Commentary Open Access
Sex versus Gender in Sea Urchins and Leeches Two Centuries after Lamarck 1816
Kutschera U*
Institute of Biology, University of Kassel, D-34132 Kassel, Germany
- *Corresponding Author:
- Ulrich Kutschera
Institute of Biology
University of Kassel
D-34132 Kassel, Germany
Tel: 40-561-804-4467
E-mail: kut@uni-kassel.de
Received date: October 17, 2016; Accepted date: October 28, 2016; Published date: November 01, 2016
Citation: Kutschera U (2016) Sex versus Gender in Sea Urchins and Leeches Two Centuries after Lamarck 1816. J Marine Sci Res Dev 6:210.doi:10.4172/2155-9910.1000210
Copyright: © 2016 Kutschera U. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Bi-parental (sexual) reproduction via the fusion of egg and sperm produced by adult female/male individuals in populations of animals and plants is a key process of Life on Earth. Here, the terms “sex” (fertilization) and “gender” (role of male/female individuals as providers of gametes) are described, based on the concepts of Carolus Linnaeus (1707-1778) and Julius Sachs (1832-1897). In 1816, Jean Lamarck (1744-1829) introduced the purple sea urchin (Paracentrotus lividus) as a new species, and six decades later, Oscar Hertwig (1849-1922) used this model organism to elucidate external fertilization at the sub-cellular level. Moreover, Hertwig referred to “gender” to denote male or female P. lividus-individuals (gonochorists). Sexual reproduction and gender-roles are also outlined with reference to the fish leech (Piscicola geometra), a protandrous hermaphrodite characterized by hypodermic insemination and a gender-ration of 50:50. Finally, gender-issues in vertebrate development and evolution are addressed.
Keywords
Gender; Leeches; Sea urchins; Sex; Lamarck
Introduction
No other words arouse more interest and excitement than “sex” and “gender”. However, clear, concise definitions of these terms sensu stricto are elusive, despite the fact that Carolus Linnaeus (1707-1778) published, in 1753, a “Sexual system” of plant systematics (with reference to gender issues in humans) that was later abandoned by botanists [1]. Recently, the term “sex” (in humans) was used with reference to organs, chromosomal complements and hormones related to reproduction. In contrast, “gender” was defined as “cultural attitudes that shape ‘feminine’ and ‘masculine’ behaviors that are learned and vary by culture, historical era, and ethnicity” [2]. In addition, in animal research, the “g-word” is used to indicate the attitude of investigators with respect to the handling of male or female laboratory animals [2]. Here, I analyze the original “Linnaean” meaning of “sex” within the context of bi-parental reproduction, with reference to Lamarck’s (1816) description of a marine model organism that has later been used to elucidate this process at the sub-cellular level. In addition, Charles Darwin’s (1809-1882) concept of different gender roles in sexually reproducing groups of males/females and hermaphroditic animals [3] is addressed.
Discussion
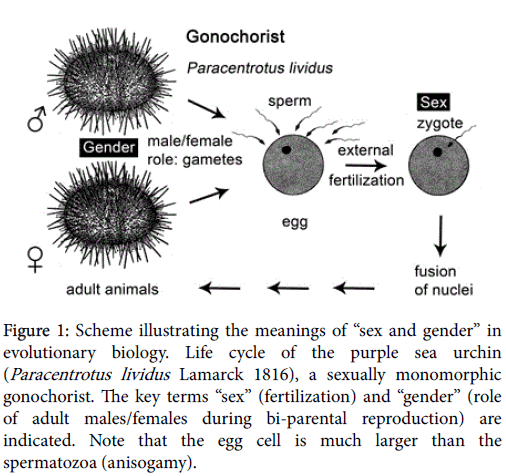
About 150 years ago, the German biologist Julius Sachs (1832 -1897), founder of experimental plant physiology [4], was working on a Textbook of Botany (1868), wherein he defined sex as “the material unification of two types of cells that lack the ability to develop in isolation, so that a product is created with the potential for development” [5]. According to Sachs, fertilization, i.e., the fusion of male and female gametes (“spermatozoid” plus “egg cell”), represents the universal “Geschlechtsact”, or “sex act”, leading, in most cellular organisms, to the generation of progeny. Hence, “sex” is a fertile cytological process: the merger of two different (haploid) gametes, or sperm-egg-fusion, to generate a totipotent cell, the (diploid) zygote, from which a genetically distinct, new individual can develop (Figure 1).
