0.308 | 0.123 | |
| marriage | -0.198 | 0.223 | 0.376 | |
| ADAScog13 | Age | 0.148 | 0.042 | 0.0004 |
| Gender | -0.746 | 0.650 | 0.252 | |
| Education (year) | -0.069 | 0.109 | 0.530 | |
| Live alone | -2.123 | 1.813 | 0.242 | |
| marriage | -0.286 | 1.317 | 0.030 | |
| memory | Age | -0.020 | 0.005 | 0.0001 |
| Gender | 0.018 | 0.080 | 0.823 | |
| Education (year) | 0.029 | 0.014 | 0.03 | |
| Live alone | 0.130 | 0.224 | 0.564 | |
| marriage | -0.072 | 0.163 | 0.661 | |
| visuospatial | Age | -0.023 | 0.005 | 0.001 |
| Gender | -0.028 | 0.082 | 0.734 | |
| Education (year) | 0.064 | 0.014 | 0.001 | |
| Live alone | 0.317 | 0.229 | 0.169 | |
| marriage | 0.292 | 0.166 | 0.080 | |
| language | Age | -0.017 | 0.005 | 0.0007 |
| Gender | -0.243 | 0.077 | 0.002 | |
| Education (year) | 0.080 | 0.213 | 0.0004 | |
| Live alone | 0.759 | 0.213 | 0.0004 | |
| marriage | 0.363 | 0.155 | 0.020 | |
| attention | Age | -0.002 | 0.003 | 0.494 |
| Gender | 0.159 | 0.046 | 0.0007 | |
| Education (year) | 0.038 | 0.008 | <0.001 | |
| Live alone | 0.035 | 0.130 | 0.785 | |
| marriage | -0.048 | 0.094 | 0.615 | |
| executive | Age | 0.009 | 0.003 | 0.011 |
| Gender | -0.152 | 0.055 | 0.006 | |
| Education (year) | -0.002 | 0.009 | 0.838 | |
| Live alone | -0.205 | 0.154 | 0.185 | |
| marriage | -0.259 | 0.112 | 0.021 |
Table 5: Multiple linear regression models of the associations of factors with cognition.
Discussion
There have been several large studies on mild cognitive impairment launched around the world to explore the risk factors and establish early diagnosis criteria. Compared to the five studies [9] showed in the appendix, the four hundred subjects enrolled in the Shanghai MCI cohort study were younger with an average age of 68.7 (SD: 8.2) and moderately female (57.5%), and there were fewer subjects with higher education (36.2%).
To know the extent of and identify the impairment is always the goal of such MCI cohort studies. Therefore, the neuropsychological tests used to identify the level and severity of impairment, which can differ substantially depending on the stage of cognitive impairment, are important. In our study, we used the MMSE, CDR, ADAS-cog13, AVLT, LM, CDT, TMT and AFT tests. These tests are often used to evaluate the domains of cognition. We also selected the CWT-C and CFT tests to measure executive function and spatial processing.
Longitudinally, the aim of the Shanghai MCI cohort study is to better characterize patients with MCI for additional clinical research studies with the objective of analyzing promising biomarkers for the early diagnosis of AD and validation of some findings of neuropsychological tests and MRI findings in ADNI as a foundation for clinical trials. Although many of the long term goals of this study are to identify the right population of MCI cases who convert to AD and the right time for intervention, use of a study design that is not population-based can limit the generalizability of certain results to the wider population. For example, the study enrolled participants with varying levels of disease severity, which restricts our ability to track the natural history of cognitive impairment in MCI from the time of disease onset. However, the large sample size at an early stage of cognitive impairment will undoubtedly allow us to describe the cognitive impairment trajectory for people with varying levels of cognitive impairment. Lack of beta amyloid imaging and cerebrospinal fluid data is one limitation, instead we focus on the neuropsychological tests.
The subjects in the Shanghai cohort study performed worse on the CDT, BNT, TMTA and B tests than that in ADNI. Lower average education is one of the reasons for such poor performance based on the fact our study showed that education, while associated with some domains of cognition, has no impact on CDR-sob or ADAS-cog13. In terms of the risk factors associated with cognition, we found that education influenced the three domains of language, attention and spatial processing. Memory, executive function and spatial processing are affected by one of either education or sex, respectively. It is well known that most neuropsychological tests are influenced by age and education [11-13]. These two factors were not found to be associated with the CDR-sob score, so this test can be used generally in any population without consideration for the impact of age or education. It is known that higher education decreases the risk of dementia. However, poor performance in some single neuropsychological tests, one domain, such as episodic memory [14-16], or in a combination of several tests predict conversion from MCI to AD and future decline of MCI [17,18]. Whether or not the influenced domains in our study predict incidence of dementia better than others is a challenge that remains for future research.
Females are consistently associated with higher levels of dementia than males. In our study, female subjects accounted for 57.7% of all subjects, and they performed worse than males in the domains of language and executive function. Whether or not differences in sex in the domains of cognitive impairment indicate future differences in the conversion from MCI to AD will be bettered answered after the followup of the cohort.
