Commentary Open Access
Sex-Gender-Conflicts in Aquatic Hermaphrodites: are Genes Immortal?
Ulrich Kutschera*
Institute of Biology, University of Kassel, D-34132 Kassel, Germany
- *Corresponding Author:
- Ulrich Kutschera
Institute of Biology, University of Kassel
D-34132 Kassel, Germany
Tel: 49-561-804-4467
E-mail: kut@uni-kassel.de
Received date: February 27, 2017; Accepted date: April 11, 2017; Published date: April 18, 2017
Citation: Kutschera U (2017) Sex-Gender-Conflicts in Aquatic Hermaphrodites: are Genes Immortal? J Marine Sci Res Dev 7:225. doi: 10.4172/2155-9910.1000225
Copyright: © 2017 Kutschera U. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
When the first edition of Richard Dawkin’s book The Selfish Gene was published (1976), the view that “altruistic behaviour” in animal populations is a common phenomenon was widely accepted. Dawkins questioned this interpretation and proposed his concept of the selfish (i.e., immortal) gene, which explains a number of phenomena, such as the evolution of anisogamy, or the roles of males vs. females during sexual reproduction in gonochorists (birds, mammals). In this 40th-2016-anniversary-analysis of Dawkins book I argue, based on observations on populations of “egoistic”, (hermaphroditic) freshwater leeches of the genus Erpobdella characterized by intraspecific cocoon cannibalism, that his theoretical deductions were basically correct. In addition, sex-gender-conflicts in leeches are discussed with reference to the avoidance of the female role in populations of these hermaphrodites. The idea of the “immortal (i.e., selfish) gene” is attributed to the 19th-century-work of the German zoologist August Weismann, and the “Post-Dawkinsian” concept of intragenomic conflicts is addressed.
Keywords
Dawkins; Cannibalism; Leeches; Selfish gene; Sex-genderconflict
Introduction
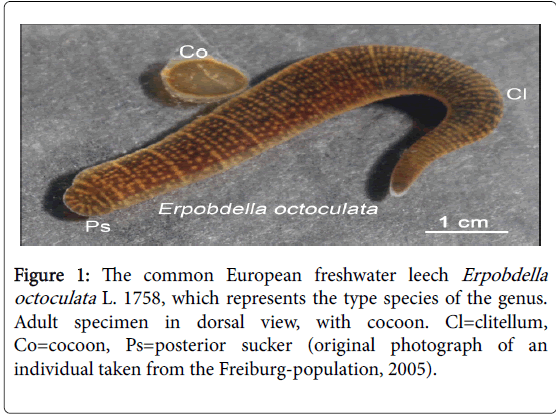
In a recent article published in this journal it has been documented that the terms “sex” (i.e., fertilization) and “gender” (i.e., the development of juveniles into adult, fertile males/females), as well as the distinction between gonochorists (such as birds or mammals) and hermaphrodites, originated in the 19th century [1]. In this contribution, I will focus on hermaphroditic invertebrates (Annelida). The majority of leeches (annelids, class Hirudinea/Clitellata) inhabit freshwater and/or marine ecosystems. They are, like earthworms, protandric hermaphrodites, and hence first act as males (distributors of sperm), and thereafter as females (providers of egg cells) [2]. Accordingly, “sex and gender-conflicts” have long been suggested to exist in these highly evolved “worms of character” (Figure 1) [3-5].
Over the past 40 years, the reproductive behaviour of several members of the Hirudinea was studied by the author, notably that of the morphologically simple, “worm-leeches” of the genus Erpobdella. These predators suck off prey organisms with the aid of their musculous pharynx [6]. The following account is based in part on unpublished observations on populations of the type-species E. octoculata L. 1758 (body length ca. 5 cm) that were maintained in freshwater aquaria (Figure 1). The results are discussed with reference to Richard Dawkin’s book The Selfish (or Immortal ) Gene (1. ed. 1976; 14th-anniversary ed. 2016) [7]. In addition, the neglected ideas of the German zoologist August Weismann (1834-1914) are addressed (Figure 1) [8,9].