Figure 1: Scheme illustrating the meanings of “sex and gender” in evolutionary biology. Life cycle of the purple sea urchin (Paracentrotus lividus Lamarck 1816), a sexually monomorphic gonochorist. The key terms “sex” (fertilization) and “gender” (role of adult males/females during bi-parental reproduction) are indicated. Note that the egg cell is much larger than the spermatozoa (anisogamy).
In egg-laying (oviparous) animals, the zygote is an independent system, whereas in mammals, after implantation into the womb, biochemical signals from the body of the mother are required for development to occur. In the following sections, the elucidation of fertilization is described, with reference to the question what “gender” means in developmental and evolutionary biology.
Two hundred years ago, the French biologist Jean-Baptiste de Lamarck (1744-1829) described a relatively large species of sea urchin, Toxopneustes lividus, that reaches diameters of up to 7.5 cm. Today known as the purple sea urchin (Paracentrotus lividus Lamarck 1816) [6], this edible marine invertebrate, which is distributed throughout the Mediterranean Sea and the Northern Atlantic, served as model organism for the elucidation of a key process in the life cycle of most multicellular eukaryotic organisms: fertilization. Paracentrotus lividus (Figure 1) is a subtidal invertebrate that feeds on algae and seagrasses; the echinoderms are attacked by a variety of predators, such as fish, gastropods and spider crabs.
Like humans, the purple sea urchin is a gonochorist: in populations, male or female individuals exist, with testes or ovaries, respectively. However, in some groups of sea urchins, hermaphrodites have been observed (individuals that contain both male and female gonads/ copulatory organs in the same body). Reproduction is achieved as follows. First, large groups of fertile P. lividus -individuals aggregate for spawning; then, the male (and female) individuals release gametes (sperm or eggs) into the water, so that external fertilization, the fusion of egg and sperm to form a zygote, can take place [7]. Purple sea urchins can be easily collected in their natural marine habitats and studied in the laboratory.
During the 1870s, the German zoologist Oscar Hertwig (1849-1922) used this model system for the analysis of fertilization at the subcellular level. Specifically, he studied what happens within the large egg-cell when a tiny sperm fuses to form a product with the potential for development (i.e., the “sex act” sensu Sachs 1868, Figure 1). Hertwig [8] collected adult, sexually mature sea urchins characterized by specific “Geschlechtsunterschiede” (gender-differences): male individuals with testes that produce/release spermatozoa, and females with ovaries that provide egg cells. Hertwig [8] established an “in vitroinsemination- system” by artificially removing sperm and eggs from the testes/ovaries of male/female P. lividus-individuals. These “sex-cells” (gametes) were mixed in a test tube and fertilization observed under a light microscope. The German zoologist was the first to document the “Befruchtungs act (fertilization [=sex]-act)” in detail. Based on numerous experiments, Hertwig [8] came to the conclusion that fertilization consists not only of the “Copulation zweier Zellen (copulation of two cells)”, but, moreover, the “Verschmelzung der beiden Zellkerne, Eikern und Spermakern (the fusion of the nuclei of egg and sperm)” [8]. Hence, the terms “sex” (fertilization) and “gender” (development of sexually mature individuals and role of these adults as males or females, respectively) can be traced back to the seminal paper of Hertwig [8] published in 1876. These classical definitions are still used today in the zoological literature with reference to the occurrence of sex within the Tree of Life, and gender roles during the reproduction of invertebrates, such as mollusks [9,10].