Compared with single subjects, married subjects performed better at ADAS-cog13 and spatial processing but performed worse at executive function. Our results differed from the Brenowitz [19] study in that compared with married participants, risk of MCI was significantly lower in widowed participants but not for divorced/separated or never-married participants. Compared to living with a spouse/partner, risk of MCI was significantly higher for subjects living with others but not for subjects living alone [20]. Although this was a clinic-based cohort and thus may not represent the community at large, it is worth noting that such factors affect some domains of cognition. Further research is needed to clarify if such factors increase the risk of MCI and/or dementia.
The Shanghai MCI cohort study is a clinically and cognitively well-characterized cohort of patients with MCI. To date, the enrolled cohort has provided baseline, cross sectional data that enhances the understanding of the extent of cognitive impairment present in MCI and progression from MCI to AD, as well as a foundation for longitudinal examination of the relationship between cognition and neuro-imaging biomarkers. Age and education influenced MMSE, ADAS-cog13 and most domains of cognition. Several social demographical factors such as living alone and being single were associated with some domains of cognition. CDR is not influenced by such factors.
Conclusion
Baseline characteristics of the Shanghai MCI cohort study indicate a younger, lower educated and moderate gender ratio compared to other studies and represent the target population. 3 years latter when the cohort will be finished some risk factors associated the conversion will be validated and some novel factors will be identified. Although the limitation in design, however the results of neuropsychological tests may provide important contribution to the future multiple-nation prevention clinical trial design for Alzheimer’s disease.
Acknowledgement
We thank Dr Zhaolan Ding and Yan Zhou (Huashan Hospital, Shanghai China) for assistance in collecting data. Thanks Foundation of Biomedical Research and Innovation for the financial support of the project.
References
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353-356.
- Jia Q, Deng Y, Qing H (2014) Potential therapeutic strategies for Alzheimer's disease targeting or beyond ß-amyloid: insights from clinical trials. Biomed Res Int 2014: 837157.
- Cummings JL, Morstorf T, Zhong K (2014) Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 6: 37.
- Holtzman DM, Morris JC, Goate AM (2011) Alzheimer's disease: the challenge of the second century. SciTransl Med 3: 77sr1.
- Tarawneh R, Holtzman DM (2012) The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring HarbPerspect Med 2: a006148.
- Mitchell AJ, Shiri-Feshki M (2009) Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. ActaPsychiatrScand 119: 252-265.
- Zhou B, Nakatani E, Teramukai S, NagaiY, Fukushima M; Alzheimer’s Disease Neuroimaging Initiative (2012) Risk classification in mild cognitive impairment patients for developing Alzheimer's disease. J Alzheimers Dis 30: 367-375.
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, et al. (2011)The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: 270-279.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, et al. (2011) The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 263-269.
- Zhou B, Zhao Q, Teramukai S, Ding D, Guo Q, et al. (2010) Executive function predicts survival in Alzheimer disease: a study in Shanghai. J Alzheimers Dis 22: 673-682.
- Gross AL, Sherva R, Mukherjee S, Newhouse S, Kauwe JS, et al. (2014) Calibrating longitudinal cognition in Alzheimer's disease across diverse test batteries and datasets. Neuroepidemiology 43: 194-205.
- Acevedo A, Loewenstein DA, Agrón J, Duara R (2007) Influence of sociodemographic variables on neuropsychological test performance in Spanish-speaking older adults. J ClinExpNeuropsychol 29: 530-544.
- Manly JJ, Jacobs DM, Sano M, Bell K, Merchant CA, et al. (1999) Effect of literacy on neuropsychological test performance in nondemented, education-matched elders. J IntNeuropsycholSoc 5: 191-202.
- Ganguli M, Snitz BE, Lee CW, Vanderbilt J, Saxton JA, et al. (2010) Age and education effects and norms on a cognitive test battery from a population-based cohort: the Monongahela-Youghiogheny Healthy Aging Team. Aging Ment Health 14:100-107.
- Nesset M, Kersten H, Ulstein ID (2014) Brief Tests such as the Clock Drawing Test or Cognistat Can Be Useful Predictors of Conversion from MCI to Dementia in the Clinical Assessment of Outpatients. Dement Geriatr Cogn Dis Extra 4: 263-270.
- Devanand DP, Liu X, Brown PJ, Huey ED, Stern Y, et al. (2012) A two-study comparison of clinical and MRI markers of transition from mild cognitive impairment to Alzheimer's disease. Int J Alzheimers Dis 2012: 483469.
- Peters F, Villeneuve S, Belleville S (2014) Predicting progression to dementia in elderly subjects with mild cognitive impairment using both cognitive and neuroimaging predictors. J Alzheimers Dis 38: 307-318.
- Tierney MC, Moineddin R, McDowell I (2010) Prediction of all-cause dementia using neuropsychological tests within 10 and 5 years of diagnosis in a community-based sample. J Alzheimers Dis 22: 1231-1240.
- Brenowitz WD, Kukull WA, Beresford SA, Monsell SE, Williams EC (2014) Social relationships and risk of incident mild cognitive impairment in U.S. Alzheimer's disease centers. Alzheimer Dis AssocDisord 28: 253-260.
- Eckerström C, Olsson E, Klasson N, Berge J, Nordlund A, et al. (2015) Multimodal prediction of dementia with up to 10 years follow up: the Gothenburg MCI study. J Alzheimers Dis 44: 205-214.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 11054
- [From(publication date):
April-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10169
- PDF downloads : 885