Selfish Behavior in Freshwater Leeches
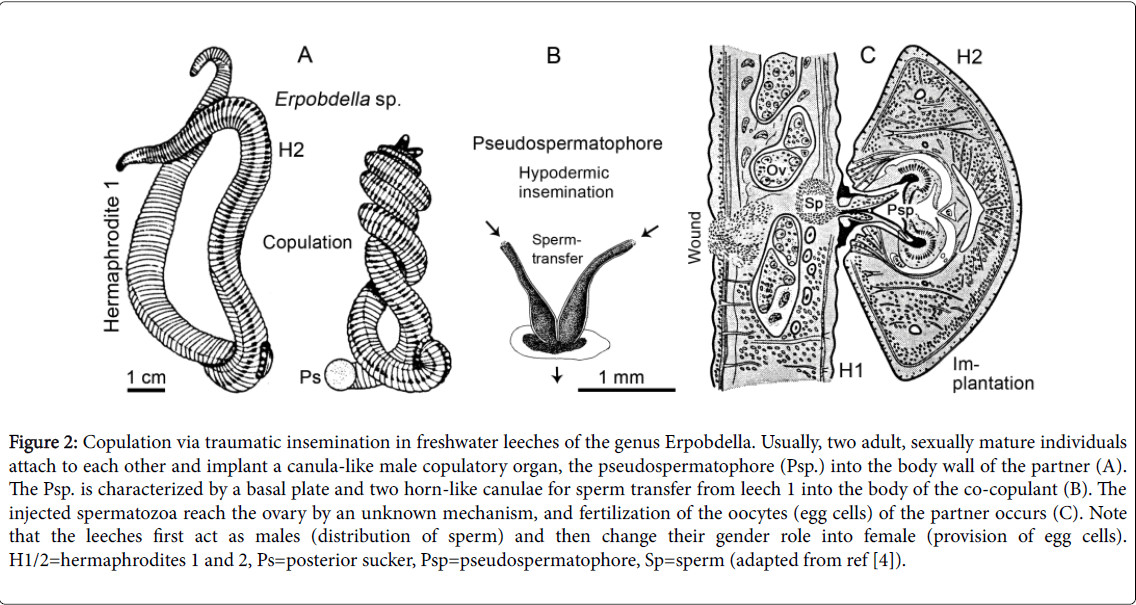
During the warm season (May to September), adult leeches, when kept isolated for a few days, readily copulate as shown in Figure 2A. The annelids first act as males (production and distribution of sperm), and thereafter as females (provision of eggs). In these protandric hermaphrodites, the transfer of sperm occurs via so-called “pseudospermatophores” (Psp.), i.e., canula-like transient copulatory organs that are produced within the male gonophore (Figure 2B and 2C). These solid structures are actively pushed into the body wall of the partner and thereby cause a large wound that is visible after the Psp. has lost its function and degrades (traumatic insemination). Hence, in Erpobdella and related taxa, the “sex-act” (fertilization) [1] is a violent sequence of events, but usually leeches 1 and 2 mutually transfer a Psp. in a “give-and-take-like”-mode of copulation [4,5,10,11]. As Figure 2C shows, sperm is pumped into the body of the partner. As a result, the male gametes reach the ovaries, where they fertilize the eggs. Since both mature adult leeches first function as distributors of sperm (male) and thereafter as providers of egg cells (female), their gender roles are in most cases identical [1] (self-fertilization does not occur in Erpobdella ). However, I have occasionally observed that individual hermaphrodites rapidly transfer their Psp. and then disappear without being “stabbed” by the corresponding Psp. of the attacked, sexually mature partner. Hence, in accordance with observations on other hirudineans [11,12], “worm-leeches” of the genus Erpobdella may have a tendency to act as males and avoid the gender role of being female (i.e., to release the fertilized eggs into a capsule, the cocoon) (Figure 3).
Figure 2: Copulation via traumatic insemination in freshwater leeches of the genus Erpobdella. Usually, two adult, sexually mature individuals attach to each other and implant a canula-like male copulatory organ, the pseudospermatophore (Psp.) into the body wall of the partner (A). The Psp. is characterized by a basal plate and two horn-like canulae for sperm transfer from leech 1 into the body of the co-copulant (B). The injected spermatozoa reach the ovary by an unknown mechanism, and fertilization of the oocytes (egg cells) of the partner occurs (C). Note that the leeches first act as males (distribution of sperm) and then change their gender role into female (provision of egg cells). H1/2=hermaphrodites 1 and 2, Ps=posterior sucker, Psp=pseudospermatophore, Sp=sperm (adapted from ref [4]).