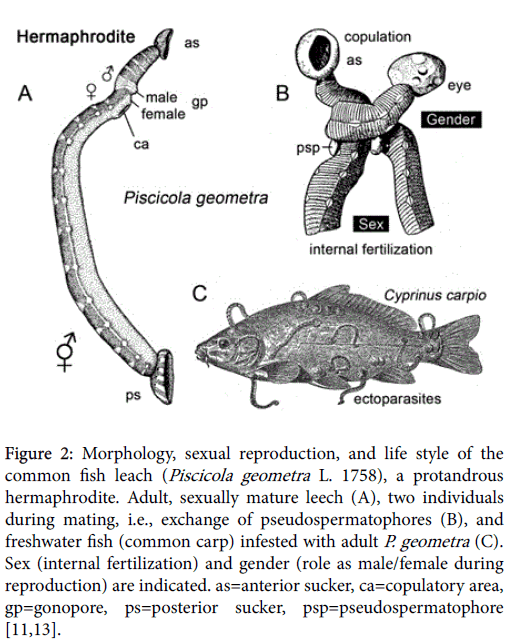
It should be mentioned that the German zoologist [8] also introduced the terms “gonochorism” and “hermaphroditism” to denote cells (or organisms) that are equipped with only one (male vs. female) or both reproductive functions (male plus female in one body). In his 1876-paper, Hertwig [8] referred to annelids (leeches) of the genus Piscicola as model organisms for the study of internal fertilization in a hermaphroditic invertebrate. Hence, “sex and gender” may also be studied in leeches (Hirudinea), predaceous or parasitic annelids that occur in both freshwater and marine ecosystems [11,12].
The common fish leech (Piscicola geometra L. 1758) occurs in ponds as well as salty waters [11]. These small (ca. 15 mm long) leeches are ectoparasites on the skin of members of the family Salmonidae and other fish species (perch, Perca fluviatilis, pike, Esox lucius, common carp, Cyprinus carpio etc.); in seawater, P. geometra have been collected from the body surface of rays and sole. The following account of “sex and gender” in this representative hermaphrodite is based on my own observations of P. geometra maintained in freshwater aquaria. During the summer (May to August), fish leeches reproduce via copulation (mutual sperm transfer), followed by the deposition of small (ca. 1,5 mm long) cocoons that each contain only one fertilized egg. P. geometra is a protandrous hermaphrodite, i.e., the male reproductive system (production of sperm) becomes active first, followed by the female function (provision of egg cells) (Figure 2A). In populations of P. geometra and other hermaphrodites, the gender-ratio (males:females) is exactly 50:50, whereas in groups of interbreeding gonochorists more males than females may occur.
Figure 2: Morphology, sexual reproduction, and life style of the common fish leach (Piscicola geometra L. 1758), a protandrous hermaphrodite. Adult, sexually mature leech (A), two individuals during mating, i.e., exchange of pseudospermatophores (B), and freshwater fish (common carp) infested with adult P. geometra (C). Sex (internal fertilization) and gender (role as male/female during reproduction) are indicated. as=anterior sucker, ca=copulatory area, gp=gonopore, ps=posterior sucker, psp=pseudospermatophore [11,13].
Selfing has not been observed in P.geometra , i.e., hypodermic cross insemination regularly occurs via the reciprocal transfer of pseudospermatophores (Psp., i.e., transitorily existing packages of sperm). Mating (Figure 1B) results in an exchange of sperm from leech 1 to individual 2, and lasts for up to 30 min. The Psp is released from the male gonopore and attached to the copulatory area of the partner. This region beneath the male/female gonopores represents an extension of the ovary [11,13]. After sperm exchange (mating), the cocopulants separate, and internal fertilization takes place. About 3 weeks later, the leeches, whose gender roles were first “male” and thereafter “female”, produce tiny cocoons from which one juvenile leech hatches (length ca. 1 mm). Like their parents, juvenile P. geometra attach to host organisms and suck the body fluids from fish, i.e., they are ectoparasitic rhynchobdellids (Figure 2C). Interestingly, in populations of brown trout (Salmo trutta L.), sexually mature male fish were found to be more severely (and frequently) infested by skin ectoparasites than mature female individuals or juvenile fish of either gender [14]. Hence, in fish (and other vertebrates), a gender-specific susceptibility to parasitic infestation has evolved, with the result that females, which produce large eggs, have a more efficient defense system than males, which release numerous tiny sperm (anisogamy [15]).