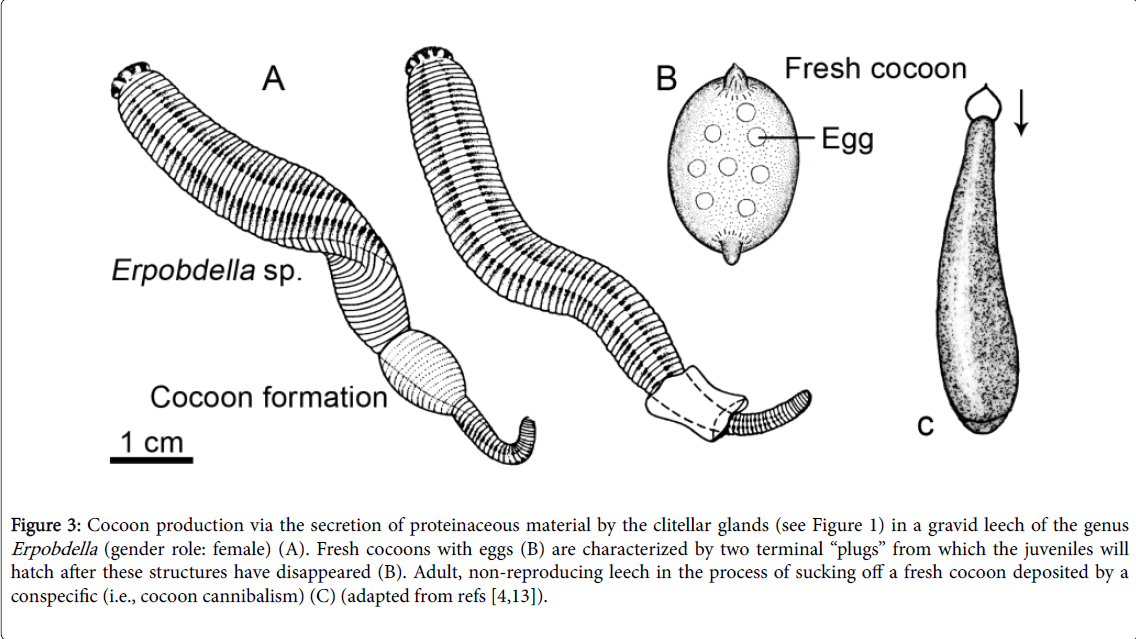
A few days after copulation, the “female” (gravid) leeches produce cocoons via the secretion of a transparent fluid from the clitellar region of their body. This process (Figure 3A) has been described in detail for different members of the Erpobdelidae and appears to be rather uniform. In 1979, I discovered that, whenever a hungry E. octoculata that is not about to produce a cocoon, creeps by chance into the vicinity of a reproducing conspecific in the female role, a rapid, fierce attack occurs. Using its pharynx, the leech sucks off the proteinaceous fluid from the emerging cocoon of the reproducing conspecific, inclusive of the enclosed eggs. Hence, intra-specific destruction and feeding on the cocoons of competing individuals is a well-documented phenomenon [11-15].
Figure 3: Cocoon production via the secretion of proteinaceous material by the clitellar glands (see Figure 1) in a gravid leech of the genus Erpobdella (gender role: female) (A). Fresh cocoons with eggs (B) are characterized by two terminal “plugs” from which the juveniles will hatch after these structures have disappeared (B). Adult, non-reproducing leech in the process of sucking off a fresh cocoon deposited by a conspecific (i.e., cocoon cannibalism) (C) (adapted from refs [4,13]).
Density Regulation vs. Selfish Individuals
During the 1980s, I had interpreted this behaviour as a means for altruistic (unselfish) “density regulation” in growing populations of Erpobdella spp. However, a few years later, and after many more observations, I re-interpreted this behaviour in the light of Dawkin’s principle of “egoistic” individuals (or genes) [7]. The predatory leeches cannot store blood etc. and therefore are always in search of food (insect larvae, small annelids etc. are rapidly sucked off with the aid of the musculous pharynx) [13-15]. Hence, whenever they detect a fresh cocoon of a reproducing individual, they “interpret” this proteinaceous, soft material as prey, so that the term “intraspecific cocoon cannibalism” is appropriate [6].
However, I have never observed that a gravid leech attacks its own cocoon (with fertilized eggs, i.e., offspring). Therefore, it is obvious that only the offspring of competitors is removed from the population, and hence the Darwinian fitness (i.e., number of surviving juveniles) of the attacking, predatory leech is maximized via this “egocentric” behaviour.
In 1979, a cannibalistic population of E. octoculata that existed in a pond in Freiburg i. Br. (Germany) was analyzed [13]. Twenty-fiveyears later, i.e., in a distant population comprised of members of the ca. 25th generation of these aggressive “1979-leeches” (Figure 1), I observed/documented the same “egoistic” behavior (unpublished results). This finding indicates that this drastic form of intraspecific competition has a genetic basis. It should be noted that in other populations of E. octoculata (and related species) the intensity of intraspecific cocoon cannibalism was not so severe as that described for the “Freiburg-leeches” [11-15].
Evolution of Sex Roles in Hermaphrodites
Dawkins [7] interpreted the “evolution of the sexes (male/female)”, specifically anisogamy, in the light of “selfish reproductive elements” (i.e., small, motile sperm cells that exploit the resources stored in large egg cells, see refs. [1,16,17]. This gametic asymmetry in male/female resource investment explained, why the occurrence of maternal care is common, whereas paternal breeding activities in males is rare (for gonochorists). It should be noted that these behavioural patterns are also apparent in hermaphrodites. These animals produce male gametes (sperm) and egg cells within the same body, so that they can perform both sex roles during reproduction [1].
As discussed above, and illustrated in Figures 2 and 3, sexually mature leeches of the genus Erpobdella (as well as those of the genera Helobdella and Glossiphonia ; unpublished results) prefer to act as males rather than function as females. However, more work is required to further analyze this evolved “gender-bias” in different groups of the Hirudinea.
Selfish Elements and the Idea of the Immortal Gene
As briefly mentioned in passing by Dawkins [7], the “Immortalityof- life-hypothesis” originated with the work of August Weismann. The German zoologist is the founder of the “germ plasm” concept of heredity and the Neo-Darwinian theory of biological evolution. In addition, he was a pioneer in hydrobiology and aging research [8,9]. According to Weismann, the “germ plasm”, which he regarded as a special part of the germ cells (eggs and sperm), serves to carry over, from generation to generation, the factors of inheritance, which are, in modern terms, the chromosomes composed of DNA and protein [18,19].
Weismann [19,13] was convinced that the germ plasm is continuous, from living organisms back to the earliest forms of life. Moreover, he distinguished between the potentially immortal germline vs. the somatic cells constituting the body of the animal, which dies soon after sexual reproduction. Weismann wrote that “Life is continuous … ever since its first appearance on earth, in the lowest organisms, it has continued without break; every individual alive today … is to be derived in an unbroken line from the first and lowest forms” [20].
In this sentence, the essence of Dawkins’s “immortal (or selfish) gene”-concept is encapsulated [20], which may explain reproductive patterns in both gonochorists and hermaphrodites (Figures 2 and 3). With respect to humans, it says that our body (i.e., Weismann’s soma) is only a vehicle for the transfer of ancient factors of heredity (replicators), which later evolved into genes, via the continuous germline. However, according to Dawkins, humans have the power to “rebel against the tyranny of the selfish replicators” (i.e., genes), despite the fact that “we are build as gene machines, and cultured as meme machines” [7].
Finally, it should be noted that Weismann [20] extended the “Darwin-Wallace-principle of natural selection” from individual organisms down to cells and heritable units. Hence, in 1913, the German biologist pre-figured the “Dawkinsian” view of gene-centered evolution [7].
Conclusion
In his book the Selfish Gene , Dawkins [7] discussed/criticized several interrelated issues: conflicts with respect to sexual reproduction [1], the significance of group selection in populations of “egocentric” animals [2], and the notion of immortal (selfish) genes [3]. Based on ca. 40 years of studies on populations of freshwater leeches (Figures 1-3), it is illustrated here that the “Dawkinsian” interpretations [1] and [2] can be verified. This unequivocal conclusion rests on numerous observations and experiments [6,11-15]. However, the idea [3], proposing selfish DNA-sequences, is more controversial. In a metaphorical sense, “genes” are potentially immortal, and natural selection of competing (“egoistic”) individuals, as well as sex-genderconflicts, are a reality in evolving populations of animals, plants and some microbes.