Summary
In summary, our analysis has shown that, with the description of the purple sea urchin (P. lividus ), Lamarck [6] characterized a marine invertebrate taxon that, six decades later, was used by Hertwig [8] as a model system to define/elucidate “sex” (fertilization; specifically, the fusion of cells/nuclei) and “gender” (function of adult individuals as males or females) (Figure 1). In leeches (Figure 2) and other hermatphroditic invertebrates, the “sex-act”, which generates variable offspring for natural selection to act upon [16], cannot be studied directly, due to internal fertilization. However, gender-issues, such as gchoice, g-conflict etc., have been studied extensively in hermaphrodites such as freshwater snails of the genus Physa [10]. Taken together, the “s- and g-words” can be traced back to the scientific work of Linnaeus, Sachs and Hertwig [1,5,8]. Accordingly, these terms should be used, when applied to humans and other vertebrates with respect to genderroles of males vs. females [17,18], in their original meanings [9,10], as described in this article.
Acknowledgement
Author thanks the referees for helpful comments on earlier versions of the manuscript and the Alexander von Humboldt-Foundation (Bonn, Germany) for financial support (AvH-Stanford 2013/14 to UK).
References
- Bremer B (2007) Linnaeus’ sexual system and flowering plant phylogeny. Nordic J of Botany 25: 5 -6.
- Richardson SS, Reiches M, Shattuck-Heidorn H, LaBonte ML, Consol T (2015) Opinion: Focus on preclinical sex differences will not address women’s and men’s health disparities. ProcNatlAcadSci USA 112: 13419-13420.
- Darwin C (1871) The Descent of Man, and Selection in Relation to Sex. John Murray, London.
- Kutschera U (2015) Comment: 150 years of an experimental plant physiology. Nature Plants 1: 1-3.
- Sachs J (1868) Textbook ofbotany. Wilhelm Engelmann, Leipzig.
- de Lamarck JB (1816) Natural History of animals without vertebrae. Tome III. Paris: Deterville/Verdière.
- Lawrence JM (2007) Edible Sea Urchins: Biology and Ecology. 2. Ed. Elsevier, Amsterdam.
- Hertwig O (1876) Contributions to knowledge of the formation, fertilization and division of the animal egg MorphologischesJahrbuch 1: 347-434.
- Speijer D, Lukes J, Elias M (2015) Sex is a ubiquitous, ancient, and inherent attritube of eukaryotic life. ProcNatlAcadSci USA 112:8827-8834.
- Wethington AR, Dillon RT (1996) Gender choice and gender conflict in a non-reciprocally mating simultaneous hermaphrodite, the freshwater snail, Physa. AnimBehav 51: 1107-1118.
- Nesemann H, Neubert E (1999) Annelida, Clitellata: Branchiobdellida, Acanthobdellea, Hirudinea. In: Schwoerbel J, Zwick P (Eds) Suesswasserfauna von Mitteleuropapp:1-178.
- Kutschera U, Weisblat DA (2015) Leeches of the genus Helobdella as model organisms for Evo-Devo studies. Theory Biosci 134: 93-104.
- Kearn GC (2004) Leeches, Lice and Lampreys. A Natural History of Skin and Gill Parasites of Fishes. Springer, Dordrecht, The Netherlands.
- Pickering AD, Christie P (1980) Sexual differences in the incidence and severity of ectoparasitic infestation of the brown trout, Salmotrutta L. J Fish Biol 16: 669-683.
- Schärer L, Rowe L, Arnqvist G (2012) Anisogamy, chance and the evolution of sex roles. Trends EcolEvol 27: 260-264.
- Kutschera U (2014) From aquatic biology to Weismannism: Science versus ideology. J Marine Sci Res Dev 4: e131.
- Janicke T, Häderer IK,Lajeunesse MJ, Anthes N (2016) Darwinian sex roles confirmed across the animal kingdom. SciAdv 2: e1500983.
- Kutschera U (2016) Men are high-metabolic chimps. Science.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 17955
- [From(publication date):
October-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 17028
- PDF downloads : 927