Finally, “Post-Dawkinsian” theorists have proposed that the genome of every organism should be interpreted as a “battlefield of conflicting interests” [21,22]. This concept of “intragenomic conflicts” via “selfish genetic elements” should be explored in more detail in order to extend the insights summarized four decades ago by the British biologist Richard Dawkins in his seminal book [7].
Acknowledgement
This article is dedicated to the memory of Olaf Breidbach (1957-2014). The author thanks three referees for helpful comments on earlier versions of the manuscript and the Alexander von Humboldt- Stiftung (Bonn, Germany) for support (AvH-Fellowship Stanford 2013/14 to U. K).
References
- Kutschera U (2016) Sex versus gender in sea urchins and leeches two centuries after Lamarck 1816. J Marine Sci Res Dev 6:1-3.
- Breidbach O, Kutsch W (eds.) (1995) The Nervous Systems of Invertebrates: An Evolutionary and Comparative Approach. BirkhäuserVerlag, Basel.
- Kutschera U, Elliott JM (2010) Charles Darwin's observations on the behaviour of earthworms and the evolutionary history of a giant endemic species from Germany, Lumbricusbadensis (Oligochaeta: Lumbricidae). ApplEnvironm Soil Sci. 2:1-11.
- Mann KH (1962) Leeches (Hirudinea). Their Structure, Physiology, Ecology and Embryology. Pergamon Press, Oxford.
- Sawyer RT (1986) Leech Biology and Behaviour. Vol. I, II, III. Clarendon Press, Oxford.
- Kutschera U, Wirtz P (2001) The evolution of parental care in freshwater leeches. Theory Biosci 120:115-137.
- Dawkins R (2016) The Selfish Gene. 40th Anniversary Edition. Oxford Landmark Science, Oxford.
- Kutschera U (2014) From aquatic biology to Weismannism: Science versus ideology. J Marine Sci Res Dev 4:e131.
- Kutschera U, Hossfeld U (2013) Alfred Russel Wallace (1823-1913): the forgotten co-founder of the Neo-Darwinian theory of biological evolution. Theory Biosci 132:207-214.
- Shain DJ (ed.) (2009) Annelids in Modern Biology. Wiley-Blackwell, New York.
- Elliott JM, Dobson M (2015) Freshwater Leeches of Britain and Ireland. Keys to the Hirudinea and a Review of their Ecology. Freshwater Biological Association Scientific Publication No. 69, Cumbria.
- Kutschera U, Weisblat DA (2015) Leeches of the genus Helobdella as model organisms for Evo-Devo studies. Theory Biosci 134:93-104.
- Kutschera U (1980) Bestandsregulation bei Egeln. Mikrokosmos 69:80-82.
- Kutschera U (1983) Dichteregulation durch intraspezifische Kokonzerstörung und Untersuchungen zur ortpflanzungsbiologie beim Egel Erpobdella octoculata L. (Hirudinea: Erpobdellidae). ZoolJbSyst 110:17-29.
- Kutschera U (2003) The feeding strategies of the leech Erpobdellaoctoculata (L.): A laboratory study. Internat Rev Hydrobiol 88:94-101.
- Parker GA, Baker RR, Smith VGF (1972) The origin and evolution of gamete dimorphism and the male/female phenomenon. J TheorBiol 36:529-535.
- Speijer D, Lukes J, Elias M (2015) Sex is a ubiquitous, ancient, and inherent attribute of eukaryotic life. Proc. Natl. Acad. Sci. USA 112:8827-8834.
- Kutschera U, Niklas KJ (2004) The modern theory of biological evolution: an expanded synthesis. Naturwissenschaften 91:255-276.
- Niklas KJ, Kutschera U (2014) Amphimixis and the individual in evolving populations: does Weismann’s Doctrine apply to all, most or a few organisms? Naturwissenschaften 101:357-372.
- Weismann A (1913) Vorträge über Deszendenztheorie. Bd. I, II. 3. Auflage. Verlag Gustav Fischer, Jena.
- Scherrer K, Jost J (2009) Response to commentaries on our paper gene and genon-concept: coding versus regulation. Theory Biosci 128:171-177.
- Burt A, Trivers R (2006) Genes in Conflict. The Biology of Selfish Genetic Elements. Belknap Press, Cambridge, Massachusetts.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 6033
- [From(publication date):
April-2017 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 5134
- PDF downloads : 899